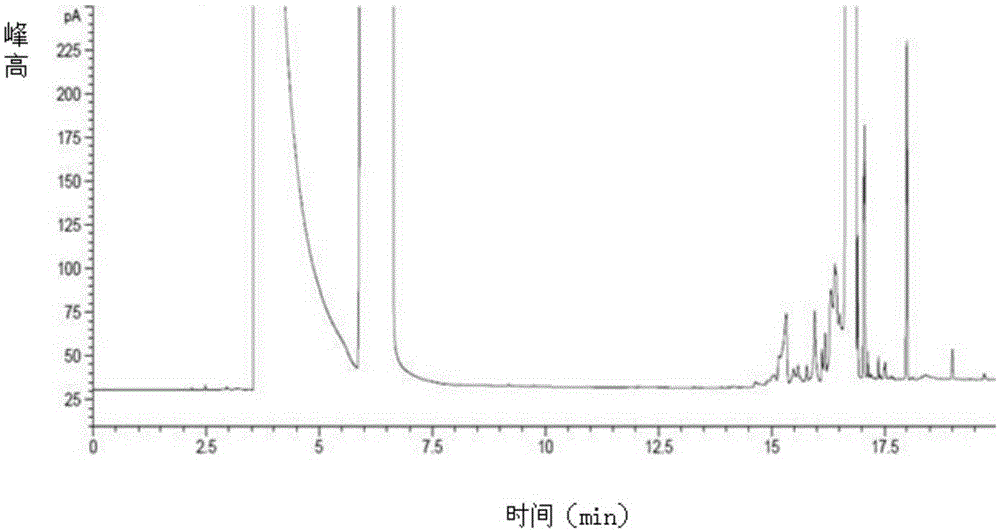
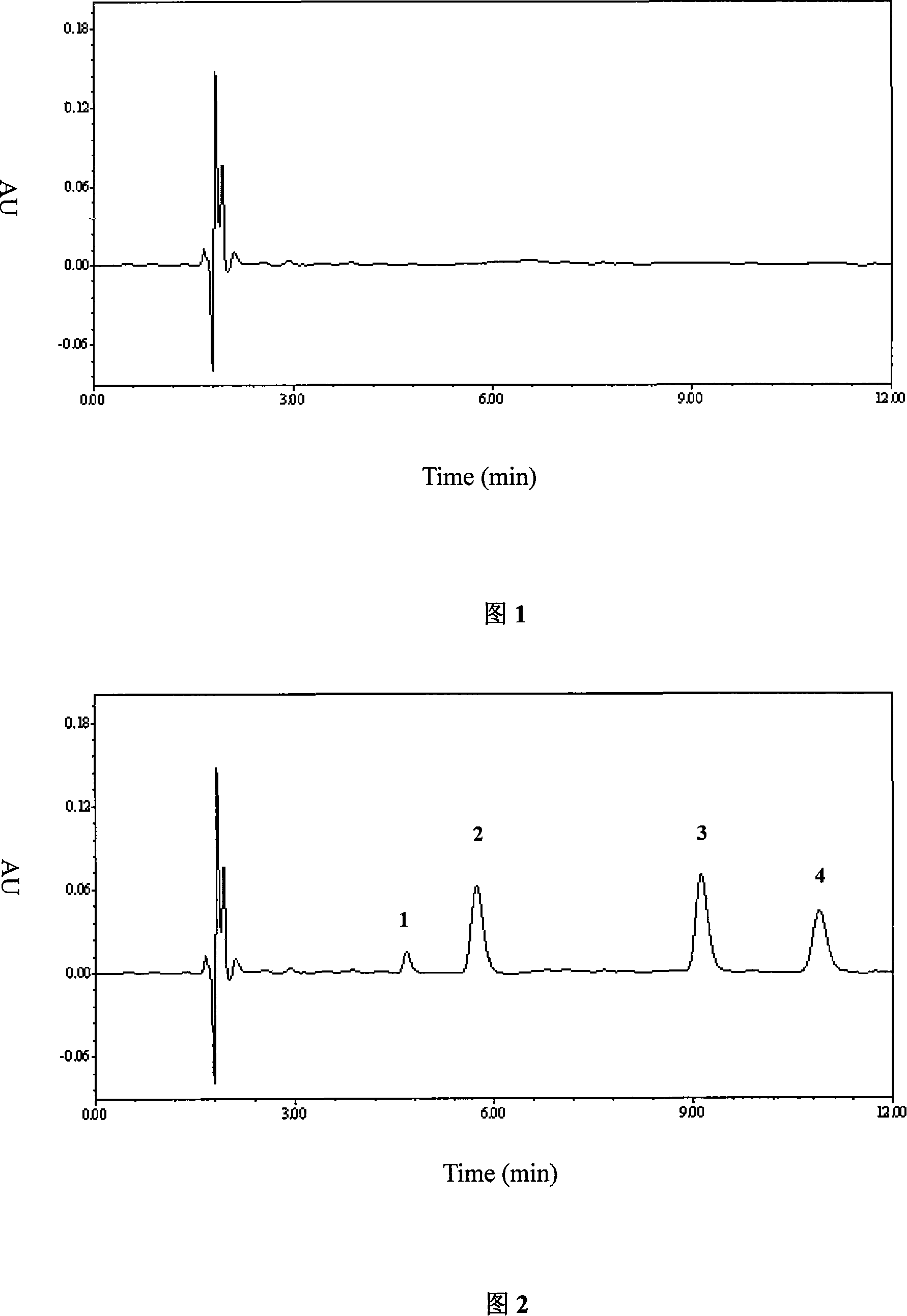
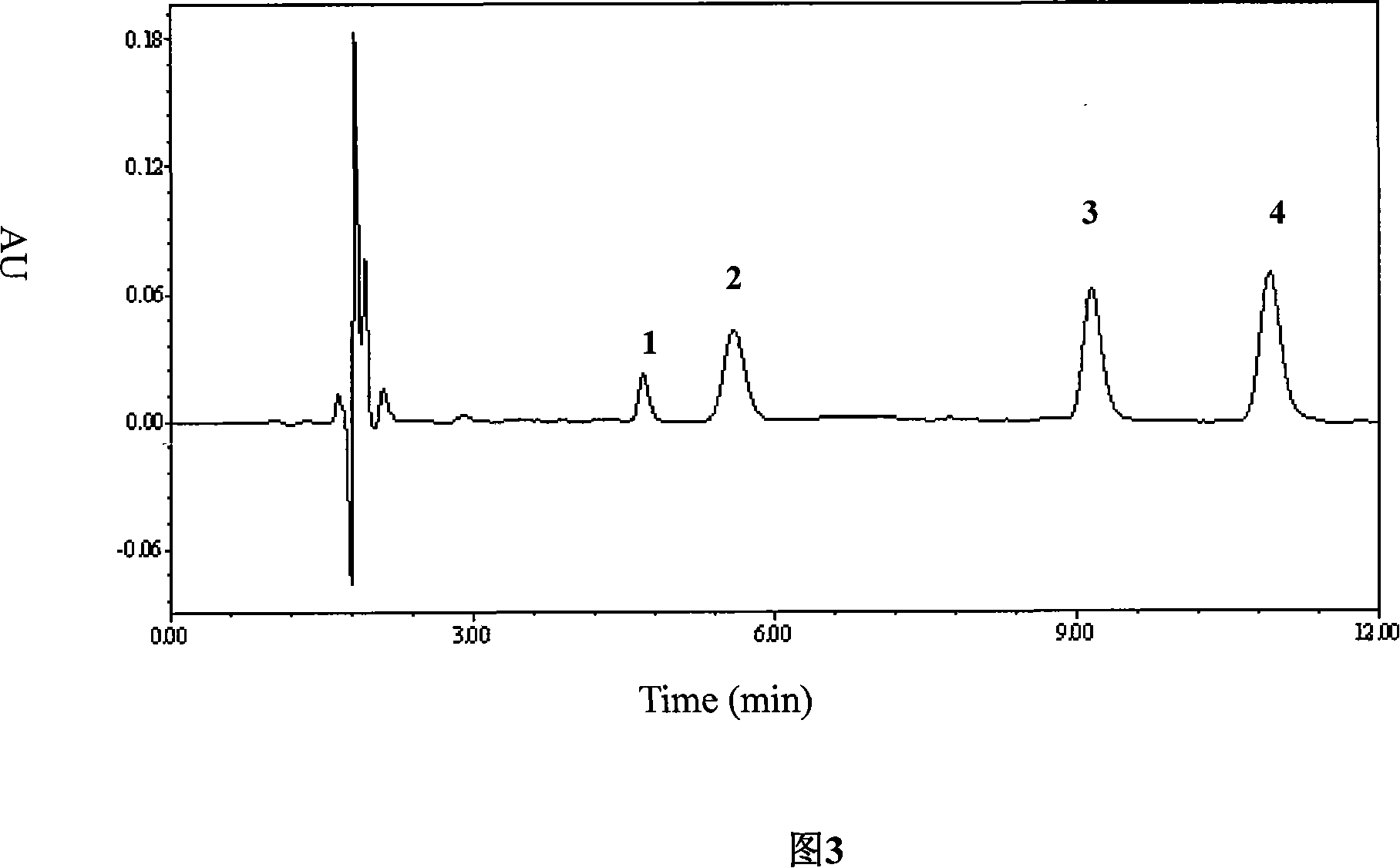
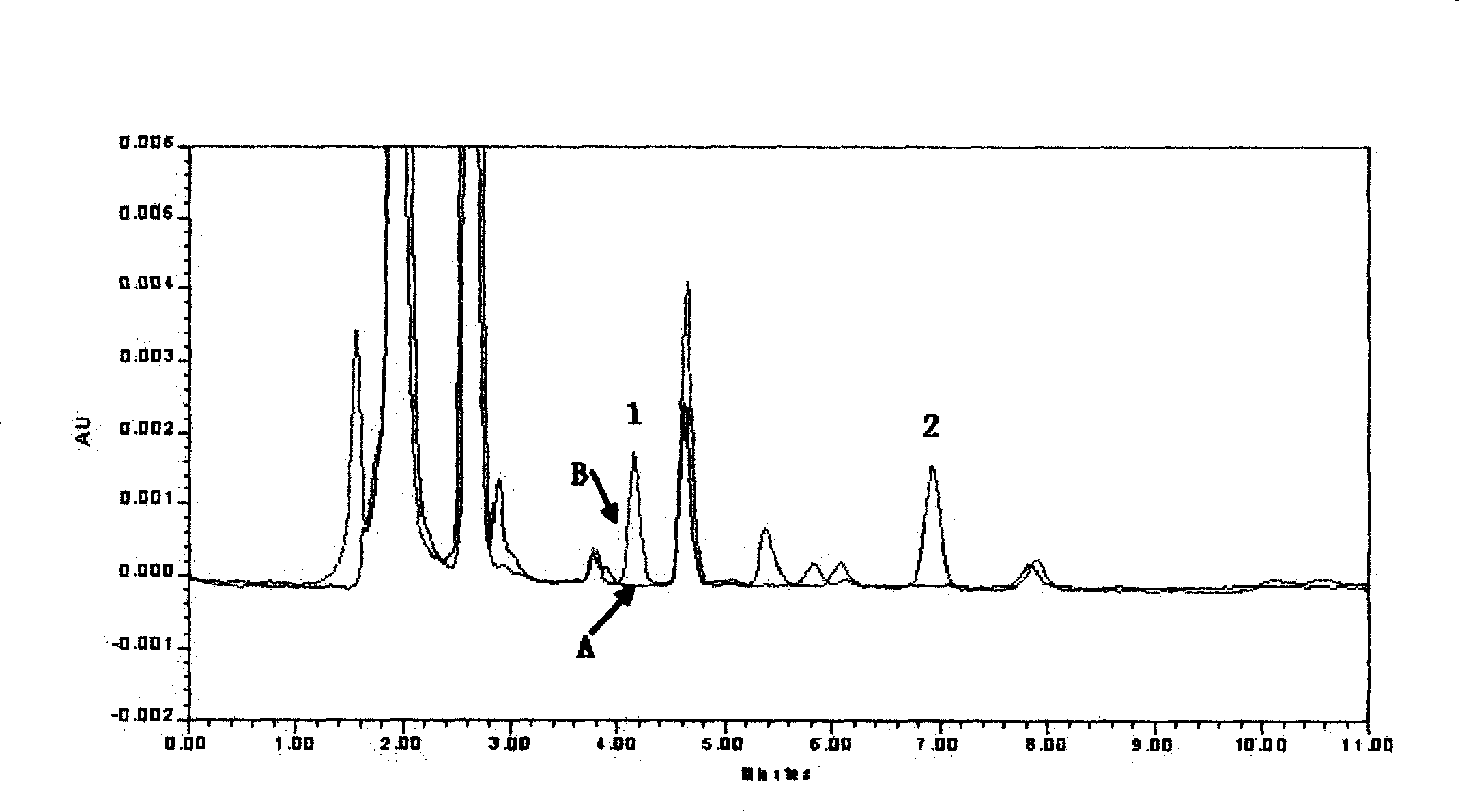
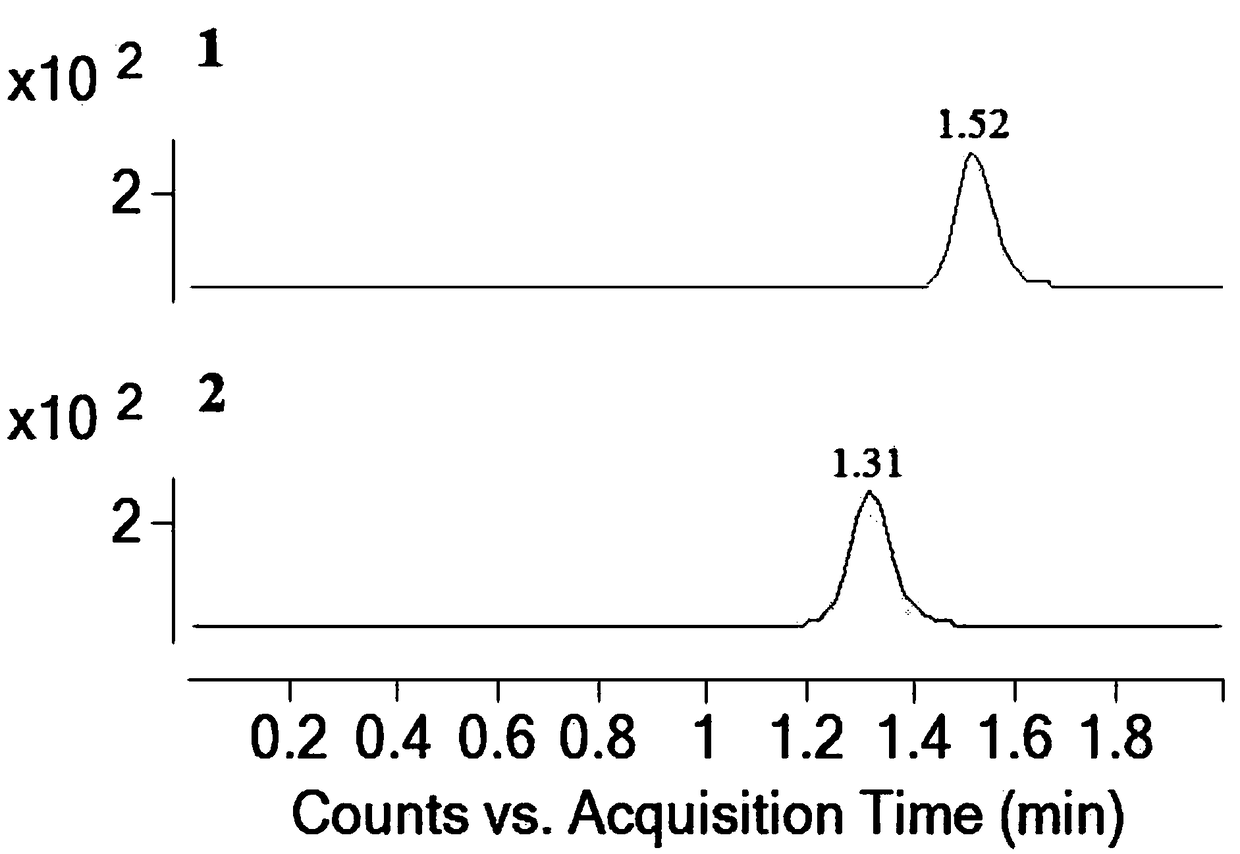
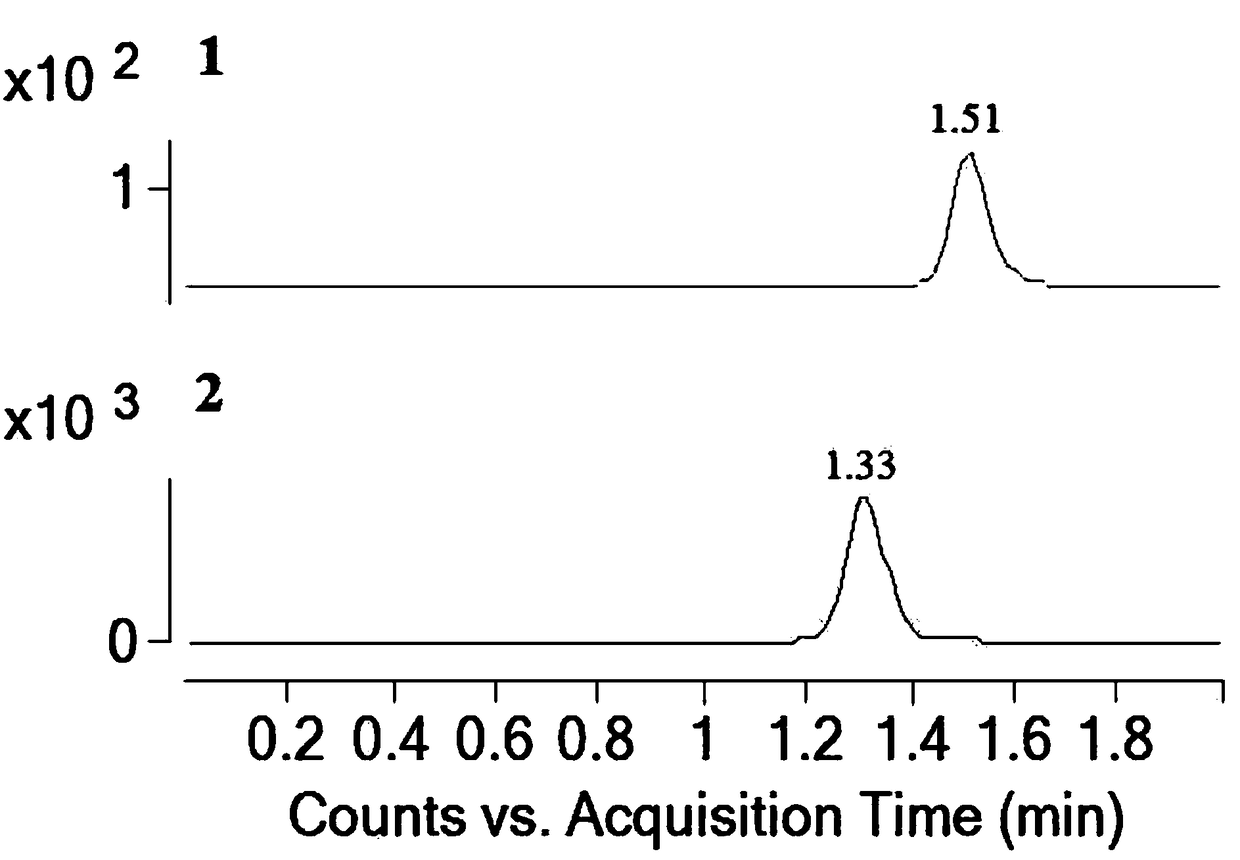
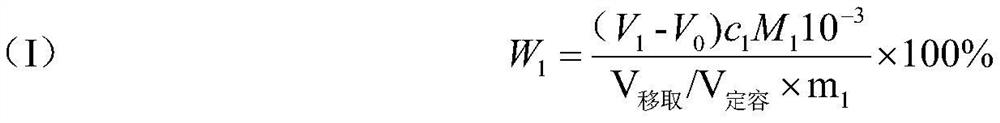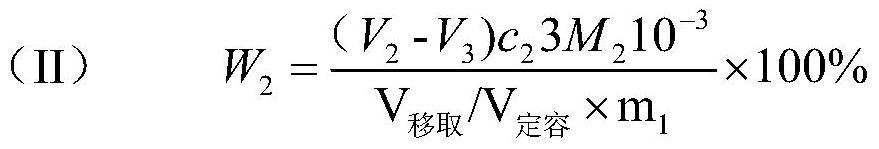Patents
Literature
31results about How to "Determination does not interfere" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
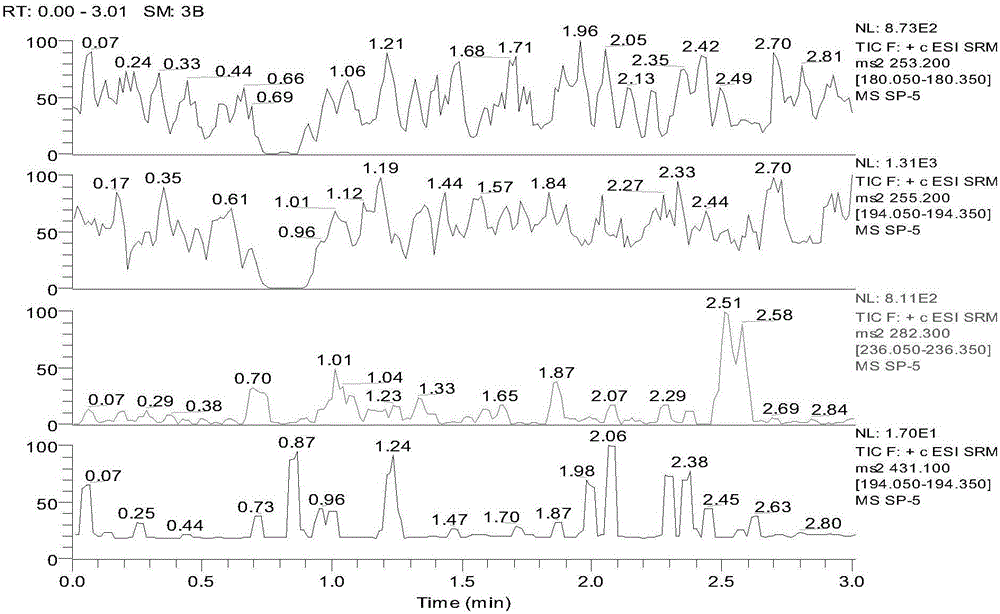
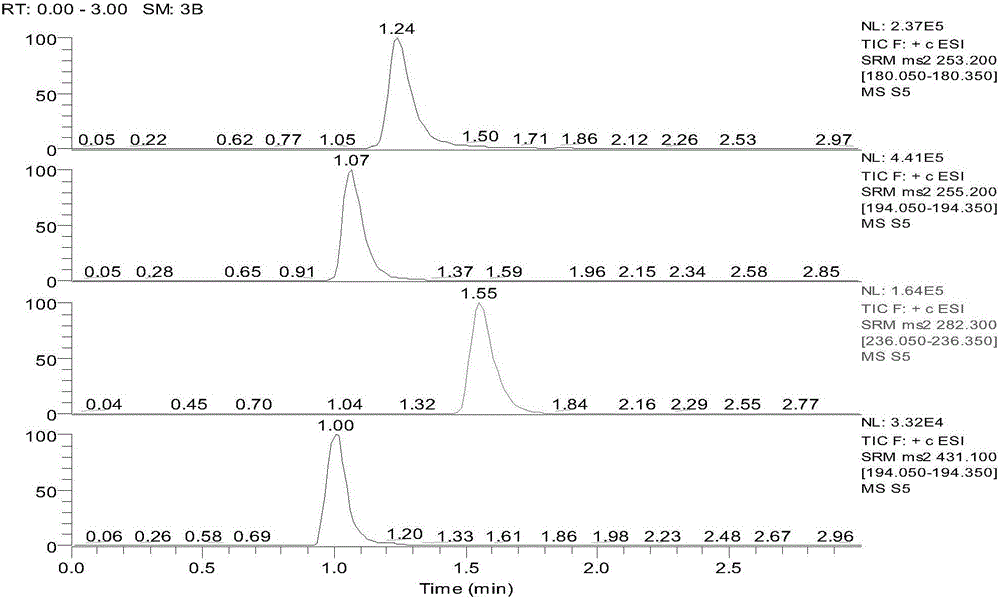
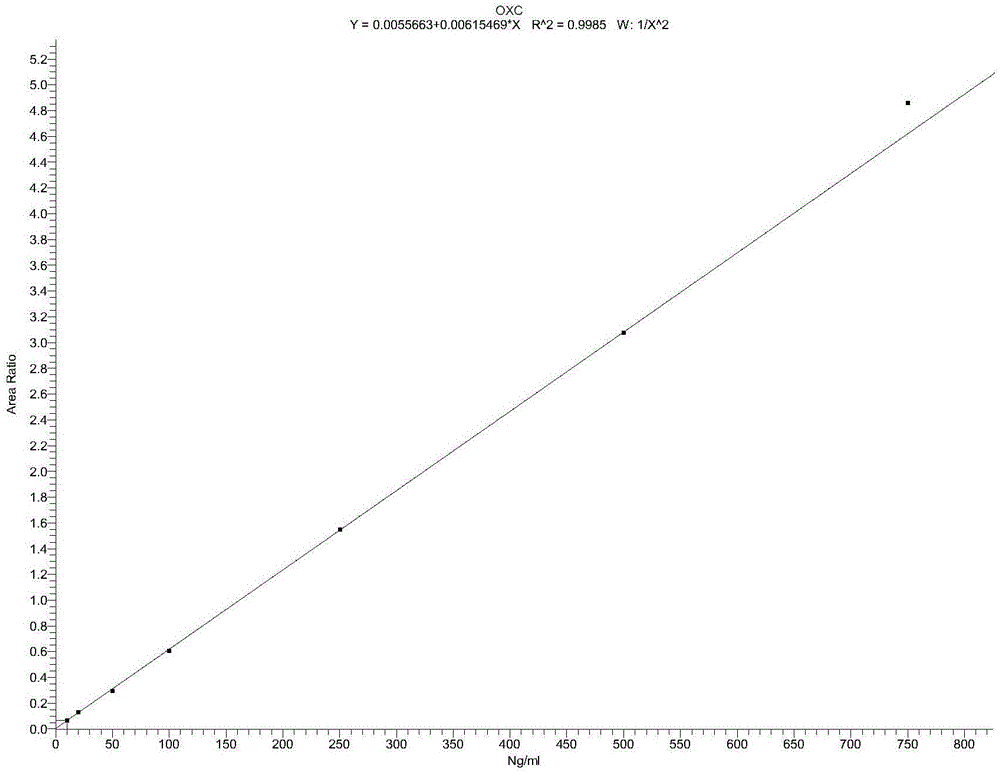
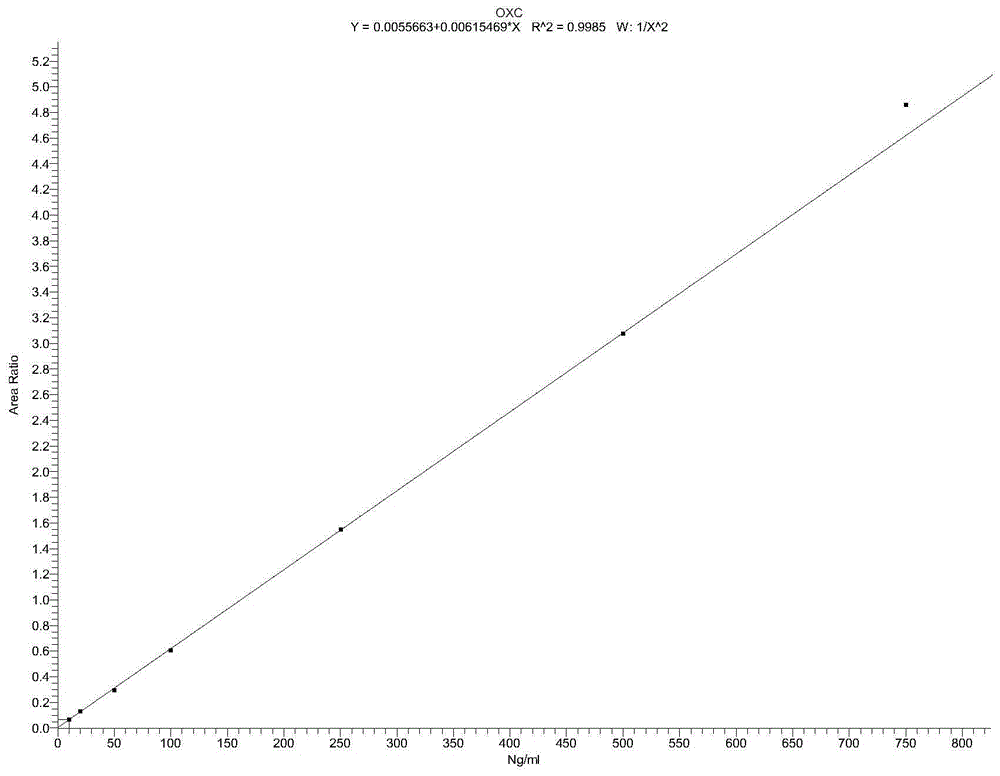
Detection method for simultaneously measuring OXC in human plasma and metabolite MHD and MHD-G
InactiveCN105136957AThe pretreatment method is simpleSuitable for routine testingComponent separationMetaboliteAnalysis study
The invention relates to a detection method for simultaneously measuring OXC in human plasma and metabolite MHD and MHD-G, and belongs to the technical field of biological detection. An experiment is carried out by taking nitrazepam as an internal standard, and can simultaneously detect the plasma concentrations of the three compounds by adopting high performance liquid chromatography-tandem mass spectrometry after pretreatment through precipitation protein. The detection method is short in time, high in specificity and sensitivity, and convenient in operation, and is successfully applied to the analysis and research of oxcarbazepine in the human plasma and the metabolite thereof.
Owner:WUXI PEOPLES HOSPITAL
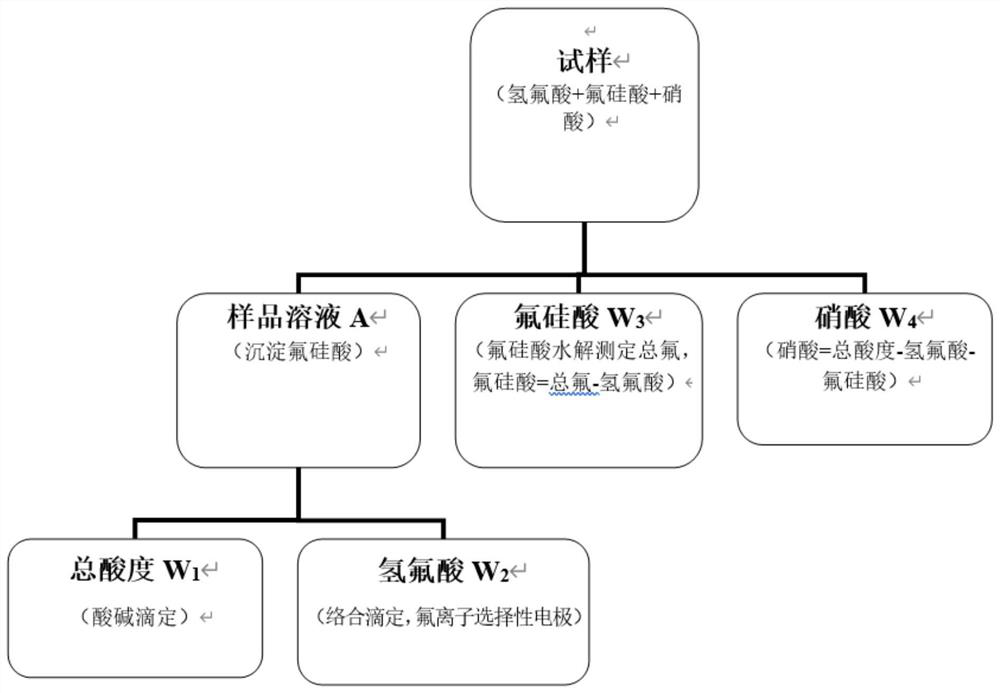
