Tamsulosin hydrochloride sustained-release pellet preparation
A technology of tamsulosin hydrochloride and sustained-release pellets is applied in the directions of bulk delivery, urinary system diseases, amide active ingredients, etc., can solve the problems of high production cost, poor production operability, complicated process, etc. Stable and effective release, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
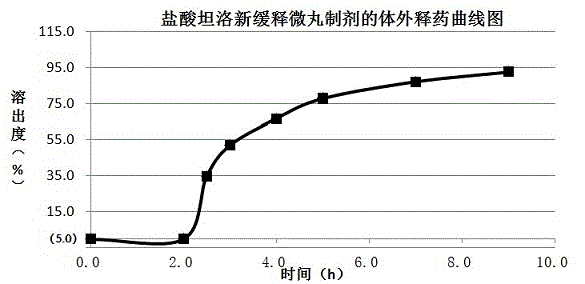
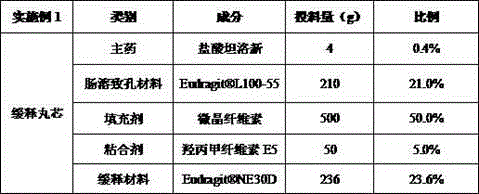
[0030] Example 1 Preparation of tamsulosin hydrochloride sustained-release capsules (based on 20,000 capsules, each capsule contains 0.2 mg tamsulosin hydrochloride)
[0031] Table 1 Example 1 Tamsulosin Hydrochloride Sustained Release Pill Core Formula Composition
[0032]
[0033] Embodiment 1 preparation method:
[0034] (1) Preparation of sustained-release pellet cores: Take tamsulosin hydrochloride, Eudragit? L100-55, microcrystalline cellulose, hypromellose E5 and Eudragit? NE30D according to the formula in the above table, and put them in a wet granulator Add water and mix evenly to make a soft material, add it to a multi-functional pellet coating machine, extrude, place it in a shot blasting pot, round it, and dry the material to make a drug-containing sustained-release pellet core;
[0035] (2) Pack the slow-release coating layer: take 33.3g of Eudragit® NE30D, the coating material of the slow-release coating layer, and make it into a 15-30% coating solution, an...
Embodiment 2
[0037] Example 2 Preparation of tamsulosin hydrochloride sustained-release capsules (based on 25,000 capsules, each capsule contains 0.2mg tamsulosin hydrochloride)
[0038] Table 2 embodiment 2 tamsulosin hydrochloride sustained-release pellet core formula composition
[0039]
[0040] Embodiment 2 preparation method is the same as embodiment 1.
Embodiment 3
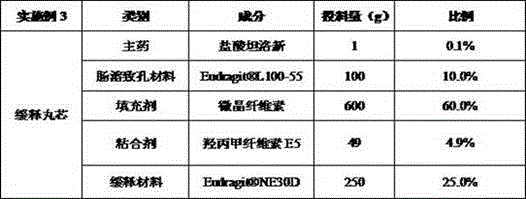
[0041] Example 3 Preparation of tamsulosin hydrochloride sustained-release capsules (based on 5000 capsules, each capsule contains 0.2mg tamsulosin hydrochloride)
[0042] Table 3 Example 3 Tamsulosin hydrochloride sustained-release pellet core formula composition
[0043]
[0044] Embodiment 3 preparation method is the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com