Huperzine A polymorphism body, preparation method thereof, pharmaceutical composition comprising polymorphism body and application thereof
A technology for huperzine A and crystal form, which is applied in the field of preparation of huperzine A polymorph and its synthesis process research field, and can solve problems such as the raw material crystal form of huperzine A is not involved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of huperzine A crystal form I.
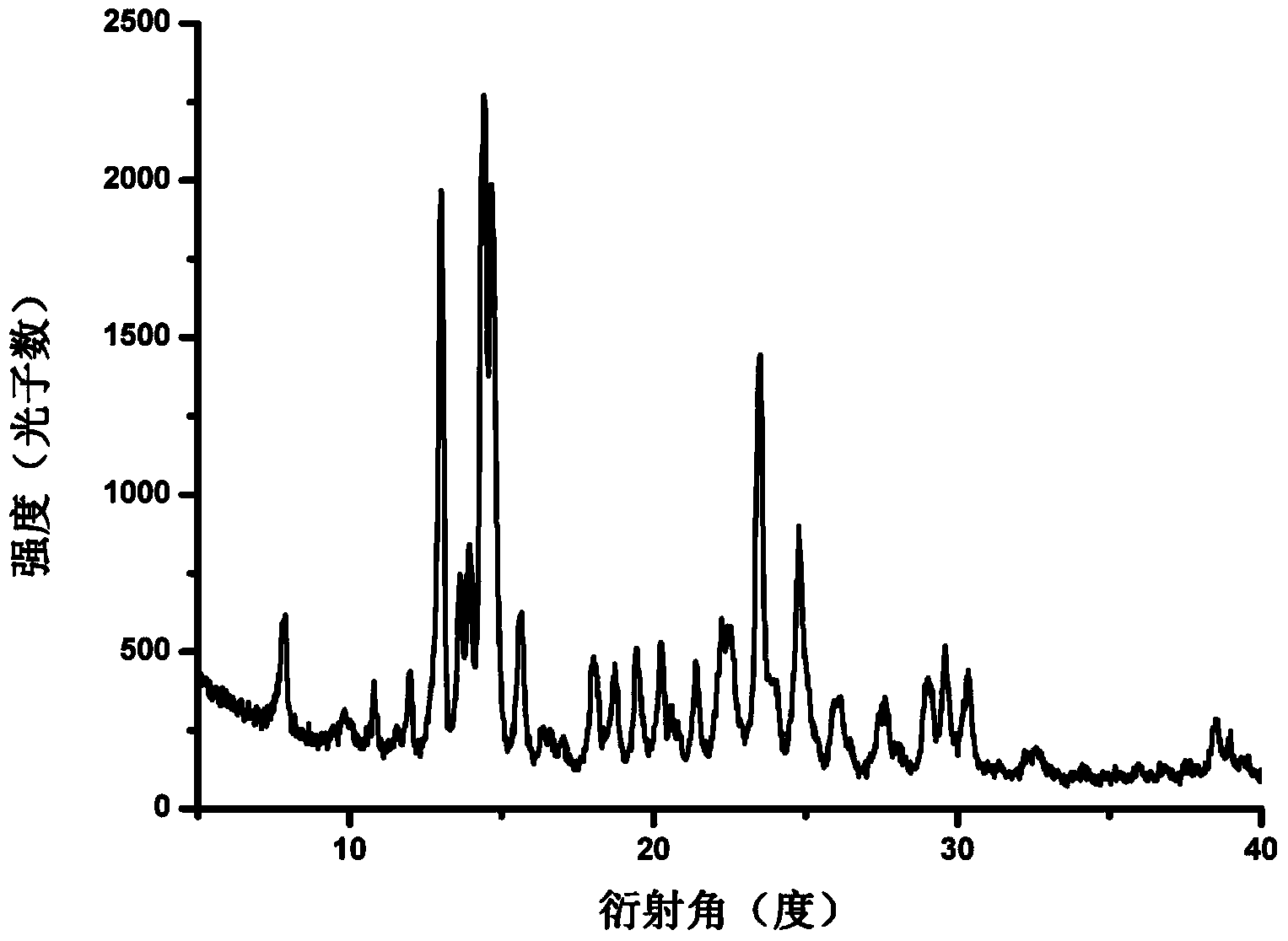
[0057] Mix 50 mg of huperzine A raw material (amorphous) with 1 ml of acetone, heat to 50° C. and keep stirring at 50° C. for 3 days, and filter to obtain a white solid. After evaporating the solvent at room temperature, the white solid was dried under reduced pressure with an oil pump for 12 hours to obtain a crystalline powder, which was determined by X-ray powder diffraction, showing that the obtained crystal form was Form I. The specific peak positions are shown in Table 1 below.