Method for determining contents of fluosilicic acid, hydrofluoric acid and nitric acid in etching acid
InactiveCN111855650AEasy to operateReliable resultsMaterial analysis by observing effect on chemical indicatorMaterial electrochemical variablesHexafluorosilicic acidLanthanum
The invention discloses a method for determining the contents of hydrofluoric acid, fluosilicic acid and nitric acid in etching acid through acid-base titration and complexometric titration. The method comprises the following steps: (1) adding potassium salt into a sample to precipitate fluosilicic acid, titrating supernatant by using a sodium hydroxide standard titration solution by taking phenolphthalein as an indicator, and calculating the total acidity of the sample solution; (2) adding potassium salt into the sample to precipitate fluosilicic acid, titrating fluorine ions in the solutionby using a lanthanum nitrate standard titration solution by taking a fluorine ion selective electrode as an indicating electrode, determining a reaction endpoint by using a secondary micro-commerce method, and calculating the content of hydrofluoric acid; (3) heating and hydrolyzing fluosilicic acid, titrating total fluorine in the solution by using a lanthanum nitrate standard titration solutionby taking a fluorine ion selective electrode as an indicating electrode, determining a reaction endpoint by using a secondary micro-commerce method, and calculating the content of fluosilicic acid byusing a subtraction method; and (4) obtaining the nitric acid content by subtracting the sum of the hydrofluoric acid content and the fluosilicic acid content from the total acidity.
