Huperzine A tablet and preparation method thereof
A technology of huperzine A tablets and huperzine A, which is applied in the direction of pharmaceutical formula, medical preparations of non-active ingredients, pill delivery, etc. It can solve the problems of slowness, half an hour to reach, and unstable quality, etc. Achieve the effects of improving dissolution rate, accelerating disintegration speed, improving bioavailability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
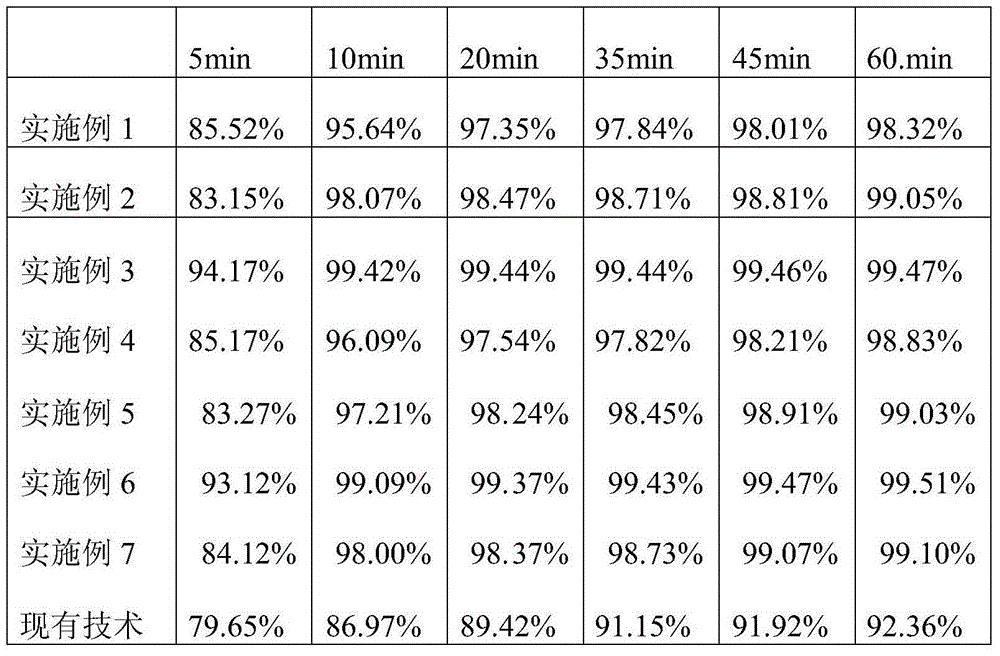
Examples
Embodiment 1
[0014] Composition: Huperzine A 0.5g, starch 320g, lactose 340g, crospovidone 50g, hypromellose 6g, magnesium stearate 3.5g, made into 10,000 tablets.
[0015] Preparation method: 1) Micronized Huperzine A, and all pass through a 120-mesh sieve; 2) Pass the remaining raw materials through a 100-mesh sieve for use; 3) Combine Huperzine A, starch, lactose, and 20g of crospovidone Mix well, then add the hypromellose solution, the solvent of the hypromellose solution is 100g ethanol and 170g water; 4) After being made into a soft material, it is granulated through a 20-mesh sieve, dried, and the inlet air temperature is controlled. Do not exceed 65°C and the water content of the granules is below 4%, then add magnesium stearate and the remaining crospovidone and mix well, then pass through an 18-mesh sieve to obtain mixed granules; 5) Determine the content of Huperzine A in the mixed granules , Determine the tablet weight according to the content, and place the mixed granules on a ta...
Embodiment 2
[0017] Composition: Huperzine A 0.5g, starch 380g, lactose 280g, crospovidone 50g, povidone K306g, magnesium stearate 3.5g, made into 10,000 tablets.
[0018] Preparation method: 1) Micronized Huperzine A, and all pass through a 120-mesh sieve; 2) Pass the filler, disintegrant, and lubricant through a 100-mesh sieve for use; 3) Put the prescription amount of Huperzine A, filled And 22g of disintegrant are mixed uniformly, and then add a prescription amount of binder solution, the solvent of the binder solution is 115g ethanol and 165g water; 4) After being made into soft material, it is granulated through a 20-mesh sieve and dried. Dry, control the inlet air temperature not to exceed 65°C, and the moisture content of the particles below 4%, then add the prescribed amount of lubricant and the remaining disintegrant, mix well, pass through an 18-mesh sieve to obtain mixed particles; 5) Determine the stone in the mixed particles The content of pyrotaxine A is determined according to...
Embodiment 3
[0020] Composition: Huperzine A 0.5g, starch 420g, lactose 240g, crospovidone 50g, povidone K306g, magnesium stearate 3.5g, and benzidine 0.5g, made into 10,000 tablets.
[0021] Preparation method: 1) Micronized Huperzine A, and all pass through a 120-mesh sieve; 2) Pass the remaining raw materials through a 100-mesh sieve for use; 3) Combine Huperzine A, starch, lactose, and 25g of crospovidone Mix well, then add the povidone K30 solution, the solvent of the povidone K30 solution is 120g ethanol and 150g water; 4) After making the soft material, pass through a 20-mesh sieve to granulate, dry, and control the inlet air temperature to not exceed At 65°C, the moisture of the granules is below 4%, then add magnesium stearate and the remaining crospovidone and benedirach to mix well, then pass through an 18-mesh sieve to obtain mixed granules; 5) Determine Huperzine A in the mixed granules According to the content, the tablet weight is determined, and the mixed granules are placed o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com