[0058] Table 1: X-ray powder diffraction data of Huperzine A crystal form I in Example 1 of the present invention
[0059]
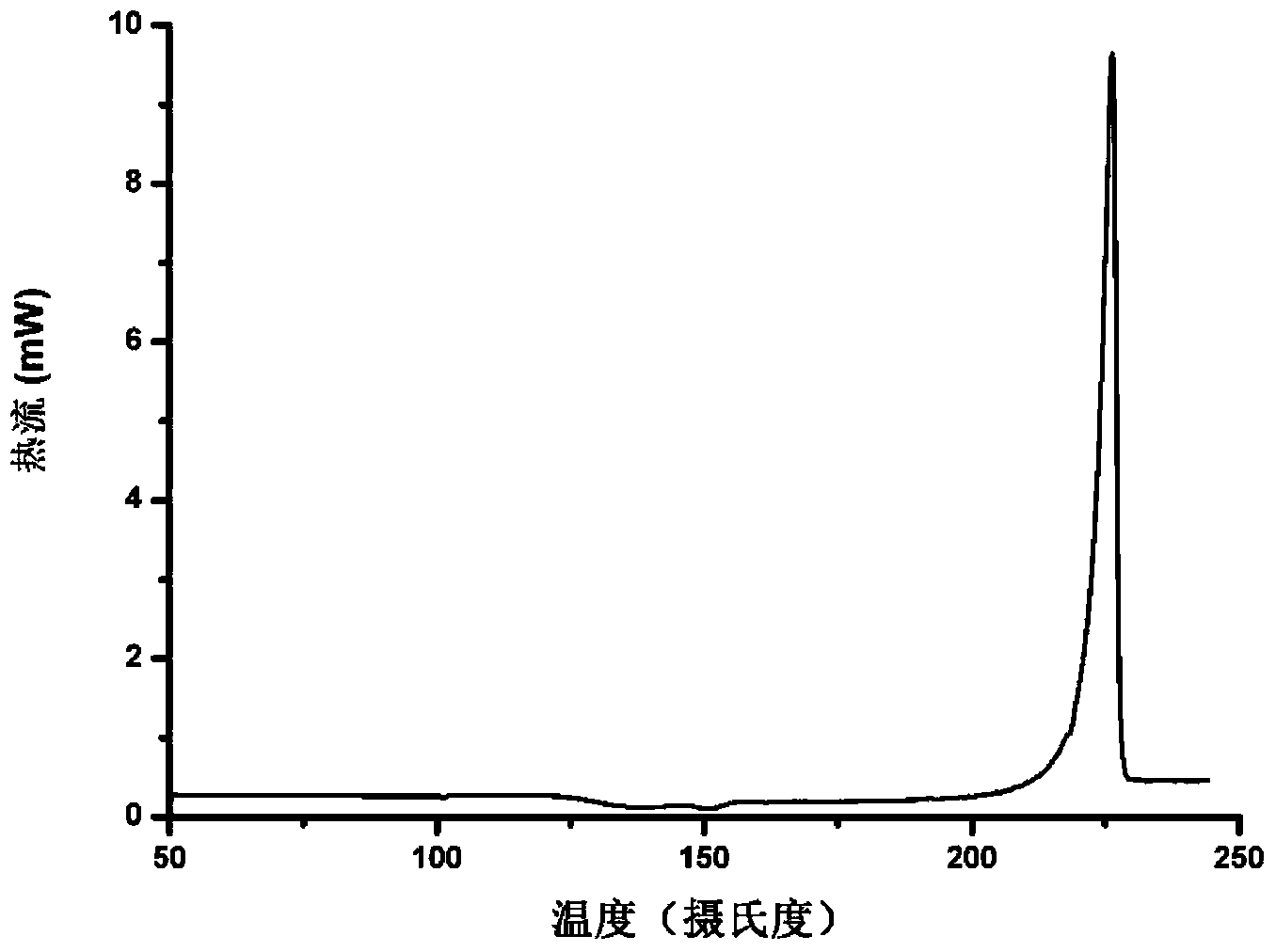
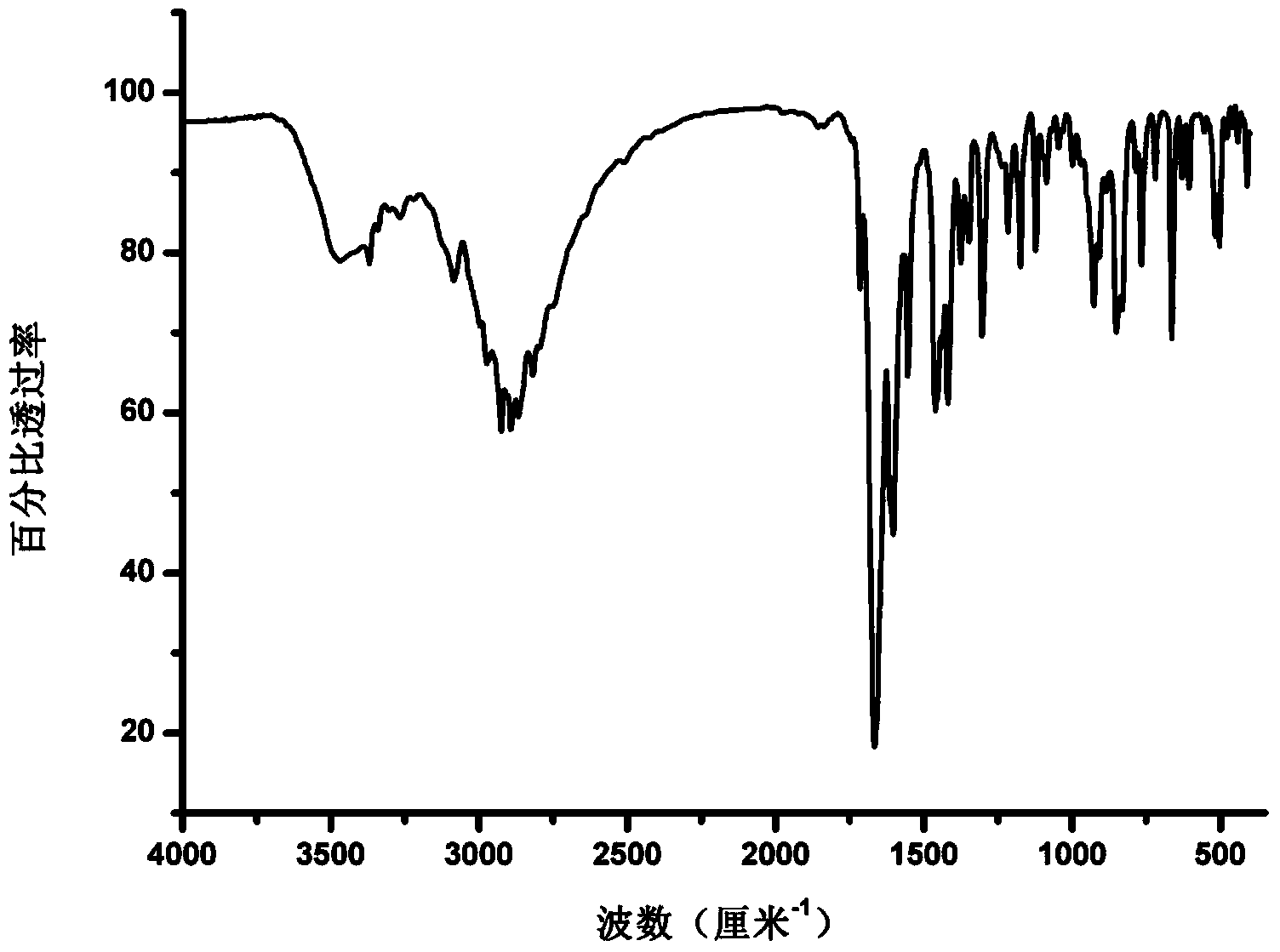
[0060] Other tests were carried out on the obtained sample, and the obtained DSC thermal spectrum, infrared spectrum and Raman spectrum were as follows: Figure 1b , 1c , 1d shown.
Embodiment 2
[0061] Example 2: Preparation of huperzine A crystal form II.
[0062] Put 25 mg of huperzine A crystal form I in an oven at 125 degrees Celsius and heat for 2 hours to obtain a crystalline powder, which was determined by X-ray powder diffraction to show that the obtained crystal form was crystal form II. The specific peak positions are shown in Table 2 below.
[0063] Table 2: X-ray powder diffraction data of Huperzine A crystal form II in Example 2 of the present invention
[0064]
[0065]
[0066] Other tests were carried out on the obtained sample, and the obtained DSC thermal spectrum, infrared spectrum and Raman spectrum were as follows: Figure 2b , 2c , as shown in 2d.
Embodiment 3
[0067] Example 3: Preparation of huperzine A crystal form III.
[0068] Mix 50 mg of huperzine A crystalline form I with 1 ml of acetonitrile, heat to 50° C. and keep stirring at 50° C. for 2 days, and filter to obtain a white solid. After evaporating the solvent at room temperature, the white solid was placed in a vacuum drying oven at 100°C, and dried for 24 hours under vacuum with an oil pump to obtain a crystalline powder, which was determined by X-ray powder diffraction, showing that the obtained crystal form was crystal Type III. The specific peak positions are shown in Table 3 below.
[0069] Table 3: X-ray powder diffraction data of Huperzine A crystal form III in Example 3 of the present invention
[0070]
[0071] Other tests were carried out on the obtained sample, and the obtained DSC thermal spectrum, infrared spectrum and Raman spectrum were as follows: Figure 3b , 3c , 3d shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com