Owner:QINGDAO UNIV OF SCI & TECH
Detection method for decitabine impurities
ActiveCN105388223AImprove stabilityEasy to separateComponent separationRelative standard deviationDissolution
The invention discloses a method for measuring the content of pyridine in decitabine impurities. A methyl alcohol-N-methyl-pyrrolidone-triethylamine mixed solution is used as a solvent, and a direct injection gas chromatographic method is adopted for measurement. The problems that decitabine raw material glucoside stability is poor, dissolution is not easily achieved, the recycling rate of an existing detection method is seriously low and interference is likely to be caused by solvent peaks are solved. According to the method, it is tested that a blank solvent has no interference peaks for residual solvent pyridine measurement, the precision is good, the relative standard deviation (RSD) is only 0.46%, the reproducibility is good, the linear relation is good, the average recycling rate is high, and pyridine possibly remaining in glucoside can be accurately and quantitatively detected. Effective quality control is achieved for preparing second-class solvent pyridine in the decitabine raw material glucoside, and the quality of decitabine and drug use safety are ensured at the same time.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Method for determining human plasma phenytoin and its precursor drug and metabolite
InactiveCN101105479ASimple and efficient operationLess plasma consumptionComponent separationColor/spectral properties measurementsFosphenytoinChromatography column
The invention belongs to the medical inspection field, relates to an analysis detection method of drug in the body of a person, and specifically relates to the method that the densities of fosphenytoin, phenytoin and 4'-hydroxyl phenytoin which is the main metabolite of fosphenytoin and phenytoin in can be detected at the same time. The method in the invention is characterized in that pilot sample is pretreated; fosphenytoin, phenytoin and 4'-hydroxyl phenytoin are separated with each other in an acidity flowing phase chromatographic column and are detected by UV detector. The method in the invention has the advantages of little sample, easy, swift and sensitive operation and short analysis period; furthermore, the method in the invention doesn't need expensive equipment and reagent, is suitable for clinical conventional detection and can be used to adjust the drug dose, which can guarantee safe and effective prescription and has the important clinical practice significance.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Method for rapidly measuring mizoribine drug concentration
InactiveCN101226172ASimple and efficient operationLess plasma consumptionComponent separationBiological testingChromatographic columnHuman blood
The invention relates to an analysis measurement method of internal drug, in particular to a method for quickly measuring mizonbine density of human blood plasma, which comprises adding sample into an interior label, after protein deposition pretreatment, isocratically eluting under water-type flow phase condition, separating via a chromatographic column, and detecting via an ultrasonic detector. The invention has low sample consumption, simple, quick and sensitive pretreatment, which not demands expensive device and organic solvent, with wide application, low environment pollution, low cost and the application for detecting clinical general blood drug density.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Method for determining blood drug level of mizoribine
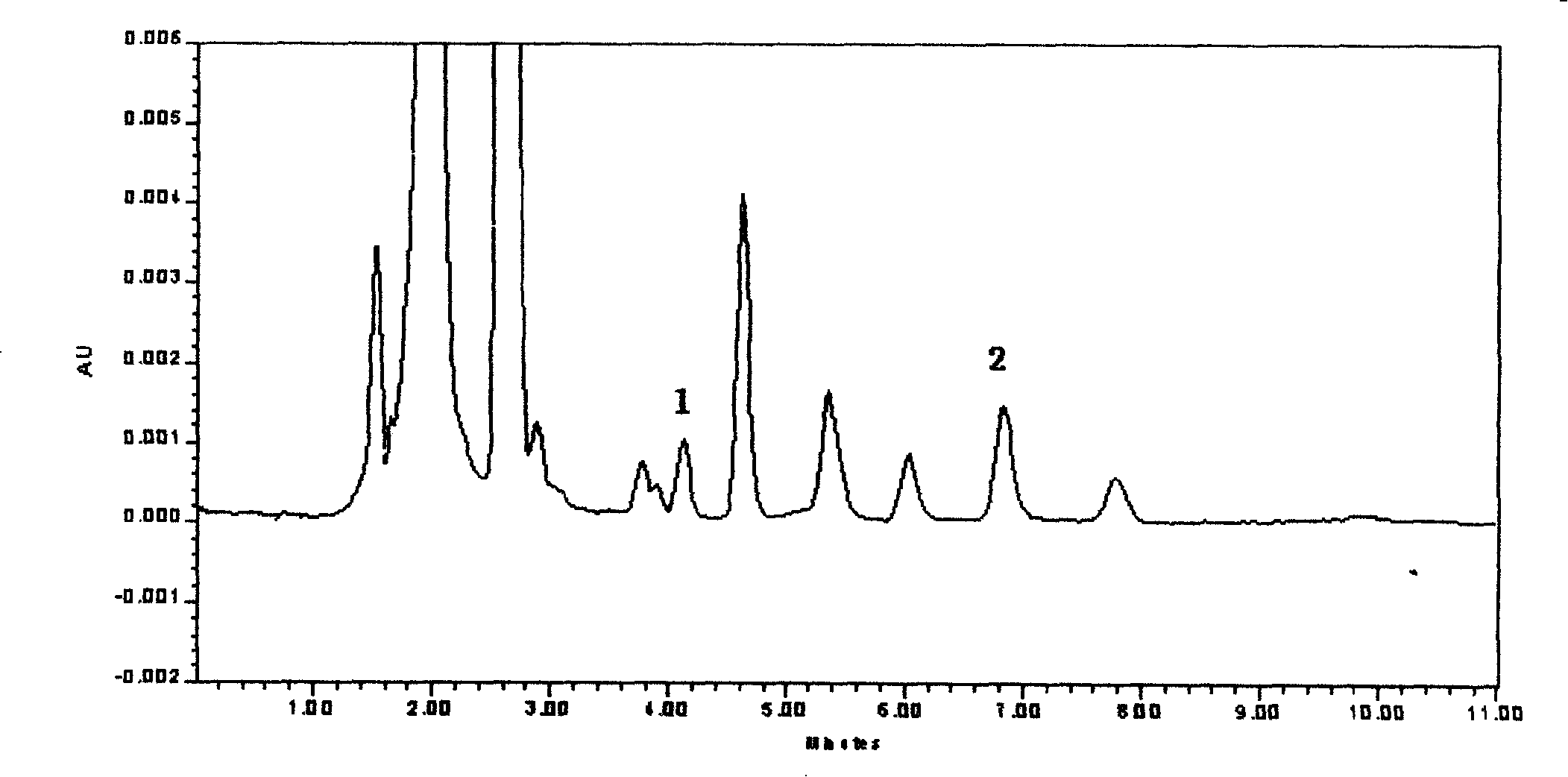
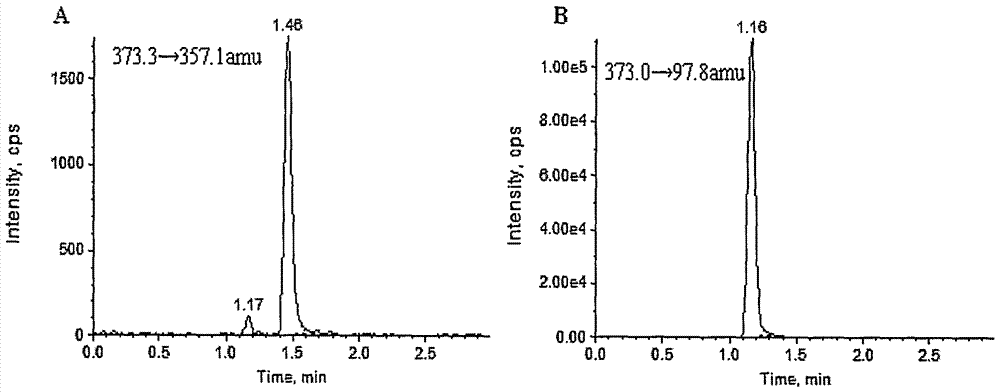
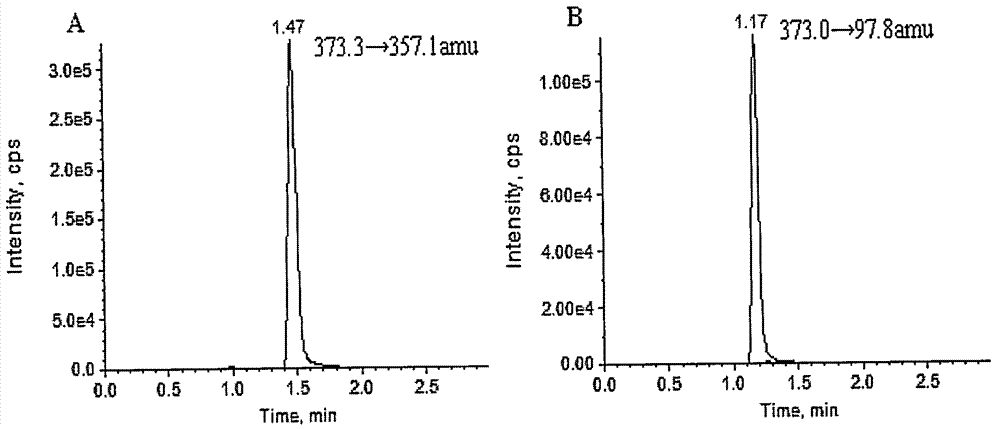
InactiveCN1804615ADetermination does not interfereStrong specificityComponent separationOther chemical processesPretreatment methodBlood plasma
The invention relates to an internal drug assay determination method in the field of medical examination, which relates to a method for measuring human plasma imidazole density. It dose equal indicative elution on acid traveling phase condition after doing first treatment to the tested sample by protein depositing and uses ultraviolet detector to test it after chromatographic column separating.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
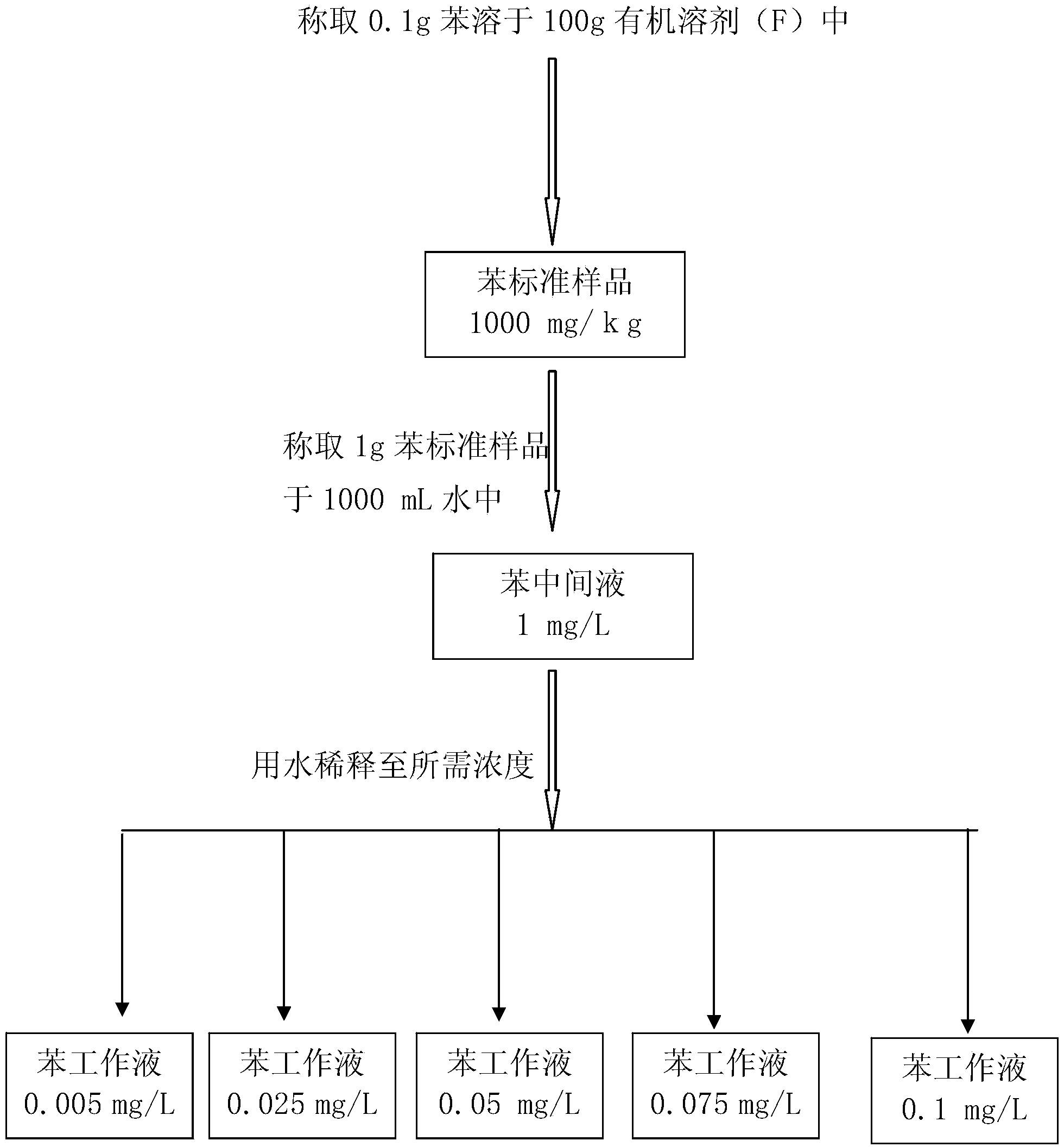
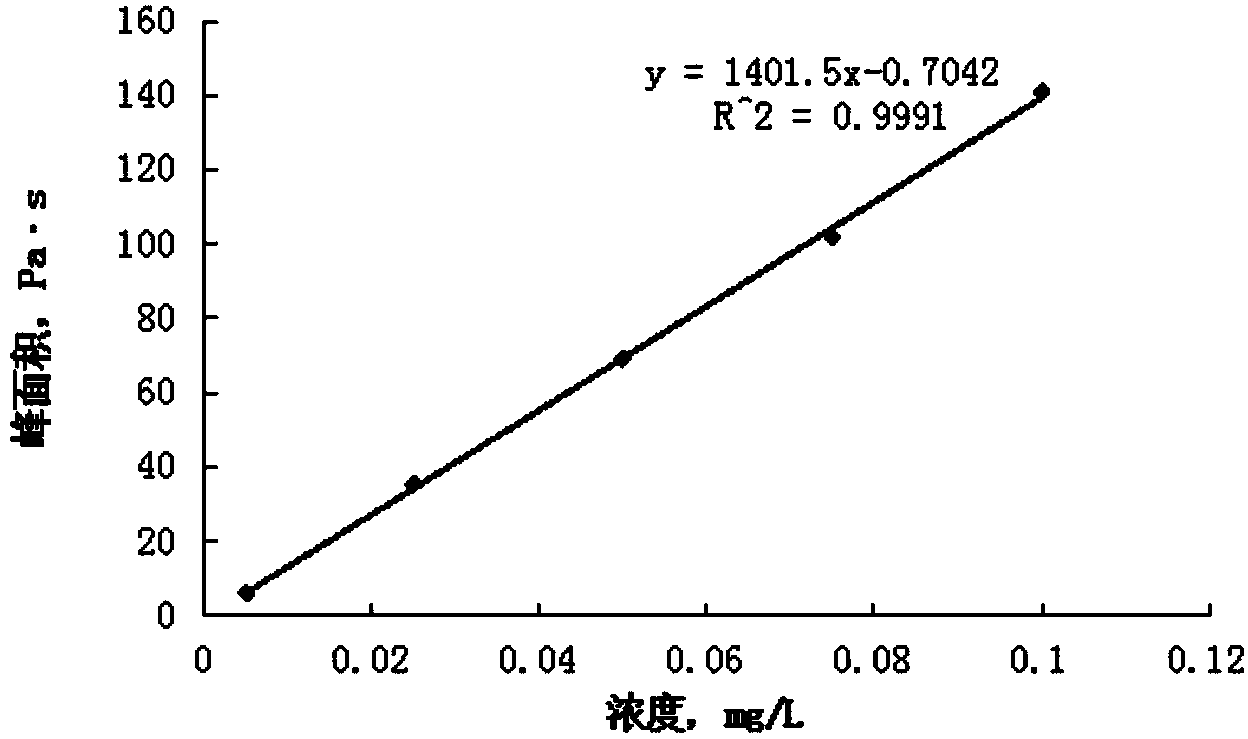
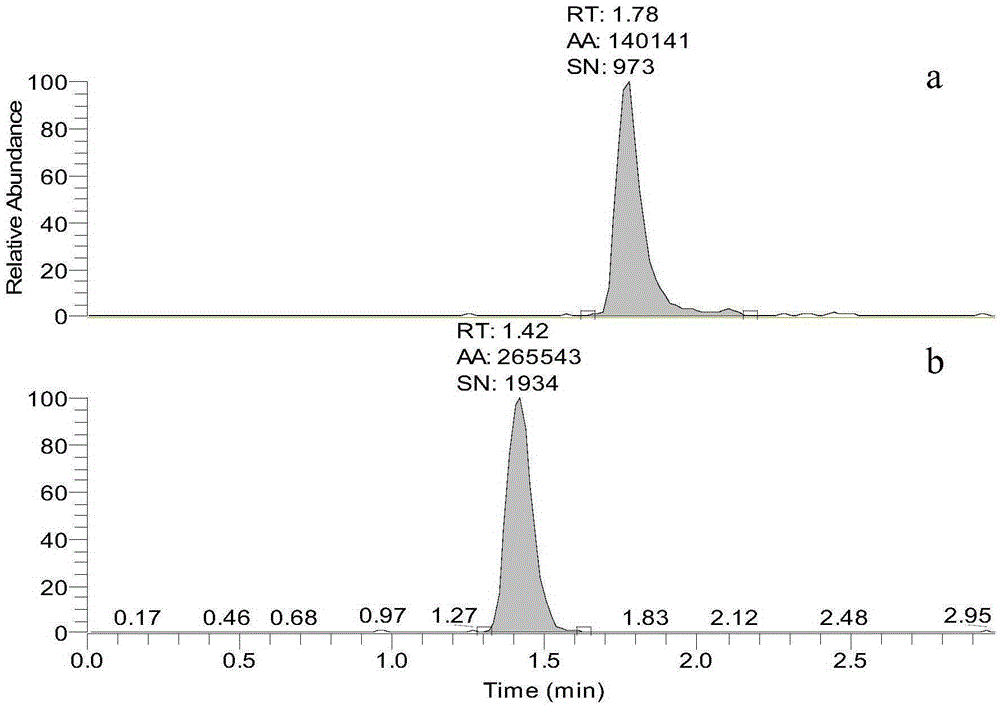
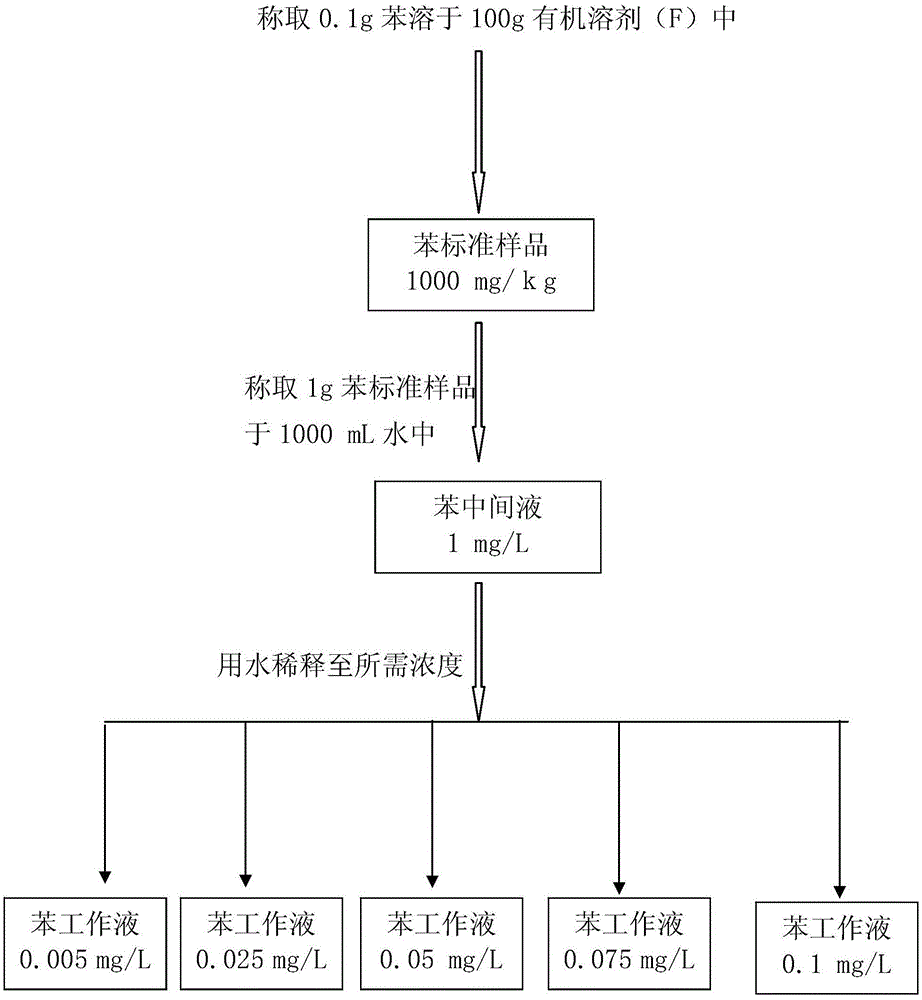
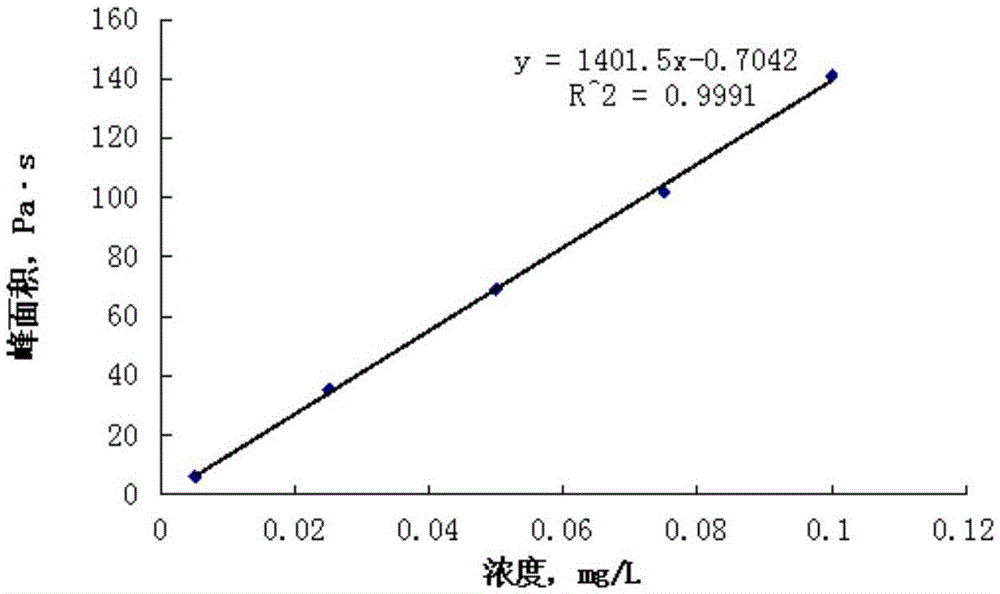
Standard sample used for headspace analysis of trace quantity benzene in water and method for measuring trace quantity benzene in water by using method thereof
ActiveCN104345104AIncrease concentrationIncrease the weighing amountComponent separationOrganic solventMutual solubility
The invention provides a standard sample used for headspace analysis of trace quantity benzene in water and a method for measuring trace quantity benzene in water by using the method thereof. The method comprises the following step of dissolving a benzene solution in a polarity sulfone organic solvent with high boiling point and capable of dissolving with water and benzene to obtain the standard sample. When in use, the standard sample is diluted by water to the required concentration; wherein the mass ratio of benzene to polarity sulfone organic solvent is 1: 100-1: 1000. According to the invention, benzene concentration in the standard sample can be increased, expiry data of the standard sample is prolonged to more than 1 year; benzene sampling weight is increased, weighing error is reduced, and the weighing relative error is reduced from 10% to 1%. The invention also provides a method for measuring trace quantity benzene in water by using the standard sample, and the standard sample does not influence the benzene measurement in headspace analysis.
