A kind of preparation method of dutasteride impurity i
A dutasteride impurity and nitrogen impurity technology, applied in the field of medicine, can solve the problems of difficult removal of impurities, side reactions, and no public literature reports on the preparation method of impurity I, and achieve the effect of improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
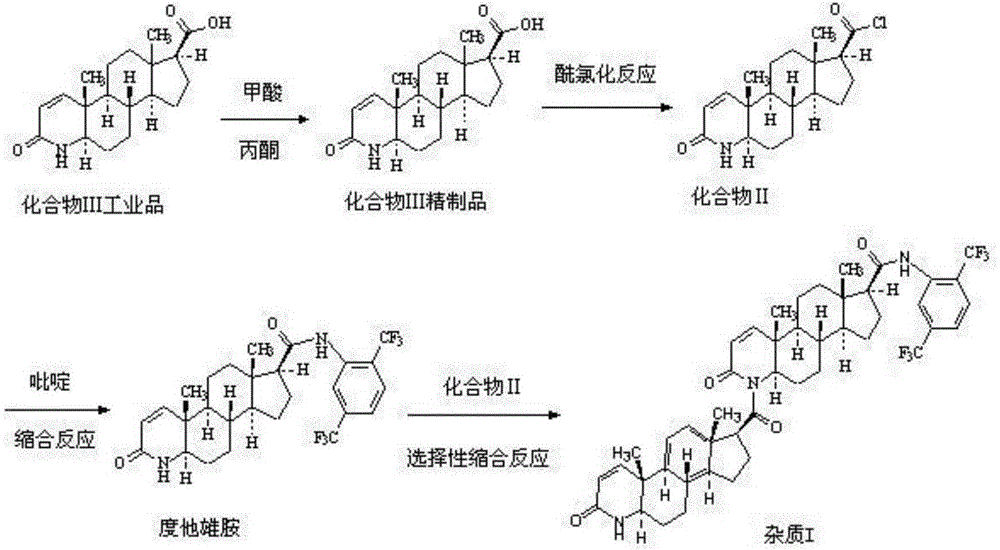
[0038] Embodiment 1 Dutasteride and compound II carry out the condition investigation of condensation reaction
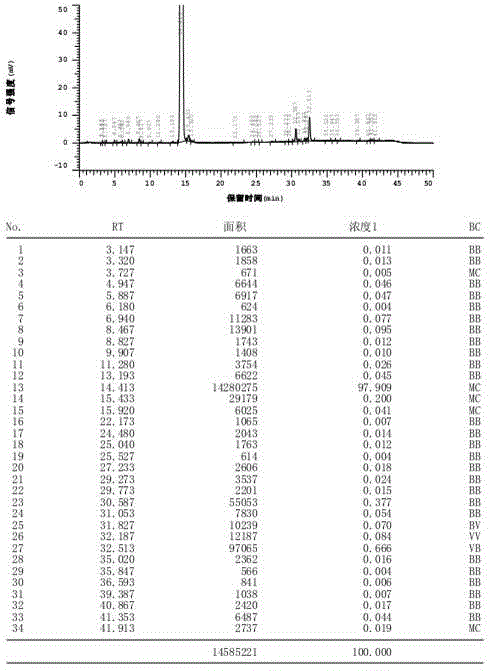
[0039] The inventor used pyridine, 2,6-lutidine, pyrrole, N-methylmorpholine, DBU, etc. as acid-binding agents to prepare impurity I, and also investigated the reaction solvent. The results are shown in Table 1 . It can be seen from Table 1 that the acid-binding agent DBU screened by the present invention has remarkable selectivity, and the condensation reaction occurs on the secondary amine at the 4-position, which can realize the preparation of impurity I, but the impurity I obtained when toluene is used as the reaction solvent The purity is only about 60%, and the time of prolonging the reflux reaction is also difficult to greatly improve the purity of the impurity I crude product; and the purity of the impurity I crude product obtained when xylene is used as the reaction solvent is as high as more than 85%, and the purity can reach 97% after column chromatograph...
Embodiment 2
[0042] The refining of the compound III of embodiment 2 technical grade
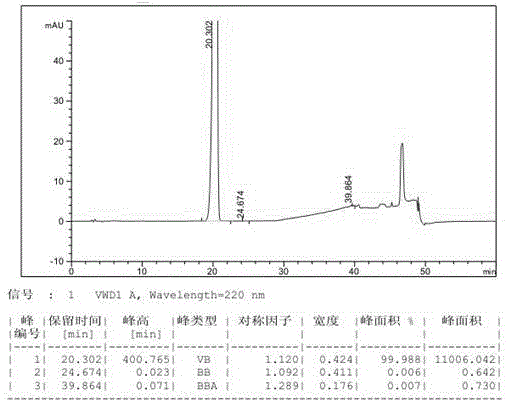
[0043] In a 3L reaction flask, add 500g of industrial grade compound III and 1.5L of anhydrous formic acid, stir and beat at 30-35°C for 5h, filter, and wash the filter cake with acetone, 0.5L each time, for a total of 2 washes, the solid is at 30-35 ℃ air-dried for 24 hours to obtain 375.0 g of the refined product of compound III, with a yield of 75.0% and a purity of 99.99%. The HPLC spectrum of its related substances is as follows: figure 1 shown.
Embodiment 3
[0044] The preparation of embodiment 3 compound II
[0045] Into a 3L reaction flask, add 250g of the refined product of Compound III obtained in Example 2 and 2.5L of dichloromethane, stir, control the temperature at 15-25°C, add 125mL of thionyl chloride dropwise, stir for 0.5h after dropping, and then Under the conditions of temperature 35-40°C and P<-0.08Mpa, the solvent was distilled off under reduced pressure, 2L of toluene was added to make a slurry for 0.5h, and filtered to obtain 370.5g of compound II.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com