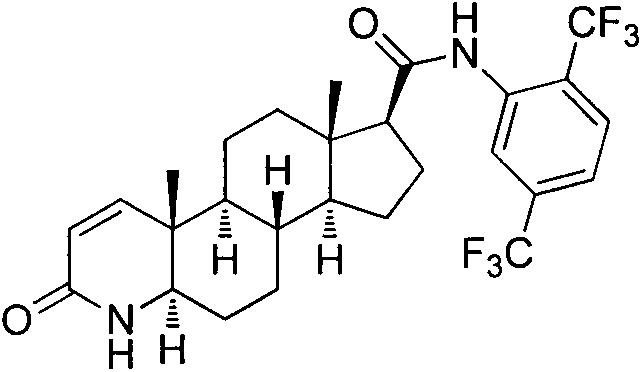
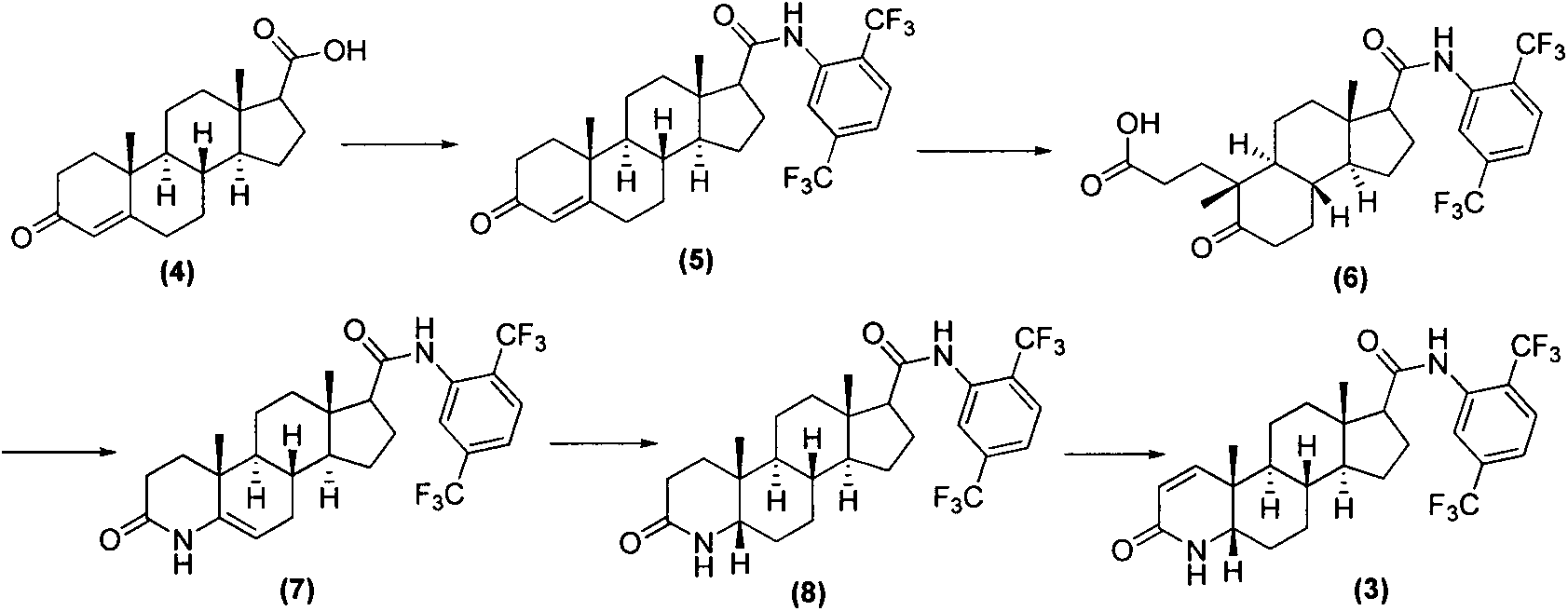
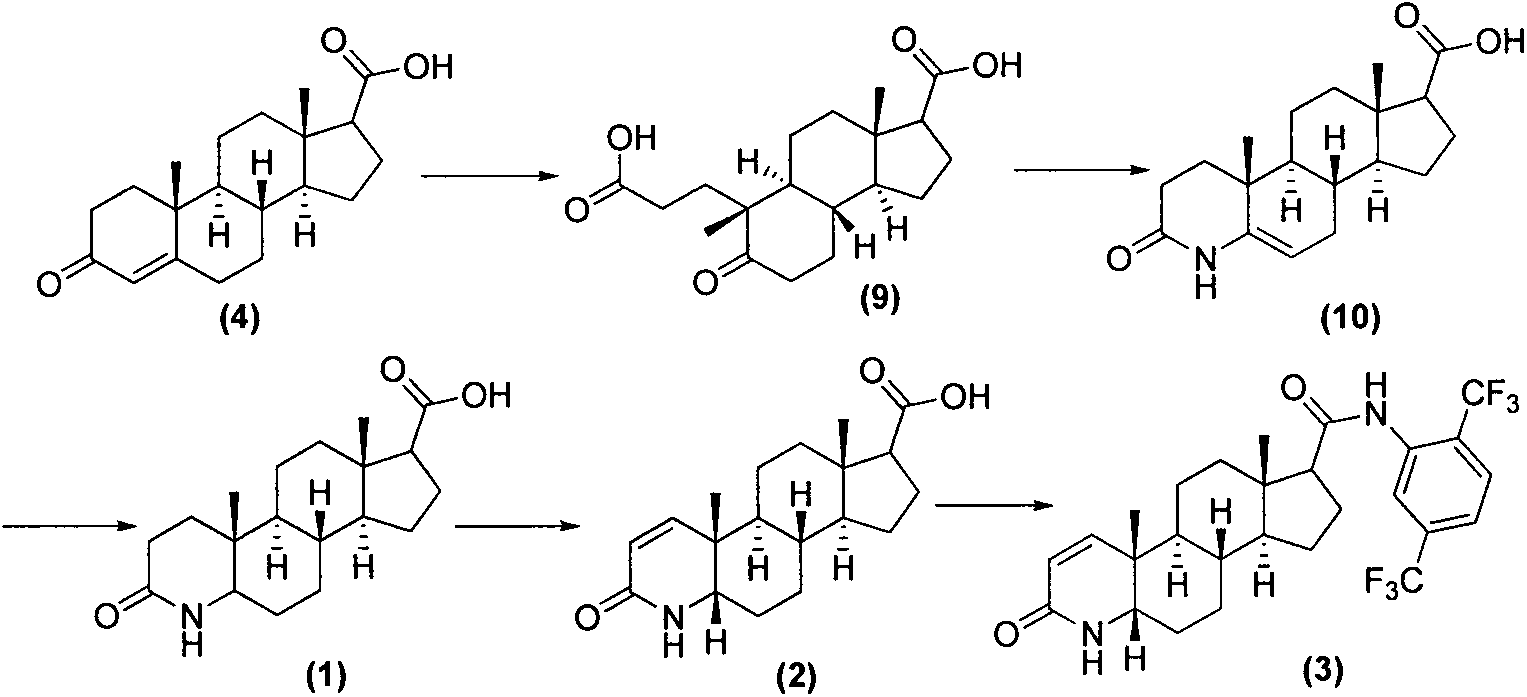
Process for preparing dutasteride
A preparation process, a technology for dutasteride, which is applied in the technological field of dutasteride preparation, can solve the problems of unqualified product quality, difficult removal of pyridine, strong irritation and the like, and achieves good product quality, low toxicity, Equipment with less corrosive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis of embodiment 1 formula (2) compound
[0033] Dissolve 10 grams of the compound of formula (1) in 100 ml of toluene, add 10 grams of dichlorodicyanobenzoquinone, add 20 ml of N, O-bis(trimethylsilyl) trifluoroacetamide dropwise at room temperature, and drop at room temperature React for 12 hours, then heat up to 125°C and react for 18 hours, cool to room temperature, spin off toluene, add 145ml of dichloromethane and 100ml of 1% sodium bisulfite aqueous solution, stir well, filter, separate the organic phase, and successively wash with 2M hydrochloric acid Wash twice, wash twice with saturated sodium chloride, decolorize with activated carbon for 1 hour, filter with suction, spin off the solvent, and recrystallize from acetonitrile to obtain 5.2 g of compound (2).