Owner:BAOWU CHARCOAL MATERIAL TECH CO LTD
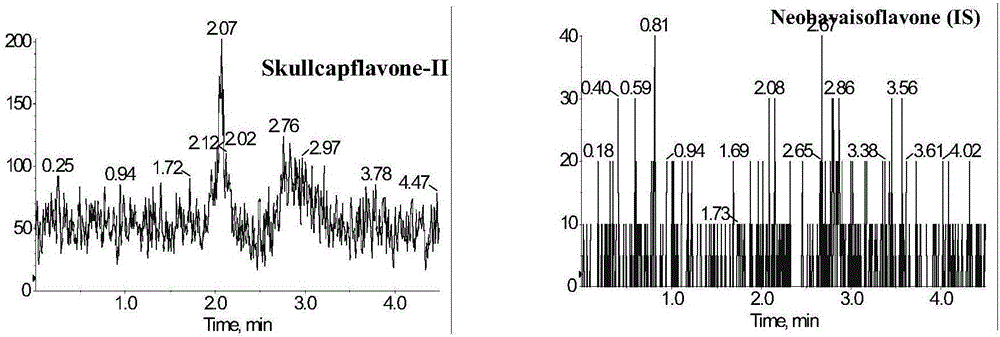
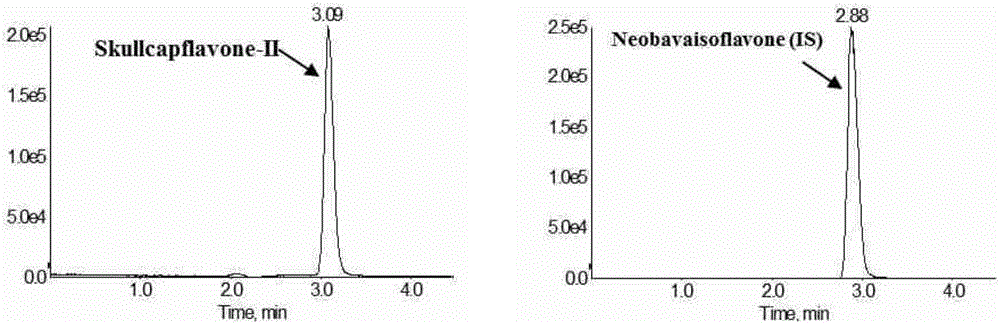
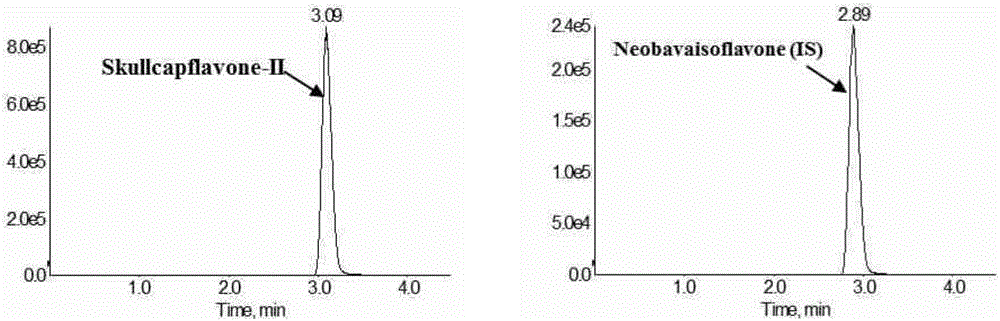
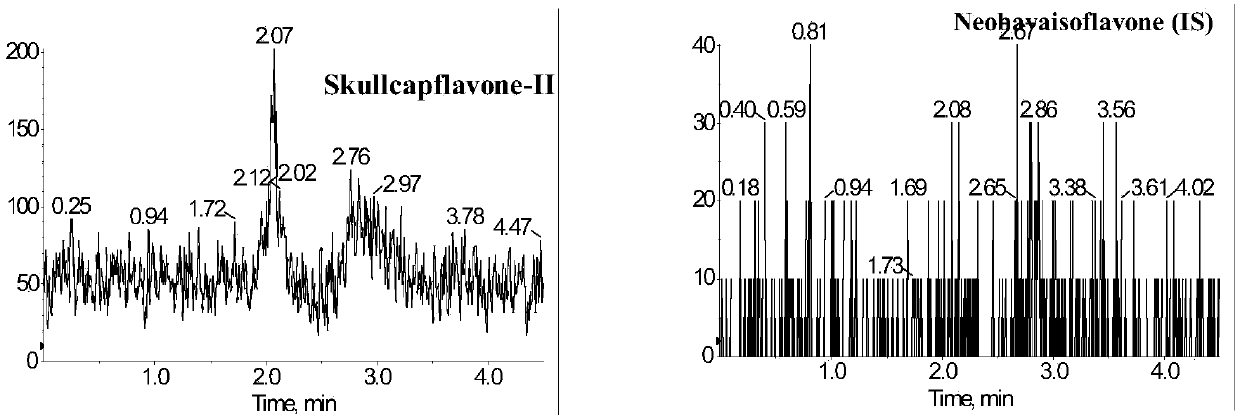
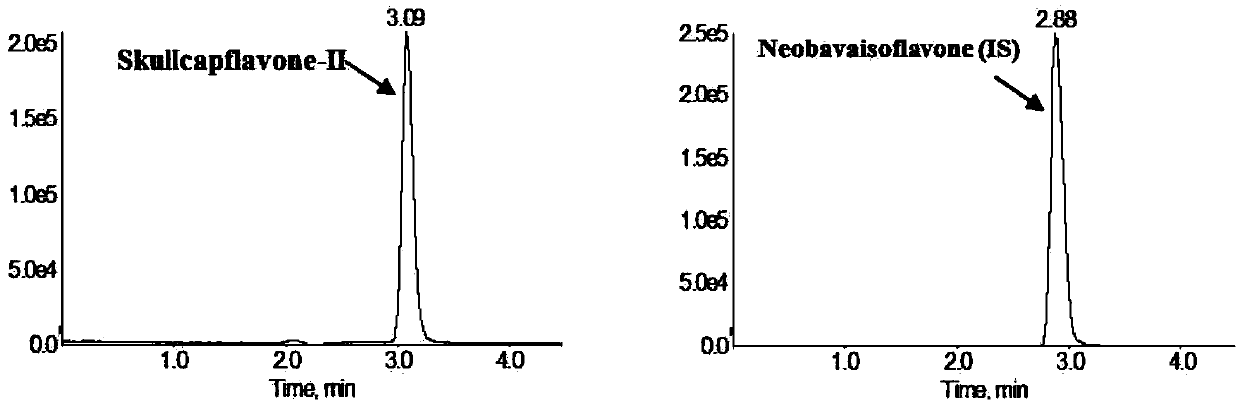
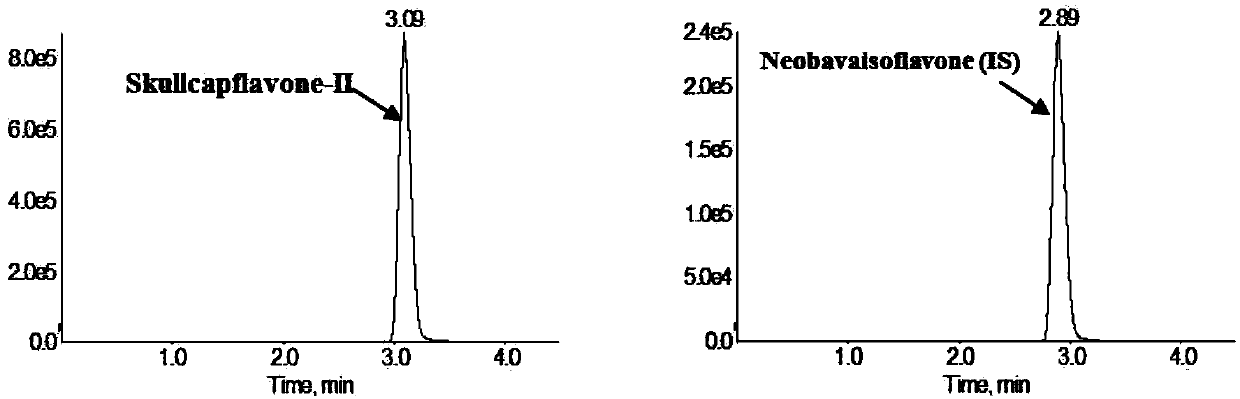
Method for measuring concentration of skullcapflavone II in plasma
InactiveCN106018580AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventPhysical chemistry
The invention discloses a method for measuring concentration of 5,2'-dyhydroxyl,6,7,8,5'-tetramethoxyflavone (skullcapflavone II) in plasma. A liquid chromatography-mass spectrometry system is used for carrying out measuring, a sample to be measured is firstly taken, a certain amount of organic solvent is added for medicine liquid-liquid extraction, after pretreatment, chromatographic column separation is carried out, and a mass spectrometry detector is used for carrying out detection. According to the method, quickness and accuracy are achieved, sensitivity is high, and operation is easy and convenient; the method is suitable for measuring the concentration of skullcapflavone II in the plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Method for simultaneous determination of rabeprazole sodium and metabolites thereof in Beagle dog plasma
InactiveCN107703221AThe pretreatment method is simpleSuitable for routine testingComponent separationMetaboliteLiquid chromatography mass spectroscopy
The invention relates to a method for simultaneous determination of levorabeprazole sodium, dexrabeprazole sodium and rabeprazole sodium metabolites comprising demethylated rabeprazole sodium, thioether rabeprazole sodium and rabeprazole sodium sulphone in Beagle dog plasma by using LC / MS / MS, and belongs to the field of biological detection. In experiments, phenacetin is used as an internal standard, and after ethyl acetate extraction pretreatment is performed, a high performance liquid chromatography-tandem mass spectrometry is adopted for simultaneous determination of the blood drug concentrations of levorabeprazole sodium, dexrabeprazole sodium and three metabolites of rabeprazole sodium in the dog plasma. The method has the advantages of high specificity, high sensitivity and simple operation, and overcomes the limitation of batch test by using two instruments, and is successfully applied to analysis and study of optical isomers of rabeprazole sodium and the metabolites thereof inthe Beagle dog plasma.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Method for determining mesalazine related substances by high performance liquid chromatography
PendingCN112630325AEnsure controllabilityEnsure medication safetyComponent separationFluid phasePhosphate
The invention relates to a method for determining mesalazine related substances by high performance liquid chromatography. The chromatographic conditions are as follows: the high performance liquid chromatography is adopted; octadecyl silica gel bonded silica gel is used as a filler (Gemimi C185mu m 150mm*4.6 mm); the volume ratio of a phosphate buffer to a TABH solution to a methanol to a water in a mixed solution serving as a mobile phase A is 250: 50: 260: 440; the volume ratio of a phosphate buffer to a TABH solution to methanol to water in a mixed solution serving as a mobile phase B is 250: 50: 500: 200; and gradient elution is performed, the flow rate is 1.1 ml / min, the column temperature is 35 DEG C, and the detection wavelength is 230 nm. According to the determination method of the related substances, main peaks and various impurities can be effectively separated, and the blank gradient does not interfere with the determination of the impurity H and the impurity N. Methodological verification is carried out on the determination conditions of the related substances, and the separation degree and the detection sensitivity meet the requirements.
