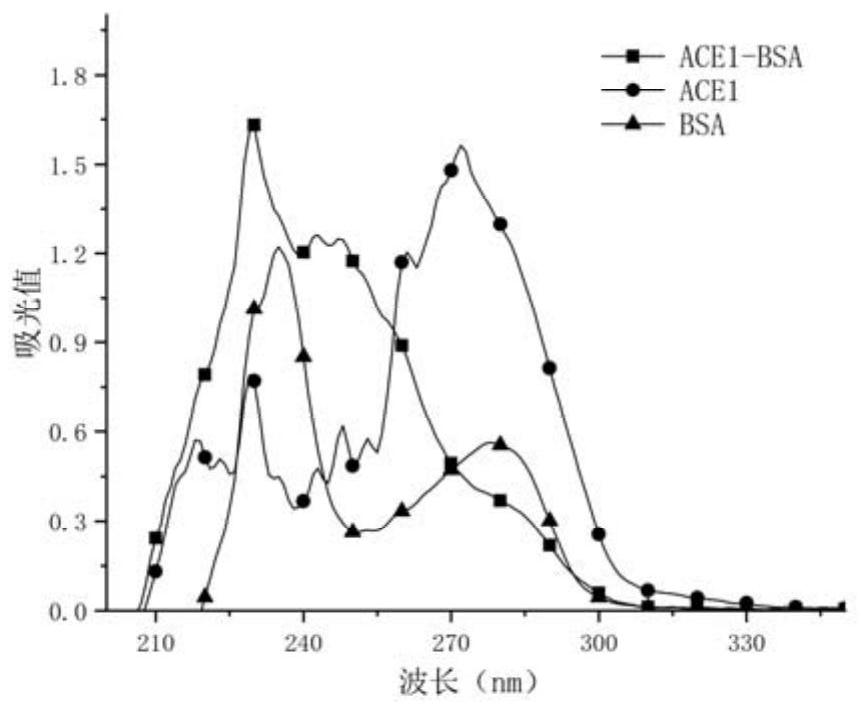
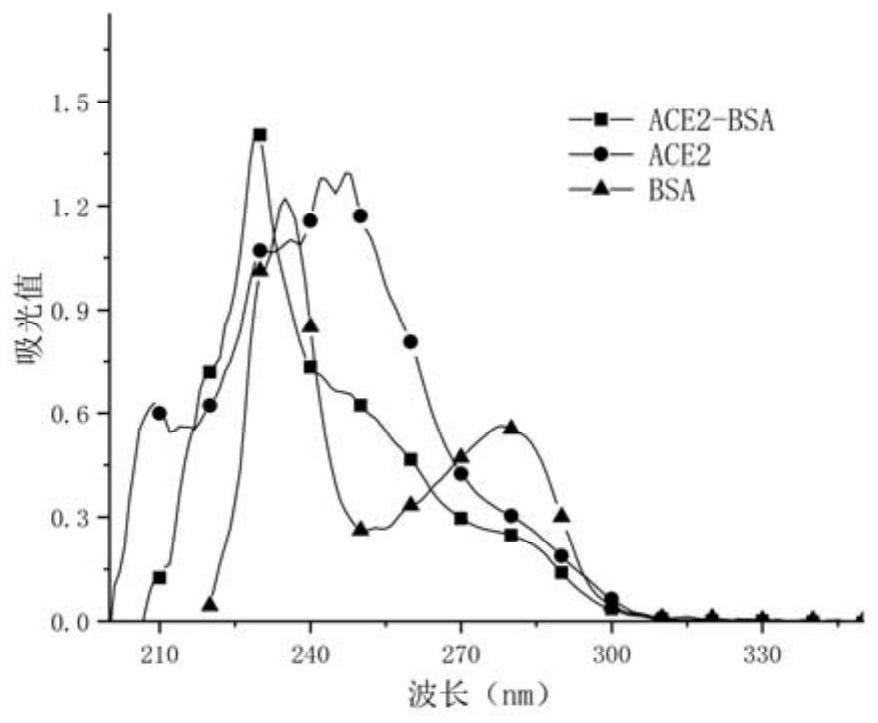
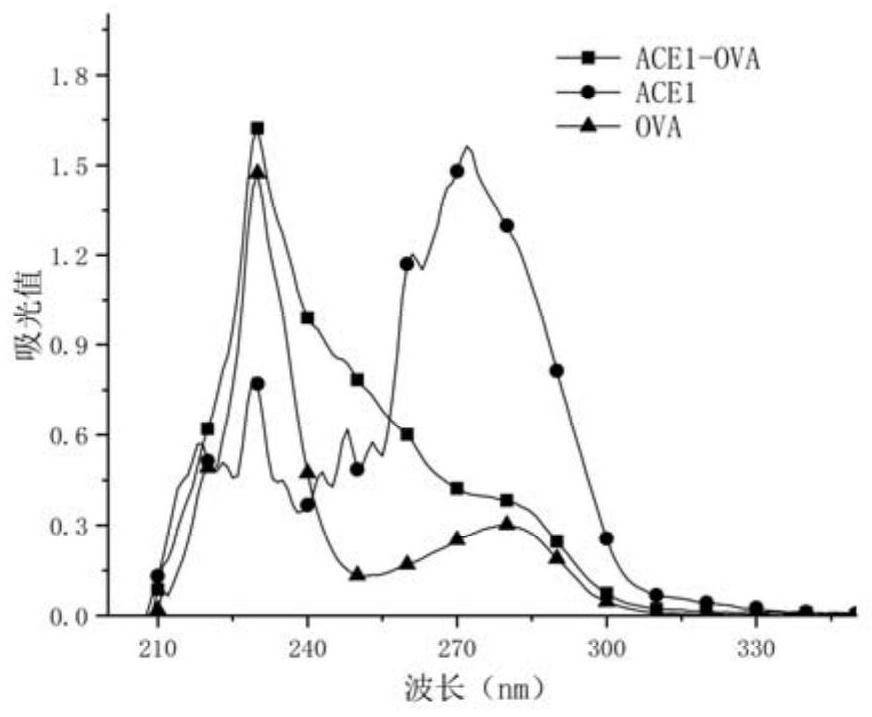
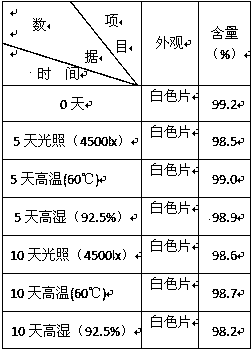
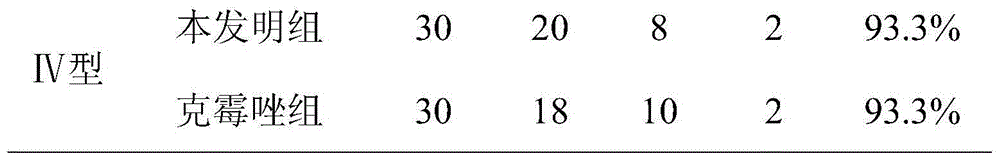
Patents
Literature
44 results about "Phenacetin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
