Hydrochloric tamsulosin sustained-release capsule and its preparation method
A technology of sustained-release capsules and tamsulosin hydrochloride, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Release and other issues, to overcome differences in gastrointestinal transfer time, promote complete and uniform absorption, and reduce fluctuations in blood drug concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
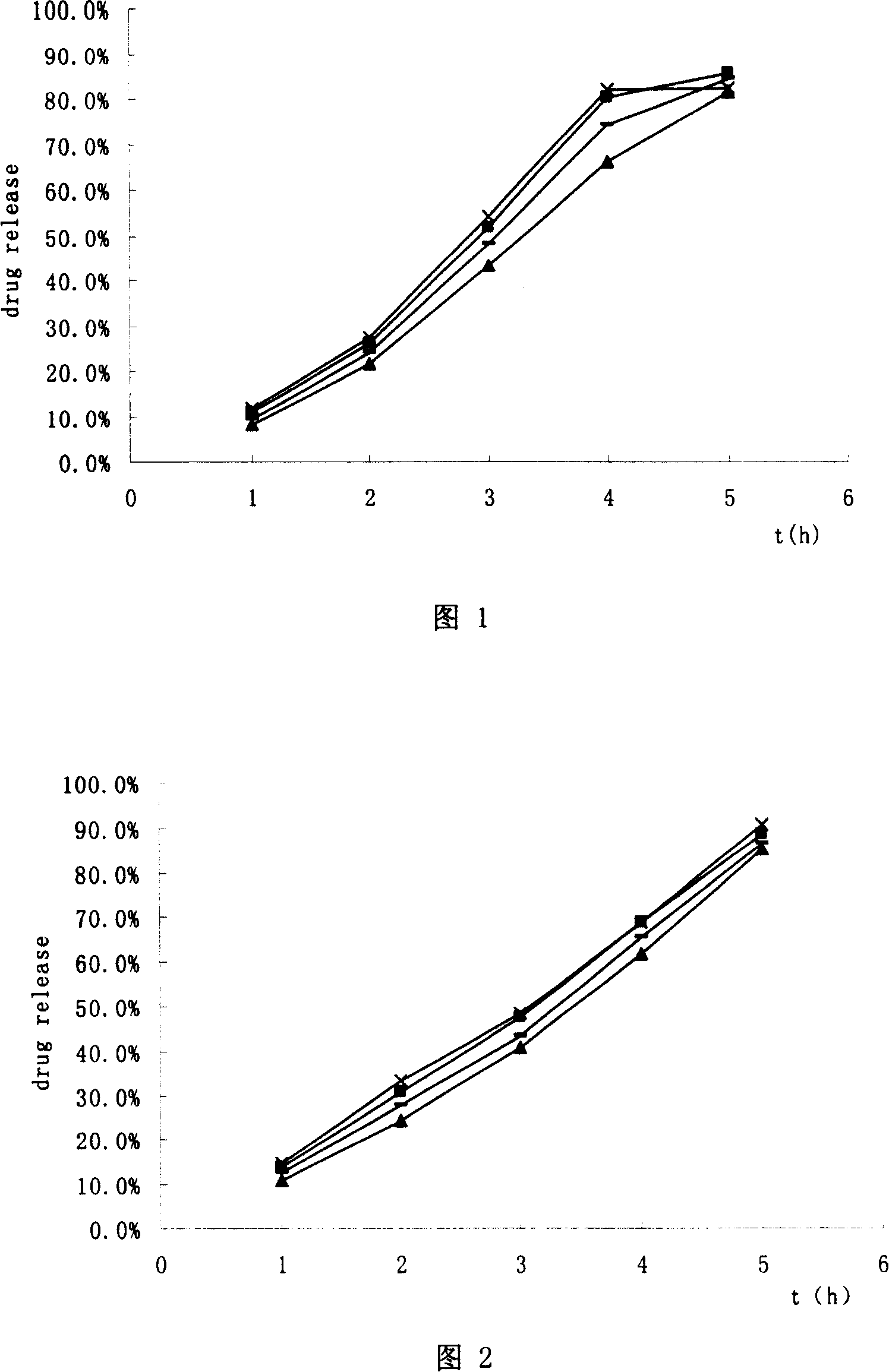
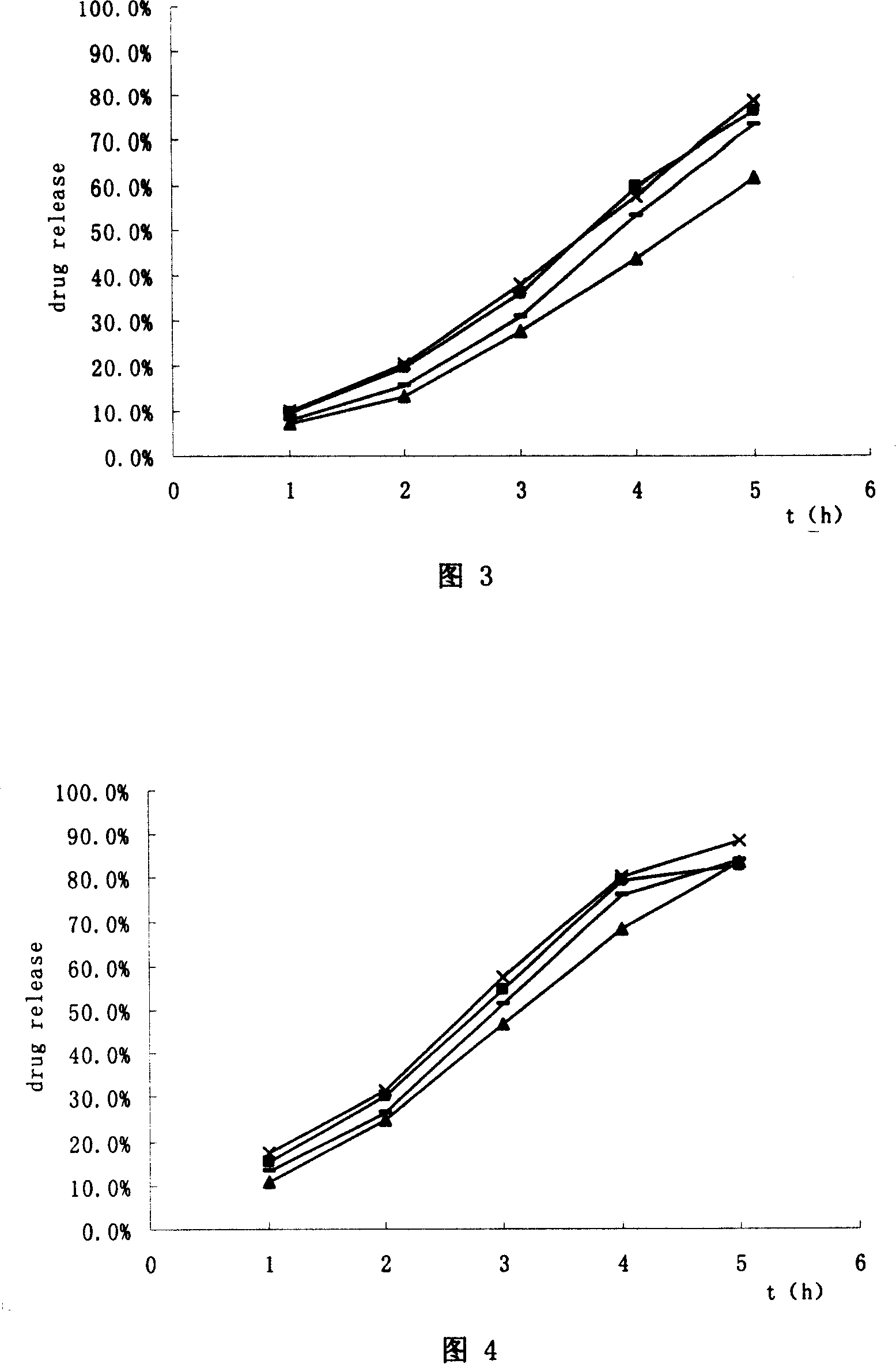
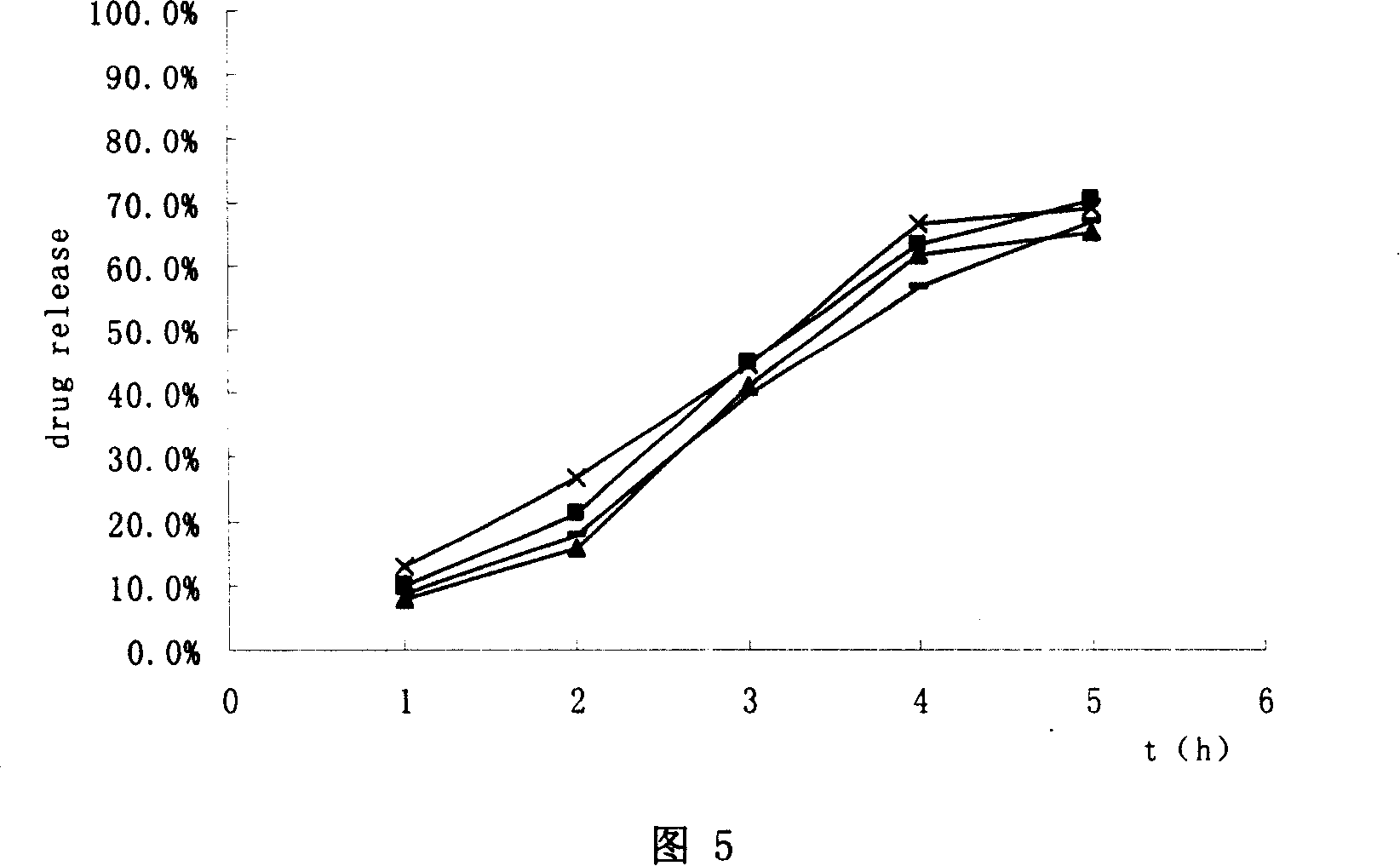
Image
Examples
Embodiment 1
[0080] Blank ball core 300g (commercially available)
[0081] Tamsulosin Hydrochloride 0.6g
[0082] Hypromellose (E5) 10g
[0083] Potassium dihydrogen phosphate 50g
[0084] Eudrugit NE 30D 240g
[0085] Triethyl citrate 5g
[0087] Preparation:
[0088] (a) Dissolve 50 g of potassium dihydrogen phosphate in 500 ml of deionized water; under mechanical stirring, add 10 g of hypromellose (E5) to dissolve into a transparent and clear solution;
[0089] (b) 0.3 g of tamsulosin hydrochloride is dissolved in the solution prepared in step (a);