Owner:ZHEJIANG ASEN PHARMA
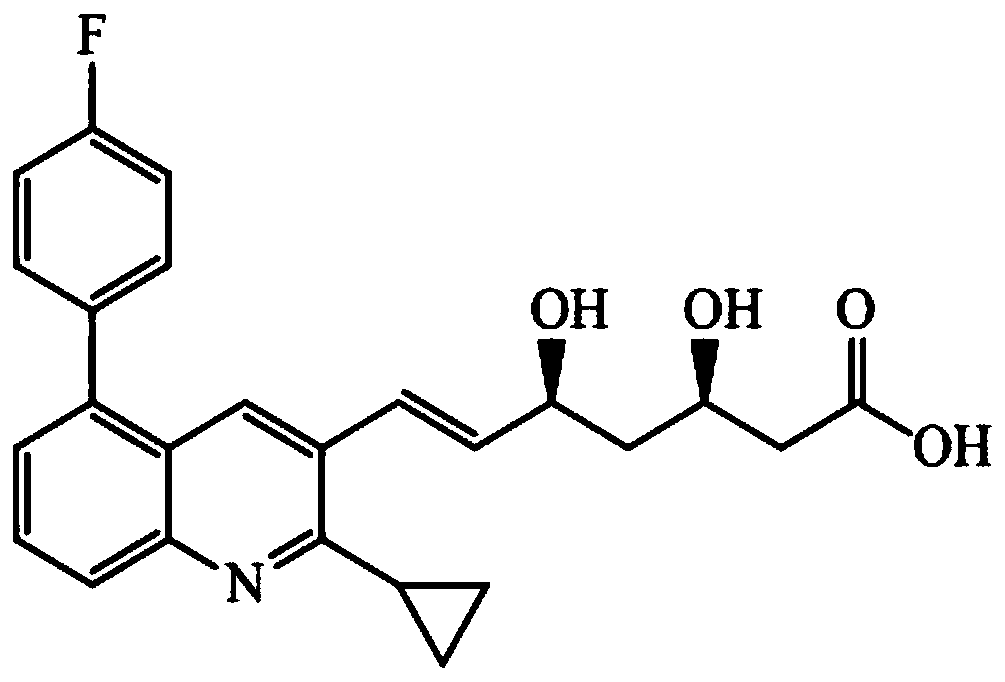
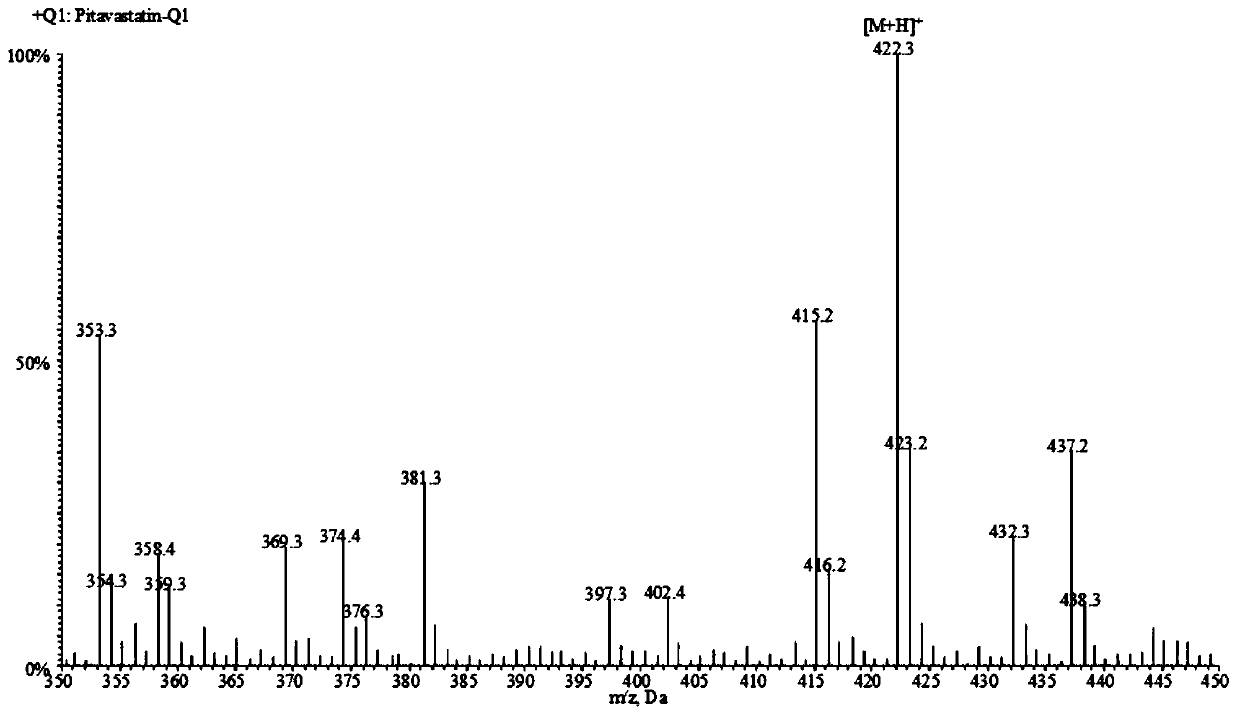
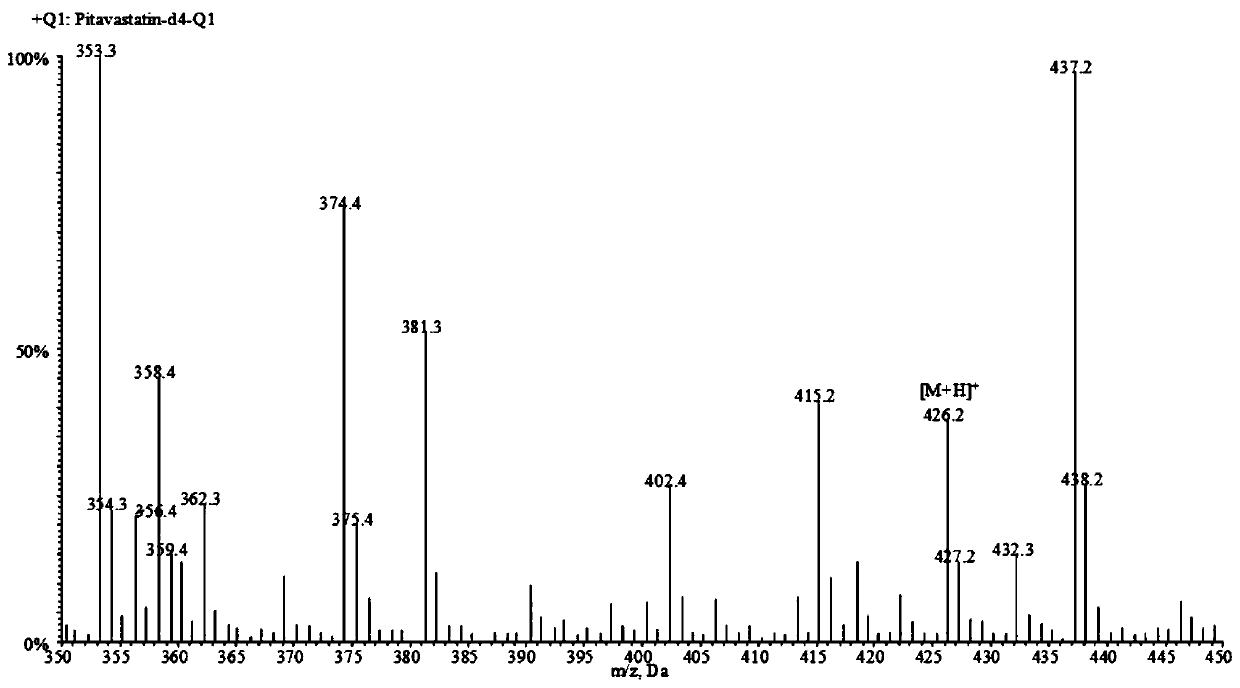
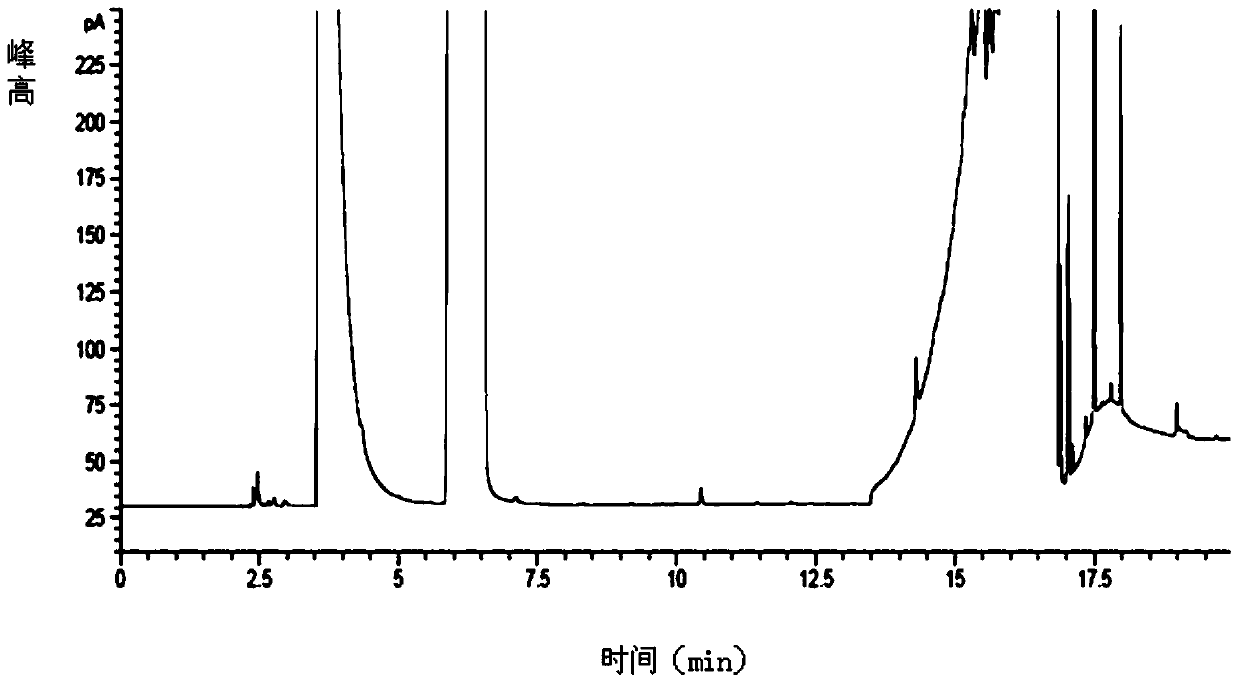
A liquid chromatography-tandem mass spectrometry method for the detection of pitavastatin in human plasma
ActiveCN107144648BGood reproducibilityImprove accuracyComponent separationPretreatment methodEclipse
The invention relates to a liquid chromatogram-tandem mass spectrum method for detecting pitavastatin in human plasma, and application to clinical pharmacokinetic research. The invention provides a method for detecting pitavastatin concentration of plasma. By the method, the pitavastatin concentration of the plasma can be analyzed through LC-MS / MS. According to the method provided by the invention, a protein precipitate pretreatment method is preferably adopted, deuterated pitavastatin serves as internal standard, Eclipse Plus Phenyl-Hexyl column isocratic elution is adopted, and electrospray ionization (ESI) tandem mass spectrum detection is adopted. By adoption of the method provided by the invention, the extraction and recovery rate of the plasma sample is 93 percent or higher and is not influenced by matrix effect, the stability of the pitavastatin is inspected by counting the pitavastatin concentration RSD% before pretreatment, the accuracy of the measured data is guaranteed, the method is high in specificity and selectivity, high in sensitivity, rapid in detection and small in use amount, and simple, reliable, high-flux and condition-controllable clinical mass-batch sample analysis requirements are met. The specificity, the stability and the like of the method provided by the invention are verified, and the method can be used for evaluating the bioequivalence of various dosage forms of pitavastatin successfully.
Owner:苏州海科医药技术有限公司
A kind of assay method of residual solvent in decitabine intermediate
ActiveCN105388223BImprove stabilityEasy to separateComponent separationRelative standard deviationPyrrolidinones
The invention discloses a method for measuring the content of pyridine in decitabine impurities. A methyl alcohol-N-methyl-pyrrolidone-triethylamine mixed solution is used as a solvent, and a direct injection gas chromatographic method is adopted for measurement. The problems that decitabine raw material glucoside stability is poor, dissolution is not easily achieved, the recycling rate of an existing detection method is seriously low and interference is likely to be caused by solvent peaks are solved. According to the method, it is tested that a blank solvent has no interference peaks for residual solvent pyridine measurement, the precision is good, the relative standard deviation (RSD) is only 0.46%, the reproducibility is good, the linear relation is good, the average recycling rate is high, and pyridine possibly remaining in glucoside can be accurately and quantitatively detected. Effective quality control is achieved for preparing second-class solvent pyridine in the decitabine raw material glucoside, and the quality of decitabine and drug use safety are ensured at the same time.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
A kind of assay method of sodium oleate content in dry emulsion for injection
ActiveCN107091890BThe assay method is sensitiveThe determination method is accurateComponent separationEmulsionHplc method
The invention discloses a determination method of sodium oleate content in an injection use dry emulsion. The method comprises the steps that an HPLC method is adopted to separate oleic acid and other components, the oleic acid content is determined according to an external standard method, and based on the relation between the oleic acid and molecular weight of sodium oleate, the sodium oleate content in the dry emulsion is calculated. The determination method is sensitive, accurate, exclusive, efficient, convenient and low in cost.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
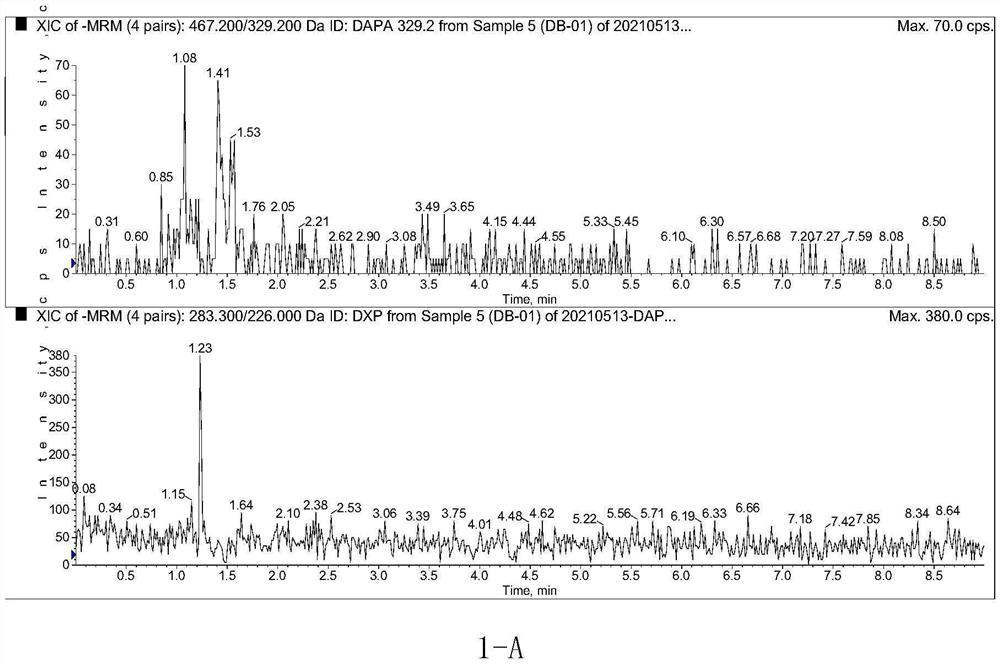
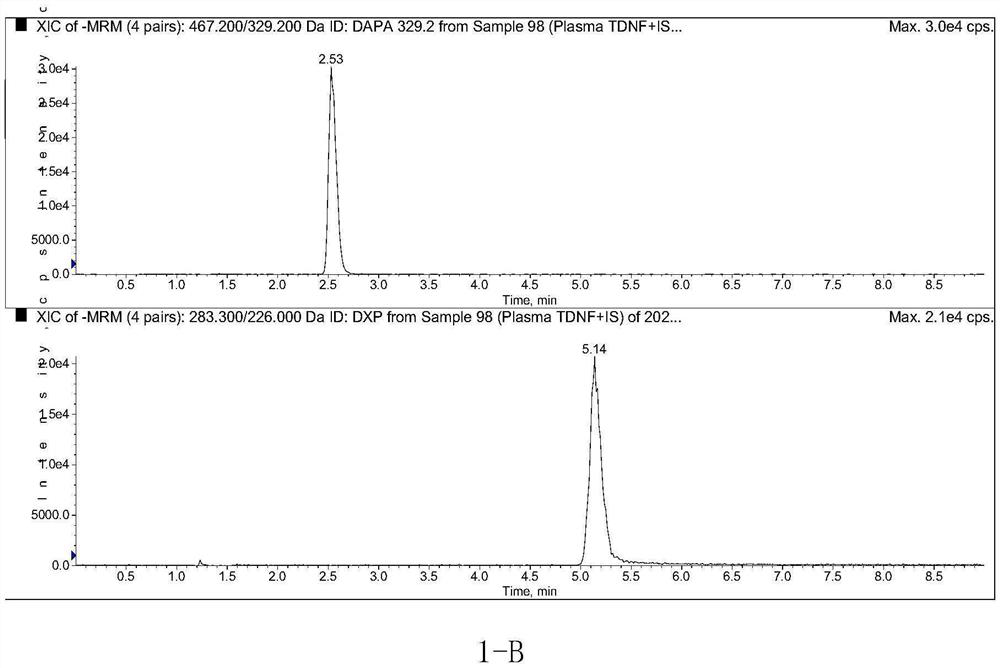
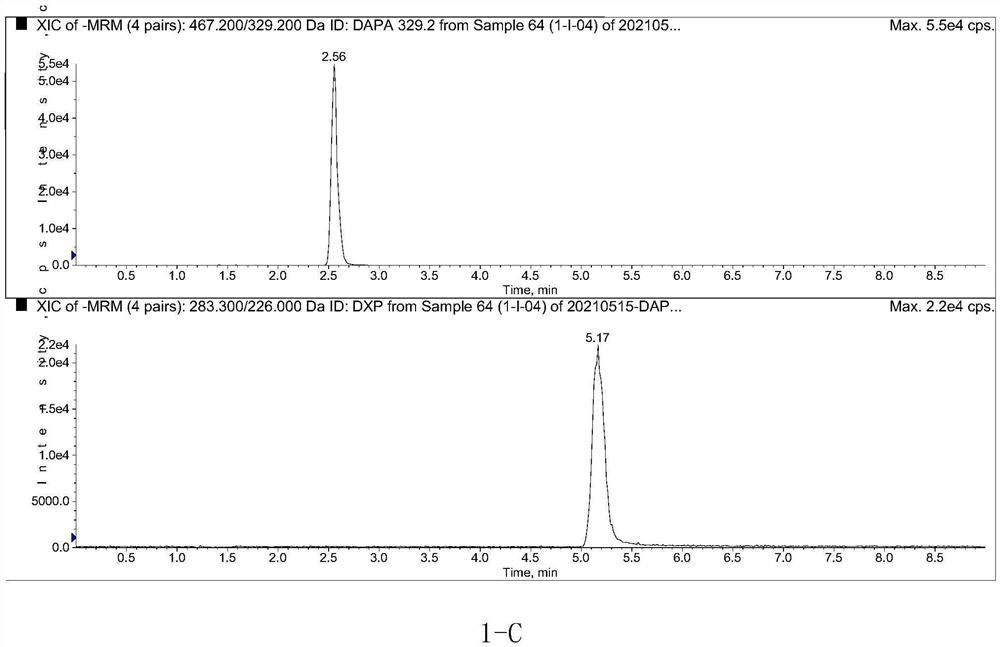
Method for determining concentration of dapagliflozin in plasma
The invention provides a method for determining the concentration of dapagliflozin in blood plasma, which comprises the following steps: pretreating a blood plasma sample, performing liquid chromatography separation on the pretreated blood plasma sample, performing mass spectrometry determination, calculating and the like. Compared with the prior art, the method has the following advantages: (1) dapagliflozin and acetate ions form charged negative ions, so the Rayleigh limit can be easily reached at the ion source, and coulomb explosion is further realized, so that the sensitivity is improved; (2) the pretreatment method is simple and convenient; (3) the specificity is high; (4) the sensitivity is high, and the lowest limit of quantitation of plasma is 1.0 ng.mL <-1 >; (5) no matrix effect exists; and (6) the method is rapid, simple and convenient to operate, accurate, high in sensitivity and low in detection limit. A basis is provided for clinical blood drug concentration determination of dapagliflozin, and the dapagliflozin is used for research, development and clinical application of new drugs. The linear range of a plasma standard curve of the method is 1.0-4000 ng.mL <-1>, and the intra-batch and inter-batch precision RSD is less than 15%.