[0034] ESI-MS m / z: 316.2 [M-H] - ; 1 H-NMR (DMSO-d 6 )δ0.63(s, 3H), 0.85(s, 3H), 0.97-1.00(m, 2H), 1.10-1.15(m, 1H), 1.16-1.29(m, 3H), 1.31-1.44(m, 2H), 1.57-1.66(m, 3H), 1.67-1.74(m, 1H), ...
Embodiment 2
[0035] Embodiment 2, the synthesis of formula (3) compound dutasteride
[0036] Dissolve 5.0 g of the compound of formula (2) in 50 ml of toluene, add 1 ml of N,N-dimethylformamide, add 1.5 g of solid triphosgene in batches under ice-bath conditions, and react at 55°C for 2 hours. Cool to room temperature, add 3.33 g of solid sodium carbonate, then add 4.0 g of 2,5-bis(trifluoromethyl)aniline, raise the temperature to 115°C, react for 12 hours, cool to room temperature, filter with suction, and wash the filtrate with water successively 2 times, 2M hydrochloric acid washing 2 times, saturated sodium carbonate washing 2 times, saturated saline washing 2 times, activated carbon decolorization for 1 hour, suction filtration, spin-drying solvent, ethyl acetate recrystallization, acetonitrile recrystallization to obtain dutasteride (3) 7.2 grams. Yield 86.8%, melting point: 246-248; ESI-MS m / z: 529.3[M+H] + ; 1 H-NMR (DMSO-d 6)δ0.66(s, 3H), 0.87(s, 3H), 0.98-1.03(m, 2H), 1.17-1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com