Preparation method of dutasteride impurity I
A technology of dutasteride impurity, aza, applied in the field of medicine, can solve the problems such as impurity being difficult to remove, no open literature report impurity I preparation method, unfavorable control, etc., to achieve the effect of improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
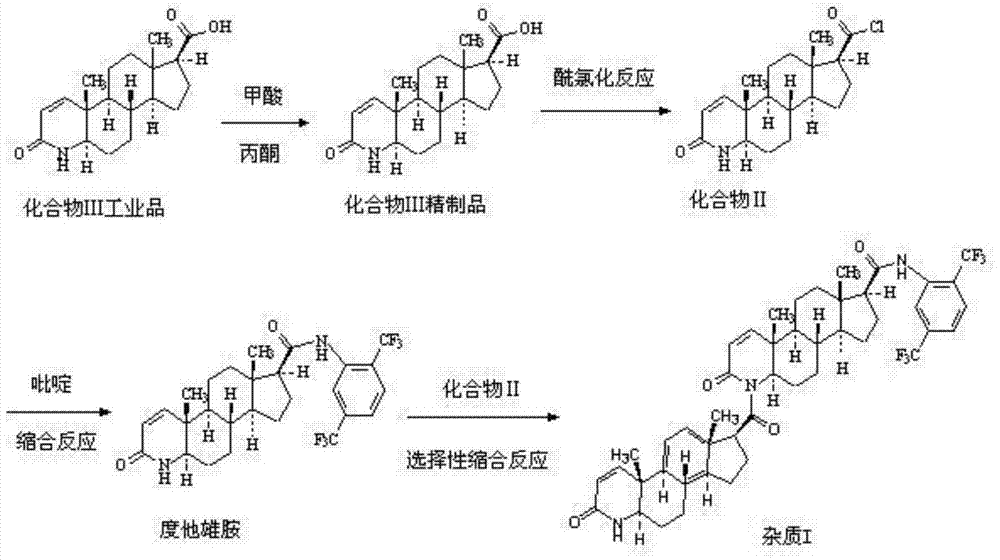
[0038] Example 1 Investigation of the conditions for the condensation reaction of dutasteride and compound II
[0039] The inventor used pyridine, 2,6-lutidine, pyrrole, N-methylmorpholine, DBU etc. as acid binding agent to carry out the preparation of impurity I, and also investigated the reaction solvent, the results are shown in Table 1 . As can be seen from Table 1, the acid binding agent DBU that the present invention screens out has significant selectivity, and condensation reaction occurs on the secondary amine at the 4th position, and the preparation of impurity I can be realized, but the impurity I obtained when using toluene as a reaction solvent The purity is only about 60%, and it is difficult to greatly improve the purity of the impurity I crude product by extending the time of the reflux reaction; and the purity of the impurity I crude product obtained when using xylene as the reaction solvent is as high as more than 85%, and the purity can reach 97% after column...
Embodiment 2
[0042] Example 2 Refinement of technical grade compound III
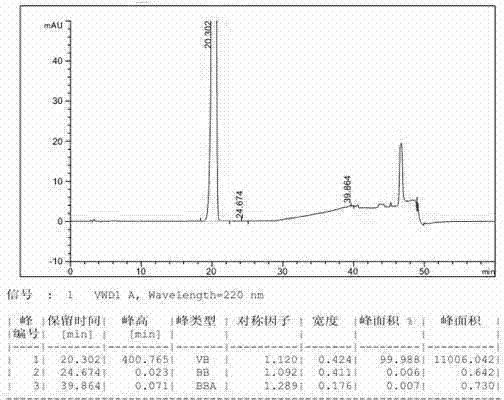
[0043] In a 3L reaction flask, add 500g of industrial grade compound III and 1.5L of anhydrous formic acid, stir and beat at 30-35°C for 5h, filter, and wash the filter cake with acetone, 0.5L each time, for a total of 2 times, and the solids are washed at 30-35°C. ℃ of blast drying for 24h to obtain 375.0g of compound III refined product, the yield is 75.0%, the purity is 99.99%, and the HPLC spectrum of its related substances is as follows figure 1 shown.
Embodiment 3
[0044] Example 3 Preparation of Compound II
[0045] In a 3L reaction flask, add 250g of compound III refined product prepared in Example 2 and 2.5L of dichloromethane, stir, control the temperature to 15-25°C, add 125mL of thionyl chloride dropwise, stir for 0.5h after dropping, and then put The solvent was evaporated under reduced pressure at a temperature of 35-40°C and P<-0.08Mpa, 2L of toluene was added to make a slurry for 0.5h, and filtered to obtain 370.5g of compound II.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com