Owner:SUZHOU SCI&TECH TOWN HOSPITAL
Method for determination of concentration of 5, 6-dihydro-7,8-dimethyl-4,5-dioxy-4-H-pyranoquinoline-2-carboxylic acid in plasma
InactiveCN106404930AThe pretreatment method is simpleFast wayComponent separationChromatographic columnChemistry
The invention discloses a method for determination of concentration of 5, 6-dihydro-7,8-dimethyl-4,5-dioxy-4-H-pyranoquinoline-2-carboxylic acid in plasma, the method uses a liquid chromatography-mass spectrometry system for the determination, and the method is as follows: first taking a to-be-tested plasma sample, adding a certain amount of an inorganic acid for acidification, adding an organic solvent into the acidified plasma sample to precipitate proteins, centrifuging at a high speed, taking supernatant to add a certain amount of deionized water for even mixing, separating by a chromatographic column, and detecting with a mass spectrometry detector. The method is rapid, accurate, high-sensitivity, simple in operation, and suitable for the determination of the concentration of the 5, 6-dihydro-7,8-dimethyl-4,5-dioxy-4-H-pyranoquinoline-2-carboxylic acid in the plasma.
Owner:THE SECOND HOSPITAL AFFILIATED TO SUZHOU UNIV
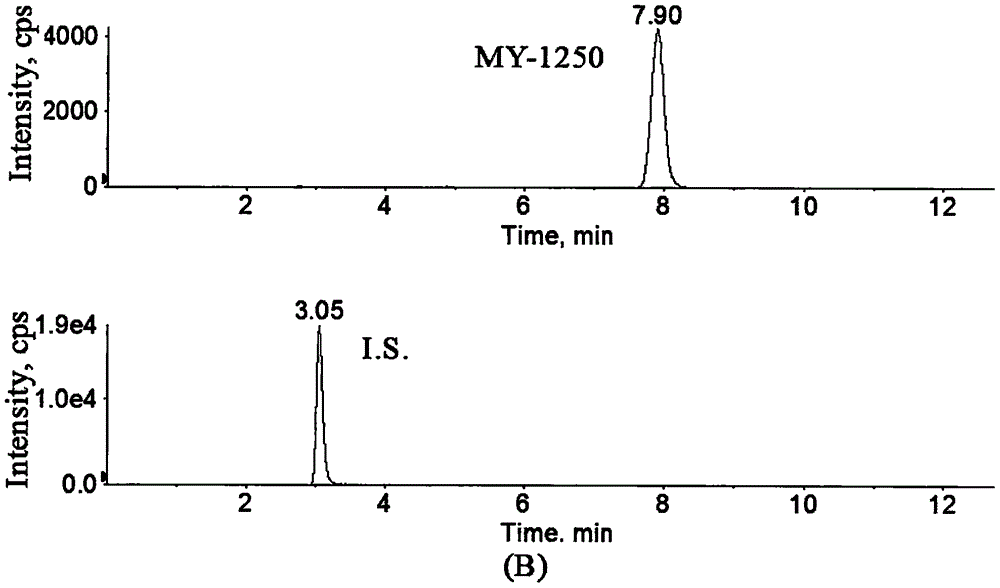
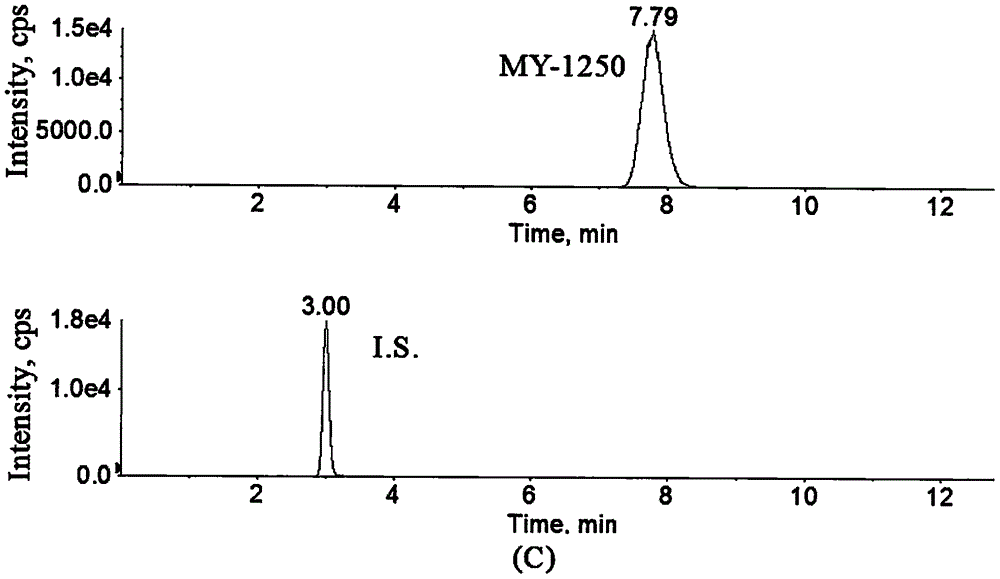
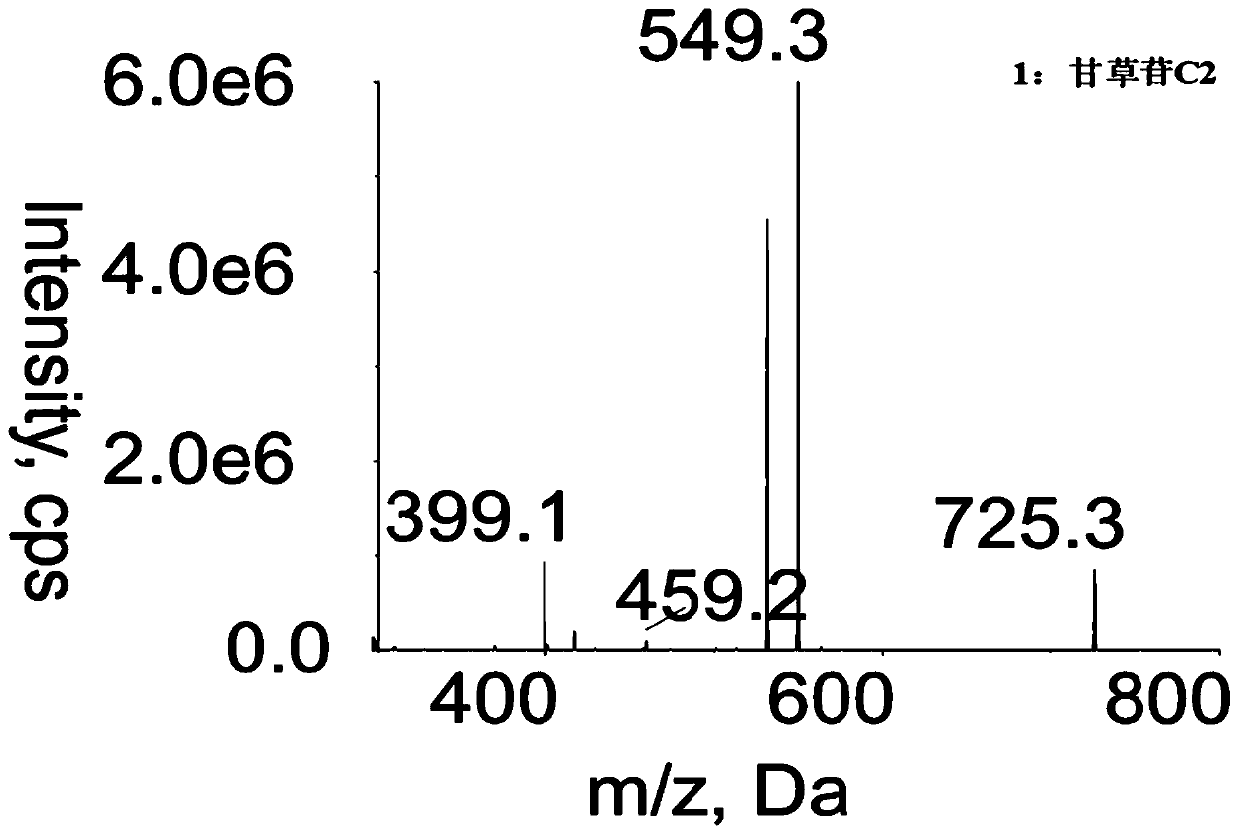
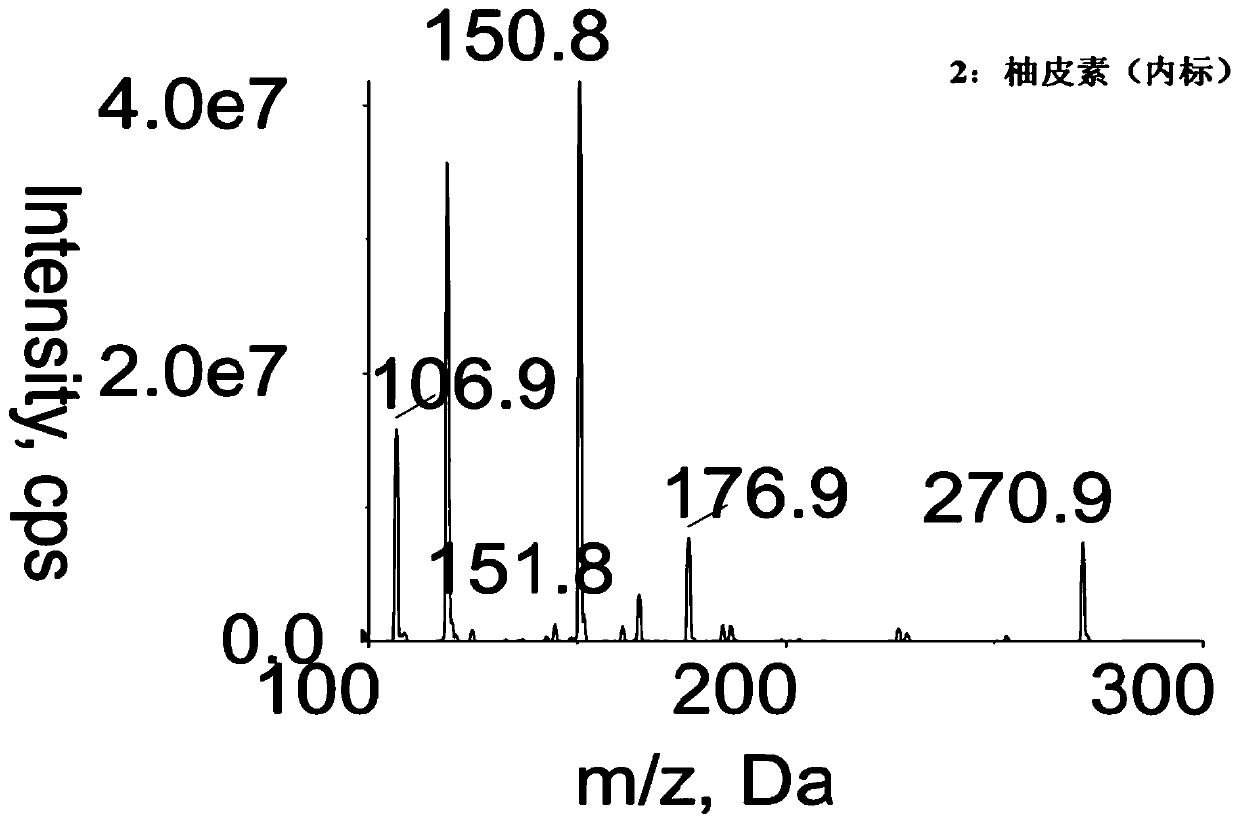
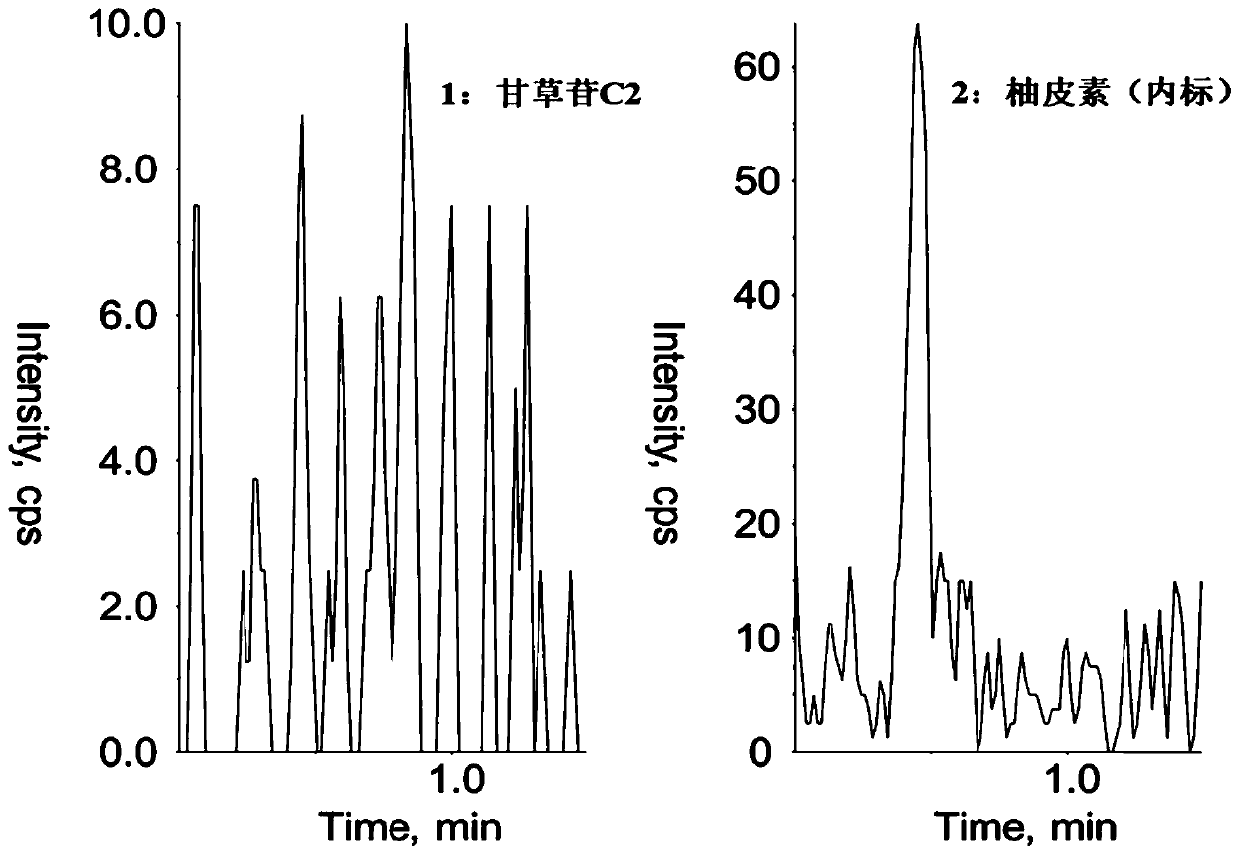
Method for determining concentration of licorice glycoside C2 in blood plasma
InactiveCN110455968ALower limit of quantitationNo distractionComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for determining concentration of licorice glycoside C2 in blood plasma. A liquid chromatography-mass spectrometry system is adopted for determination, firstly, a to-be-determined sample is taken, a certain quantity of organic solvent protein precipitation solutions are added, a chromatographic column is used for separation after pretreatment, and a mass spectrometry detector is used for detection. The method is rapid, accurate, high in sensitivity, simple and convenient to operate and suitable for determining the concentration of licorice glycoside C2 in the blood plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
A method for separating and determining degraded impurities in dutasteride raw materials and preparations by hplc method
ActiveCN109490444BDetermination does not interfereStrong specificityComponent separationHplc dadHplc method
The invention specifically relates to a method for separating and measuring degraded impurities in dutasteride raw materials and preparations by using high performance liquid chromatography, and the impurities are generated under forced degradation conditions. The method of the invention adopts octadecylsilane bonded silica gel as a chromatographic column, and uses mobile phase A and mobile phase B to carry out linear gradient elution. Since there are no quality standards for dutasteride soft capsules in the pharmacopoeias of various countries at present, and there are no relevant documents or standard reports, so this set of HPLC methods is a set of methods completely self-built. The present invention explores the detection wavelength, diluent Conditions such as mass concentration determine a set of methods suitable for the detection of the substance. The method has little interference during detection, has good specificity, good reproducibility and high accuracy, and can play a role in providing technical support for the quality control of dutasteride soft capsules.