[0090] (c) adding the blank ball core to the fluidized bed, carrying out fluidized bed coating with the drug-containing solution prepared in step (b), and drying to obtain drug-containing pellets of tamsulosin hydrochloride;
[0091] (d) Disperse the prescribed amount of triethyl citrate and talcum powder in 100ml of water, shear with a high-shear homogenizer for 5 minutes, add the prescribed amount of Eudrug...
Embodiment 2
[0101] Blank ball core 300g (commercially available)
[0102] Tamsulosin Hydrochloride 0.6g
[0103] Potassium dihydrogen phosphate 50g
[0104] Hypromellose (E5) 15g
[0105] Surelease 400g
[0106] PEG4000 15g
[0108] Preparation:
[0109] (a) Dissolve 50 g of potassium dihydrogen phosphate in 500 ml of deionized water; under mechanical stirring conditions, add 15 g of hypromellose (E5) to dissolve into a transparent and clear solution;
[0110] (b) 0.3 g of tamsulosin hydrochloride is dissolved in the solution prepared in step (a);
[0111] (c) adding the blank ball core to the fluidized bed, carrying out fluidized bed coating with the drug-containing solution prepared in step (b), and drying to obtain drug-containing pellets of tamsulosin hydrochloride;
[0112] (d) Dissolve 15g of PEG4000 in 250ml of deionized water, add 25g of talcum powder under mechanical stirring, then add the prescribed amount of Surelease aqueous dispersion solution,...
Embodiment 3
[0118] Blank ball core 300g (commercially available)
[0119] Tamsulosin Hydrochloride 0.6g
[0120] PVP K30 20g
[0121] Potassium dihydrogen phosphate 50g
[0122] Eudrugit RL100 30g
[0123] Eudrugit RS100 70g
[0124] Dibutyl sebacate 10g
[0125] Talc powder 25g
[0126] Preparation:
[0127](a) Dissolve 50 g of potassium dihydrogen phosphate in 500 ml of deionized water; under mechanical stirring, add 20 g of PVPK30 to dissolve into a transparent and clear solution;
[0128] (b) 0.3 g of tamsulosin hydrochloride is dissolved in the solution prepared in step (a);
[0129] (c) adding the blank ball core to the fluidized bed, carrying out fluidized bed coating with the drug-containing solution prepared in step (b), and drying to obtain drug-containing pellets of tamsulosin hydrochloride;
[0130] (d) Add the Eudrugit RL100 and Eudrugit RS100 of the prescription amount in 95% ethanol solution under the condition of mechanical stirring, and dissolve completely to Eudr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com