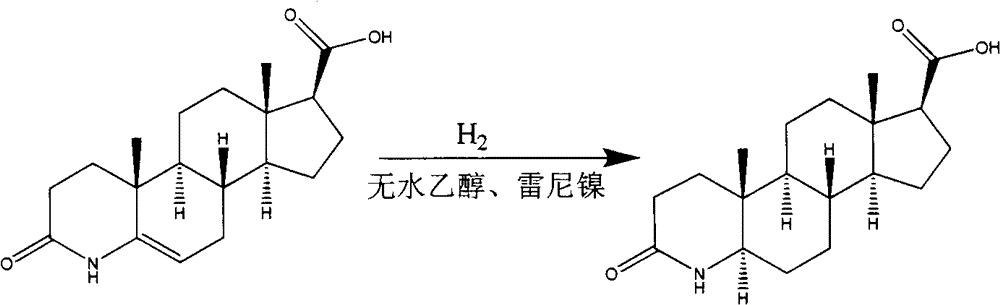
Preparation method of dutasteride intermediate
A technology of crystals and compounds, applied in the field of drug synthesis, can solve the problems of unsuitability for industrial production, complicated operation, etc., and achieve the effects of cost reduction, simplified operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0038] Preparation of Example 13-Carbonyl-4-aza-5α-androst-17β-carboxylic acid crude product
[0039]
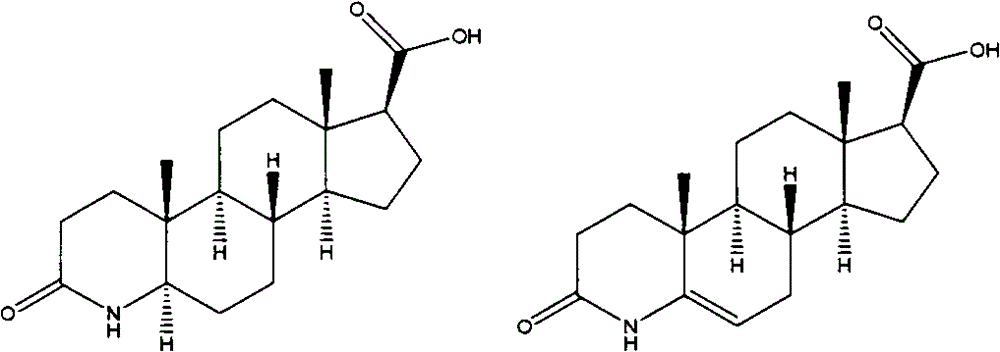
[0040] Formula I Formula II
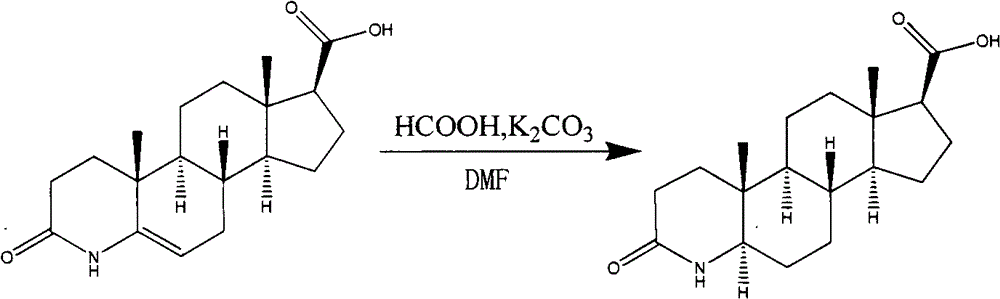
[0041] In a 5L reaction flask equipped with a reflux condenser, 3-carbonyl-4-aza-5-androstene-17β-carboxylic acid (compound of formula II) (66.0g, 208mmol) and K 2 CO 3 (430.0g, 3.12mol) was added into DMF (2500ml), stirred, and heated to 140°C. Then slowly drop formic acid (1716ml) in the above-mentioned solution, drip in about 2 hours, continue to react for 4-6 hours, until TLC detects that the raw material point disappears. Then concentrated under reduced pressure to about 2100ml, cooled to room temperature, added 2100ml of water, stirred overnight in an ice-water bath, washed with water, and dried to obtain 53.2g of light yellow solid. ESI: 320.2[M+H] + . As detected by HPLC, the 5-beta isomer was 24.7%.
Embodiment 2
[0043] Preparation of crude 3-carbonyl-4-aza-5α-androst-17β-carboxylic acid
[0044]
[0045] In a 5L reaction flask equipped with a reflux condenser, 3-carbonyl-4-aza-5-androstene-17β-carboxylic acid (66.0g, 208mmol) and K 2 CO 3 (430.0g, 3.12mol) was added into DMF (2500ml), stirred, and heated to 150°C. Then, in the above-mentioned solution, formic acid (1452ml) was slowly added dropwise for about 1.7 hours, and the reaction was continued for 4-6 hours until the TLC detection raw material point disappeared. Then concentrated under reduced pressure to about 1950ml, cooled to room temperature, added 1950ml of water, stirred overnight in an ice-water bath, washed with water, and dried to obtain 52.8g of light yellow solid. ESI: 320.2[M+H] + . As detected by HPLC, the 5-beta isomer was 24.8%.
Embodiment 33
[0046] Example 33- Preparation of the crude product of carbonyl-4-aza-5α-androst-17β-carboxylic acid
[0047]
[0048] In a 5L reaction flask equipped with a reflux condenser, 3-carbonyl-4-aza-5-androstene-17β-carboxylic acid (66.0g, 208mmol) and K 2 CO 3 (430.0g, 3.12mol) was added into DMF (2500ml), stirred, and heated to 145°C. Then, in the above-mentioned solution, formic acid (1848ml) was slowly added dropwise for about 2.2 hours, and the reaction was continued for 4-6 hours until the TLC detection raw material point disappeared. Then concentrated under reduced pressure to about 2200ml, cooled to room temperature, added 2200ml of water, stirred overnight in an ice-water bath, washed with water, and dried to obtain 53.0g of light yellow solid. ESI: 320.2[M+H] + . As detected by HPLC, the 5-beta isomer was 24.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com