Owner:CHONGQING HUAPONT PHARMA
Method for determining human plasma phenytoin and its precursor drug and metabolite
InactiveCN100580448CFew samplesEasy pretreatmentComponent separationColor/spectral properties measurementsFosphenytoinMetabolite
The invention belongs to the medical inspection field, relates to an analysis detection method of drug in the body of a person, and specifically relates to the method that the densities of fosphenytoin, phenytoin and 4'-hydroxyl phenytoin which is the main metabolite of fosphenytoin and phenytoin in can be detected at the same time. The method in the invention is characterized in that pilot sample is pretreated; fosphenytoin, phenytoin and 4'-hydroxyl phenytoin are separated with each other in an acidity flowing phase chromatographic column and are detected by UV detector. The method in the invention has the advantages of little sample, easy, swift and sensitive operation and short analysis period; furthermore, the method in the invention doesn't need expensive equipment and reagent, is suitable for clinical conventional detection and can be used to adjust the drug dose, which can guarantee safe and effective prescription and has the important clinical practice significance.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
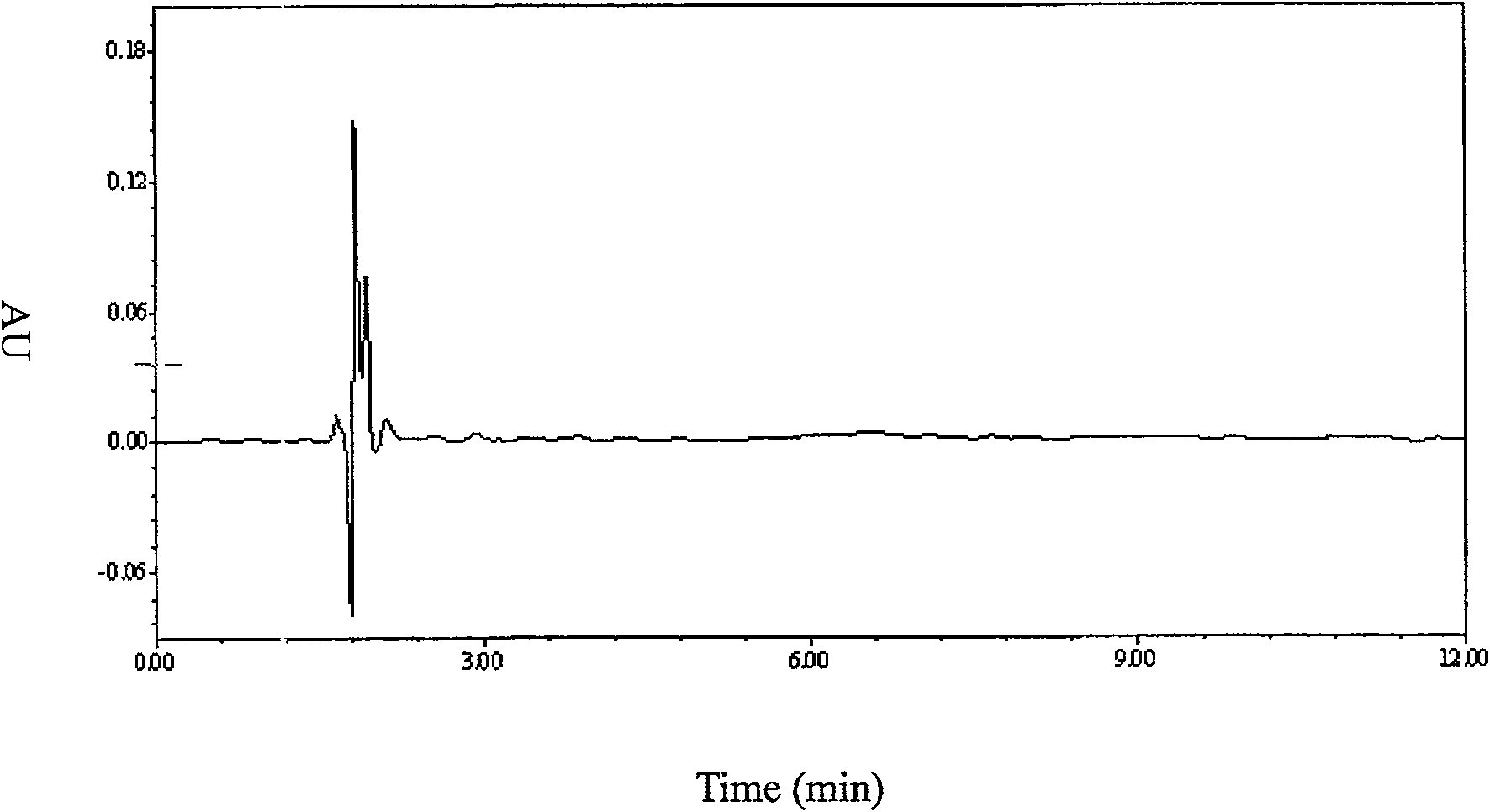
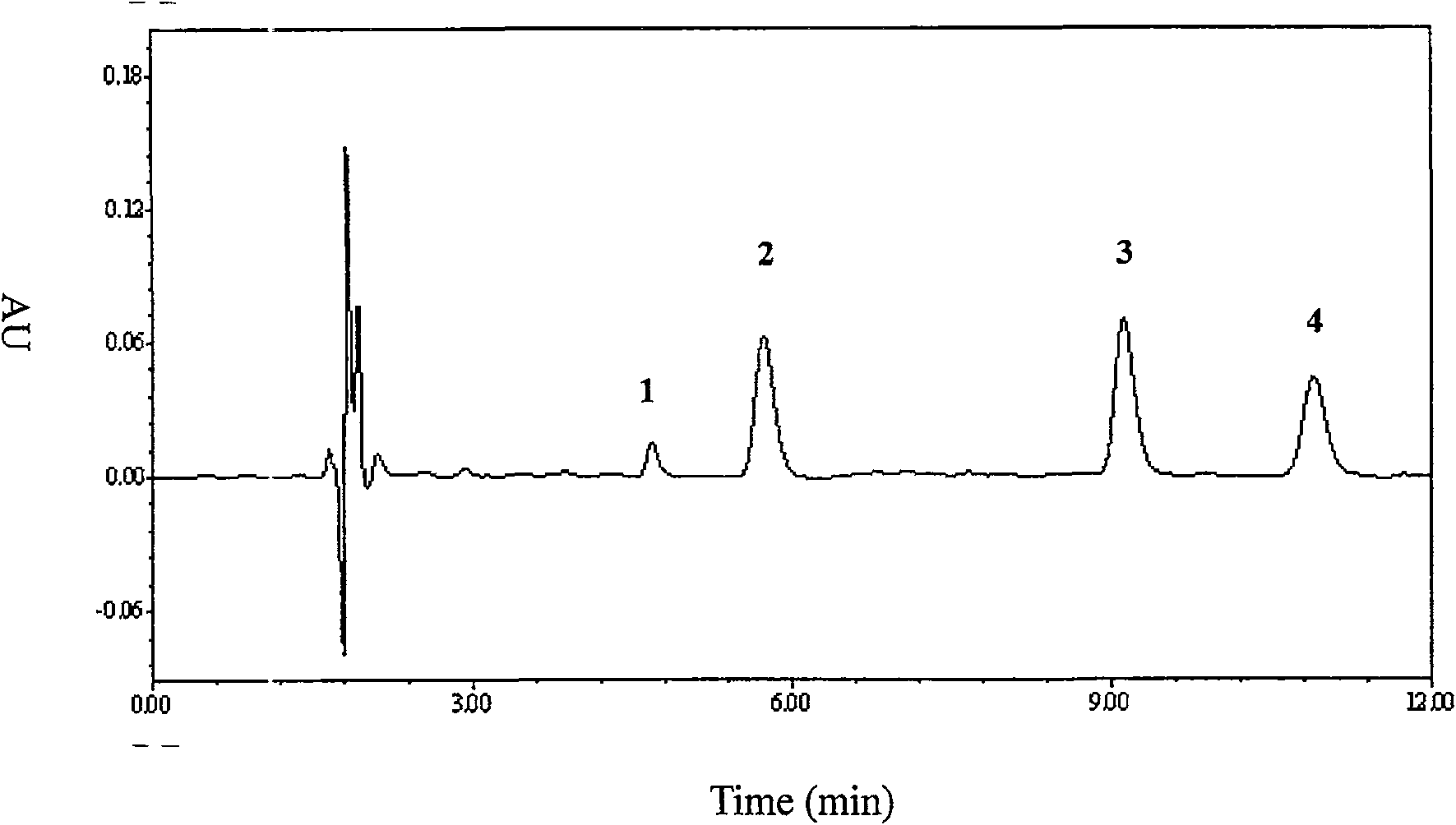
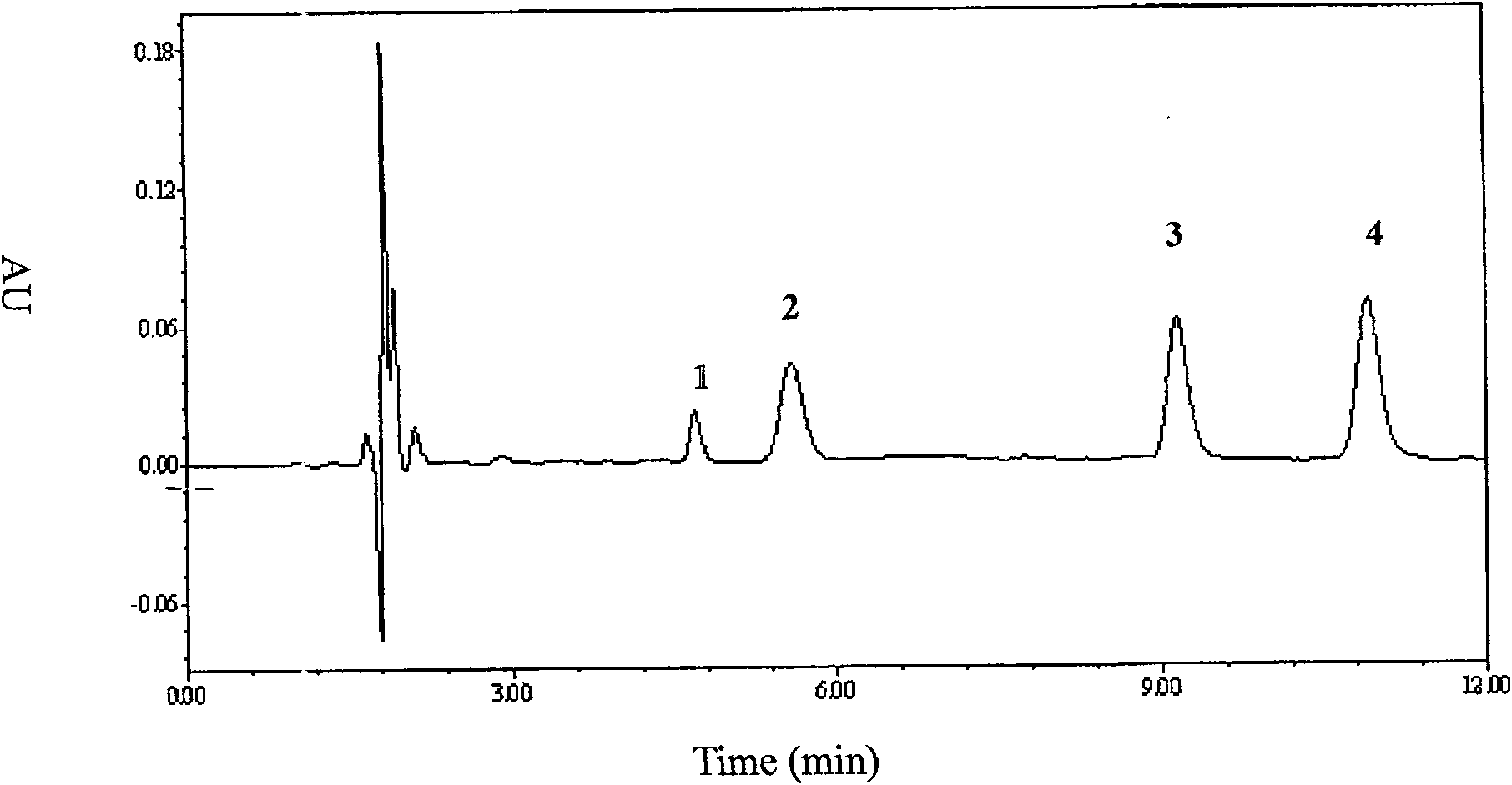
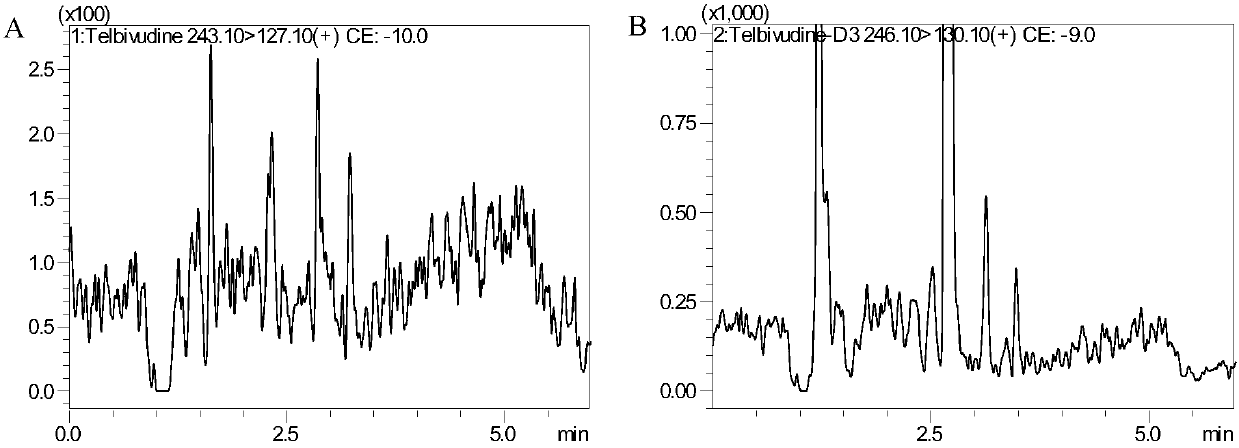
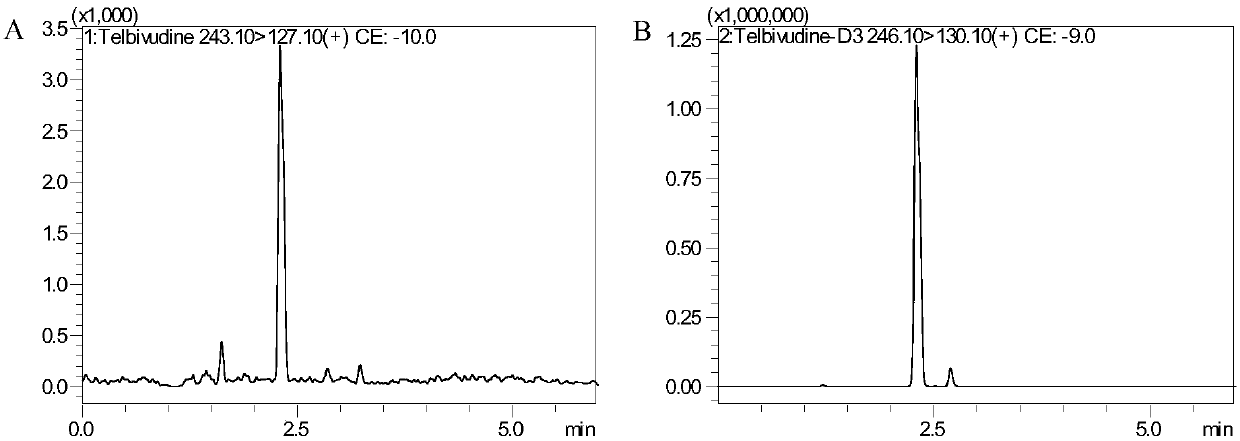
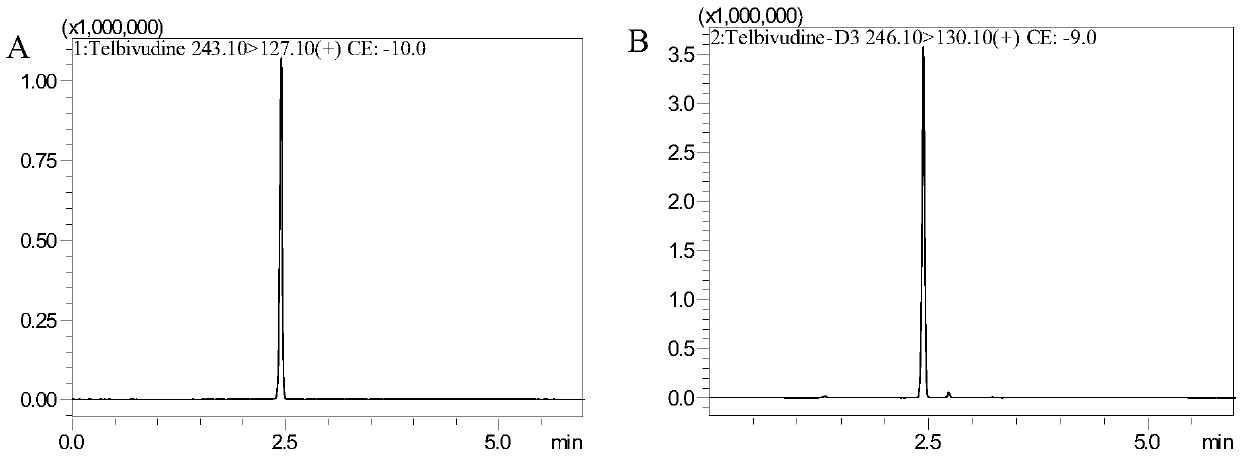
Method for detecting content of telbivudine in blood plasma
InactiveCN107655982AThe preprocessing method is simpleImprove securityComponent separationNitrogen gasBlood plasma
The invention belongs to the field of medical examination and relates to a method for detecting the content of telbivudine in blood plasma by liquid chromatography-tandem mass spectrometry. Accordingto the method, without the need of blow-drying with nitrogen, a sample to be detected directly undergoes acetonitrile dilution / protein precipitation and a supernatant is taken for sample introduction,elution separation is carried out through a mobile phase in a chromatographic column, and detection is finally carried out by a tandem mass spectrometry detector. According to the method, deuterium 3-telbivudine (D3-LdT) is adopted as the internal standard, and a certain amount of acetonitrile precipitated protein is added into a blood sample. After virus inactivation in the blood plasma sample processing, telbivudine is not obviously degraded. Accuracy of the method is guaranteed, and safety of operators is enhanced. By measuring concentration of telbivudine and the internal standard throughthe tandem mass spectrometry, linearity range is within 10-10000 ng / mL, thus meeting requirements of human pharmacokinetics research. Less samples are sampled by the method, pretreatment is simple, fast and sensitive. Only universal equipment and reagents are needed. Analytical period is short, and cost is low. The method of the invention is suitable for the regulation of a hepatitis B patient treatment scheme and monitoring of routine plasma concentration.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
A kind of method for measuring the concentration of sclareol in plasma
InactiveCN104569215BThe pretreatment method is simpleSuitable for routine testingComponent separationDrug metabolismInternal standard
The invention relates to a method for determining concentration of sclareol in blood plasma and belongs to the technical field of biological detection. Nitrazepam is taken as an internal standard, a protein precipitation method is adopted, and the concentration of sclareol in the determined blood plasma is calculated by virtue of blood plasma sample pre-treatment, chromatograph, mass spectrometry and an internal standard method. The method for determining the concentration of sclareol in the blood plasma is rapid, accurate, high in sensitivity and easy to operate, and a methodological foundation is laid for drug metabolism dynamic research of sclareol in an animal body.
Owner:WUXI PEOPLES HOSPITAL
Standard sample for headspace analysis of trace benzene in water, preparation method thereof and method for determining trace benzene in water using it
ActiveCN104345104BIncrease concentrationIncrease the weighing amountComponent separationOrganic solventPhysical chemistry
Owner:BAOWU CHARCOAL MATERIAL TECH CO LTD
Pretreatment method for detecting content of phenolic impurities in sample
ActiveCN113092622AFix stability issuesPrevent oxidationComponent separationVitamin CPretreatment method
The invention discloses a pretreatment method for detecting the content of phenolic impurities in a sample. The method comprises the following steps: weighing the sample, putting the sample into a volumetric flask, adding an acetonitrile solution of vitamin C, dissolving the sample, uniformly shaking, fixing the volume by using a formic acid solution of the vitamin C, filtering, and carrying out instrumental analysis on filtrate, wherein the content of the vitamin C in the acetonitrile solution of the vitamin C and the formic acid solution of the vitamin C is 90-110 [mu]g / ml. By adopting the pretreatment method disclosed by the invention, extremely low detection limit and quantitation limit can be achieved in phenol impurity detection, and the repeatability is good; the stabilizing time of the reference substance solution can be prolonged to 30 hours, the stabilizing time of the standard recovery solution can be prolonged to 11 hours, meanwhile, the vitamin C and the phenol target object can be effectively separated in a corresponding chromatographic system, and the determination of the target compound is not interfered.
Owner:广州国标检验检测有限公司
A method for measuring the concentration of sodium tanshinone IIA sulfonate in human plasma
The invention belongs to the field of medical examination, and relates to the analysis and measurement method of in vivo drugs, particularly to a method capable of measuring the concentration of Sodium Tanshinone IIA Sulfonate (STS) in human plasma. The method uses deuterium 5-dehydroepiandrosteronesulfate (DHEAS-D5) as an interior label, and the condition of a yellow light safety lamp without UV (light at the wavelength of 420 nm or below is removed) is adopted to control STS degradation during the blood sample treatment process, so that the accuracy of the method is ensured; after the blood sample is acidized using formic acid, a certain amount of organic solvent methyl alcohol and acetonitrile mixed liquor is added to enable protein to precipitate; a tandem mass spectrometry is used to measure the concentration of STS and the interior label; the quantitative linear range is 2-1000 ng / mL, the requirements of human pharmacokinetic studies are met. The method has the advantages that the less sample is required, the pretreatment is simple, quick, and sensitive, only general-type equipment and reagents are required, the analysis period is short, and the cost is low; the method is applicable to the detection of clinical blood routine STS concentration.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
A detection method for simultaneous determination of oxc and metabolites mhd and mhd-g in human plasma
InactiveCN105136957BThe pretreatment method is simpleSuitable for routine testingComponent separationMetaboliteAnalysis study
The invention relates to a detection method for simultaneously measuring OXC in human plasma and metabolite MHD and MHD-G, and belongs to the technical field of biological detection. An experiment is carried out by taking nitrazepam as an internal standard, and can simultaneously detect the plasma concentrations of the three compounds by adopting high performance liquid chromatography-tandem mass spectrometry after pretreatment through precipitation protein. The detection method is short in time, high in specificity and sensitivity, and convenient in operation, and is successfully applied to the analysis and research of oxcarbazepine in the human plasma and the metabolite thereof.
Owner:WUXI PEOPLES HOSPITAL
Method for determining concentration of nilotinib in blood plasma
PendingCN111521711AThe pretreatment method is simpleSuitable for routine determinationComponent separationNew medicationsInternal standard
The invention provides a method for determining the concentration of nilotinib in blood plasma. The method comprises the following steps: pretreating a blood plasma sample, and carrying out liquid chromatography separation, mass spectrometry determination, calculation and the like on the pretreated blood plasma sample. Compared with the prior art, the method has the following advantages: (1) the pretreatment method is simple and convenient; (2) the specificity is high; (3) the sensitivity is high: the lowest quantification limit of plasma is 2.5 ng.mL<-1>; (4) isotope internal standard: no matrix effect exists; and (5) the method is rapid, simple, convenient and accurate to operate, high in sensitivity and low in detection limit. A basis is provided for clinical blood drug concentration determination of nilotinib, and the method serves research, development and clinical application of new drugs. According to the method, the linear range of a plasma standard curve is 2.5-2000 ng.mL<-1>,and the within-run precision RSD and between-run precision RSD are both smaller than 15%.
Owner:THE SECOND HOSPITAL AFFILIATED TO SUZHOU UNIV
A method for measuring skullcapflavone II concentration in blood plasma
InactiveCN106018580BThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for measuring the concentration of 5,2`-dihydroxyl, 6,7,8,5`-tetramethoxyflavone (skullcapflavone II) in blood plasma. A certain amount of organic solvent is added to the sample to carry out the liquid-liquid extraction of the drug. After pretreatment, it is separated by a chromatographic column and detected by a mass spectrometer. concentration.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Method for determining blood drug level of mizoribine
InactiveCN100414293CDetermination does not interfereStrong specificityComponent separationOther chemical processesPretreatment methodBlood plasma
The invention relates to an internal drug assay determination method in the field of medical examination, which relates to a method for measuring human plasma imidazole density. It dose equal indicative elution on acid traveling phase condition after doing first treatment to the tested sample by protein depositing and uses ultraviolet detector to test it after chromatographic column separating.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Infrared light detection method for rapidly detecting industrially discharged toluene
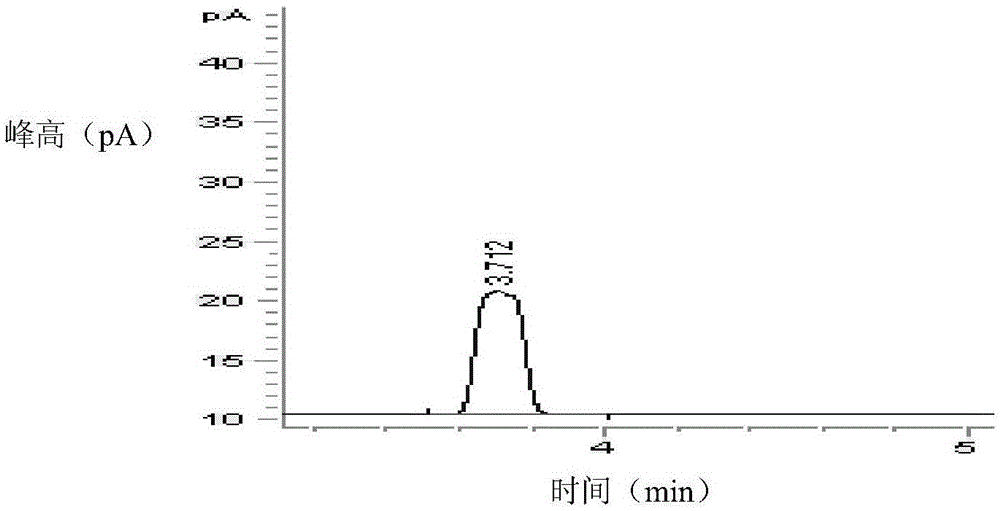
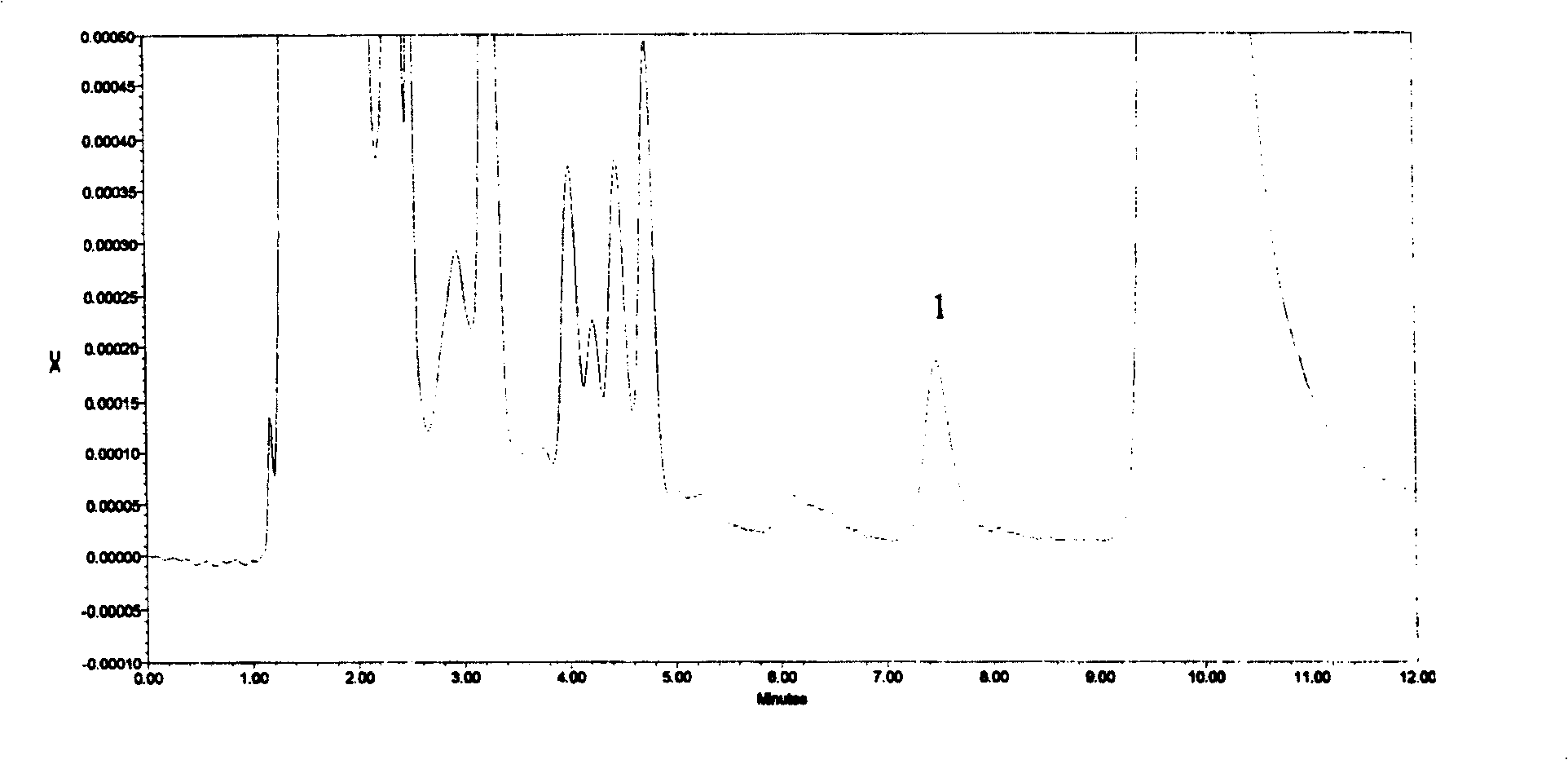
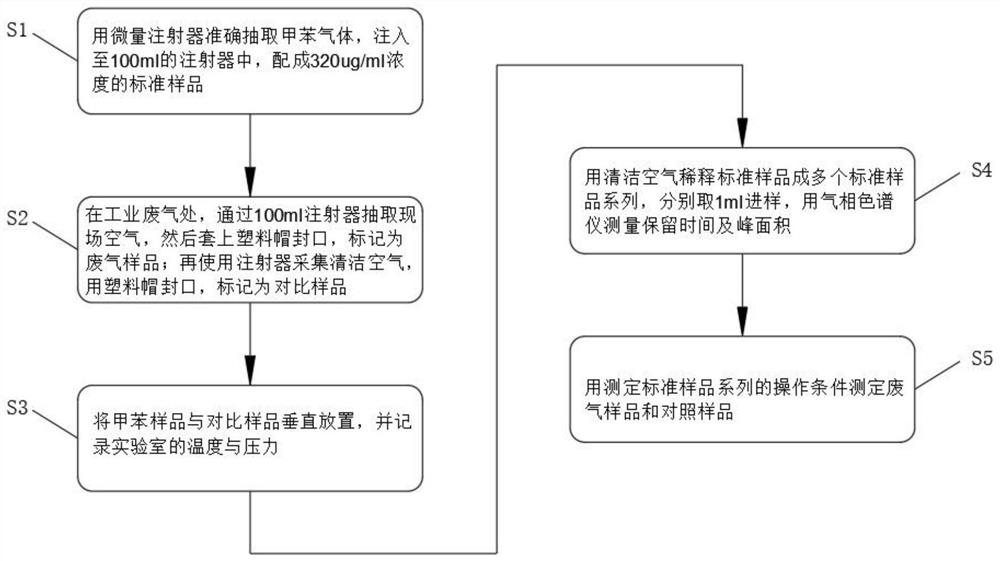
PendingCN113984698ADetermination does not interfereGood effectMaterial analysis by optical meansGas liquid chromatographicToxic industrial waste
The invention relates to the technical field of industrial emission, in particular to an infrared light detection method for rapidly detecting industrially discharged toluene. The method comprises the following steps of accurately extracting a toluene gas by using a microsyringe, and injecting the toluene gas into the syringe; at an industrial waste gas part, extracting the field air through an injector and marking as a waste gas sample; collecting the clean air by using the injector, and marking the clean air as a contrast sample; diluting a standard sample into a plurality of standard sample series by using the clean air, respectively sampling, and measuring the retention time and peak area by using a gas chromatograph; and determining the exhaust gas sample and the control sample by using the operation conditions for determining the standard sample series. According to the infrared light detection method for rapidly detecting the industrially discharged toluene, the toluene in the air is collected by using the microsyringe, and is directly fed into the gas chromatography and a hydrogen flame ionization detector for determination, so that the effect is good, the reproducibility of the method is better, and meanwhile, other substances in the air do not interfere with the determination under the conditions of the method.
Owner:YANGTZE DELTA REGION INST OF UNIV OF ELECTRONICS SCI & TECH OF CHINE HUZHOU
A kind of pretreatment method for detecting phenolic impurity content in samples
ActiveCN113092622BFix stability issuesPrevent oxidationComponent separationVitamin CPretreatment method
The invention discloses a pretreatment method for detecting the content of phenolic impurities in a sample. The method comprises the following steps: weighing the sample, placing it in a volumetric flask, adding an acetonitrile solution of vitamin C, dissolving the sample, shaking well, and taking vitamin C dilute formic acid solution, filter, and the filtrate is used for instrumental analysis; the acetonitrile solution of vitamin C and the formic acid solution of vitamin C, wherein the content of vitamin C is 90-110 μ g / ml. By adopting the pretreatment method of the present invention, extremely low detection limits and quantitative limits can be achieved in the detection of phenolic impurities, and the repeatability is good; the stability time of the reference substance solution can also be extended to 30 hours, and the recovery of the solution after adding the standard can be achieved. The stability time is extended to 11 hours, and vitamin C and phenolic target substances can be effectively separated in the corresponding chromatographic system without interfering with the determination of target compounds.
Owner:广州国标检验检测有限公司
A method for measuring the concentration of 8-epiflavin e-acetate in blood plasma
ActiveCN106153766BThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solvent8-epidiosbulbin E acetate
The invention discloses a method for determining the concentration of 8-epiflavin E acetate in plasma. The method adopts a liquid chromatography-mass spectrometry system for determination. First, a sample to be tested is taken, a certain amount of an organic solvent protein precipitation agent is added to carry out protein precipitation, and after pretreatment , separated by a chromatographic column and detected by a mass spectrometer detector, the method of the invention is fast, accurate, highly sensitive, easy to operate, and suitable for determining the concentration of 8-epiflavin E acetate in plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com