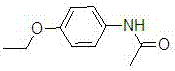
Phenacetin (or acetophenetidin) is a pain-relieving and fever-reducing drug, which was widely used between its introduction in 1887 and the 1983 ban imposed by the U.S. Food and Drug Administration.
Specific probe substrate composition of cytochrome P450 enzyme and application of specific probe substrate composition
InactiveCN104195218AStrong specificityReduce distractionsComponent separationMicrobiological testing/measurementLiquid chromatography mass spectroscopyIonic Channels
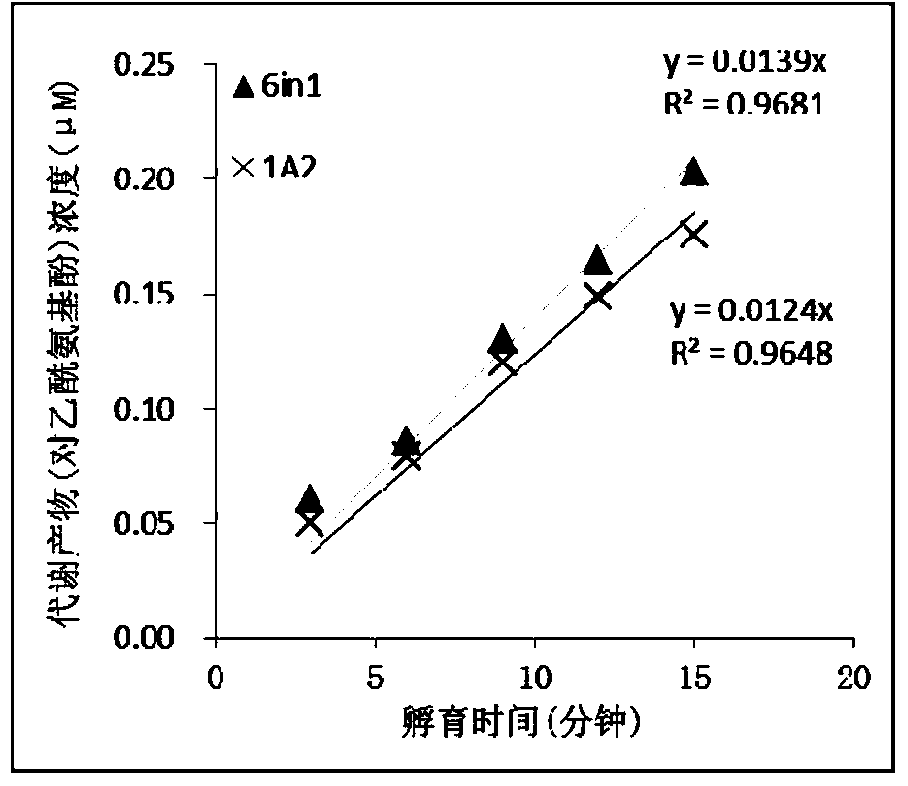
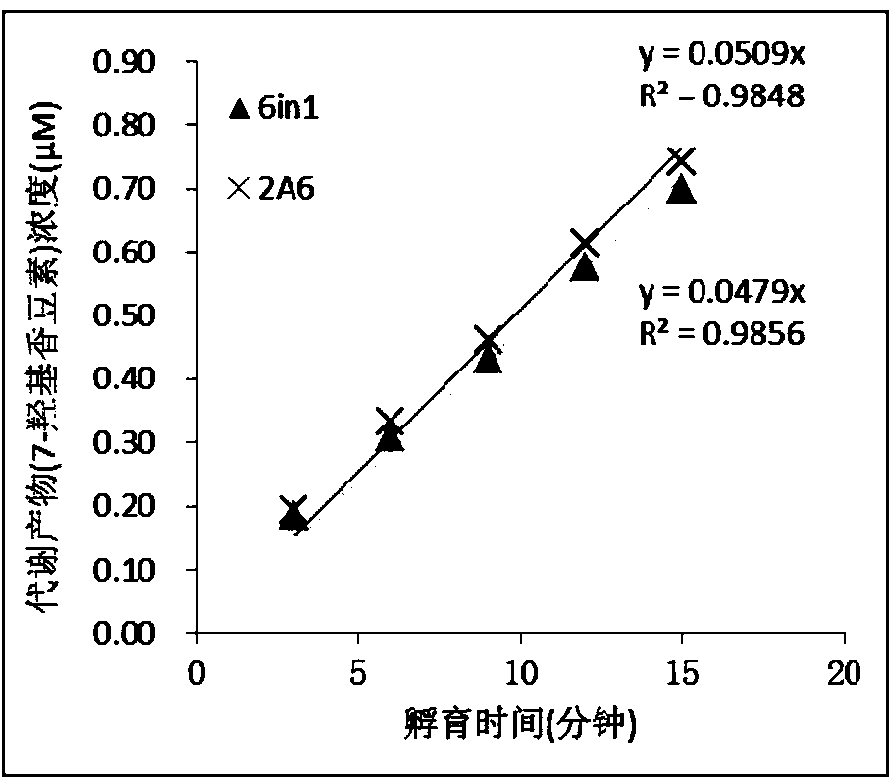
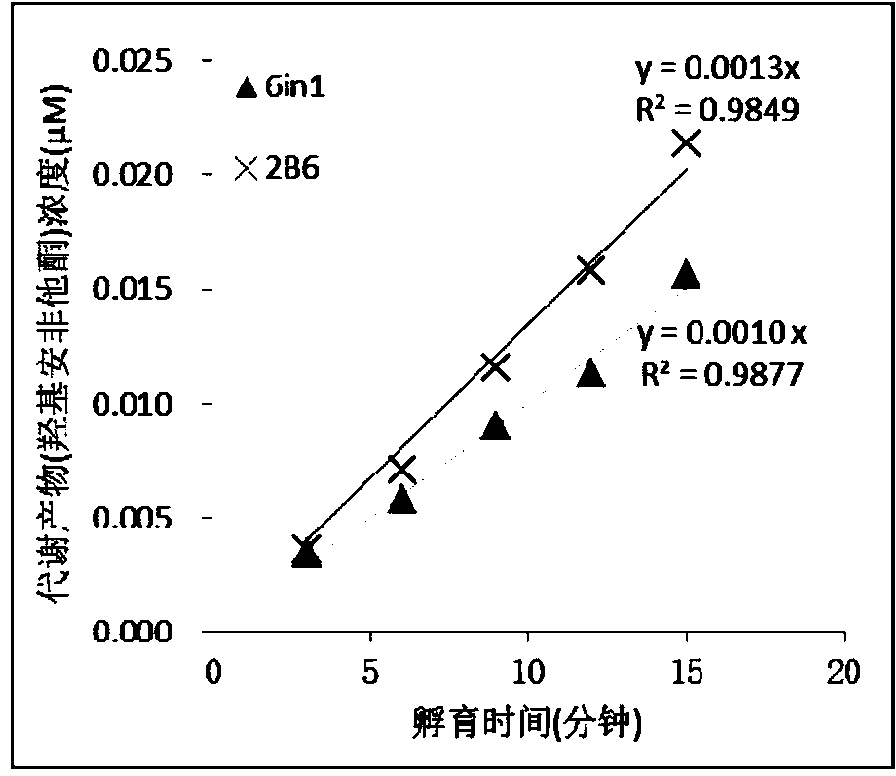
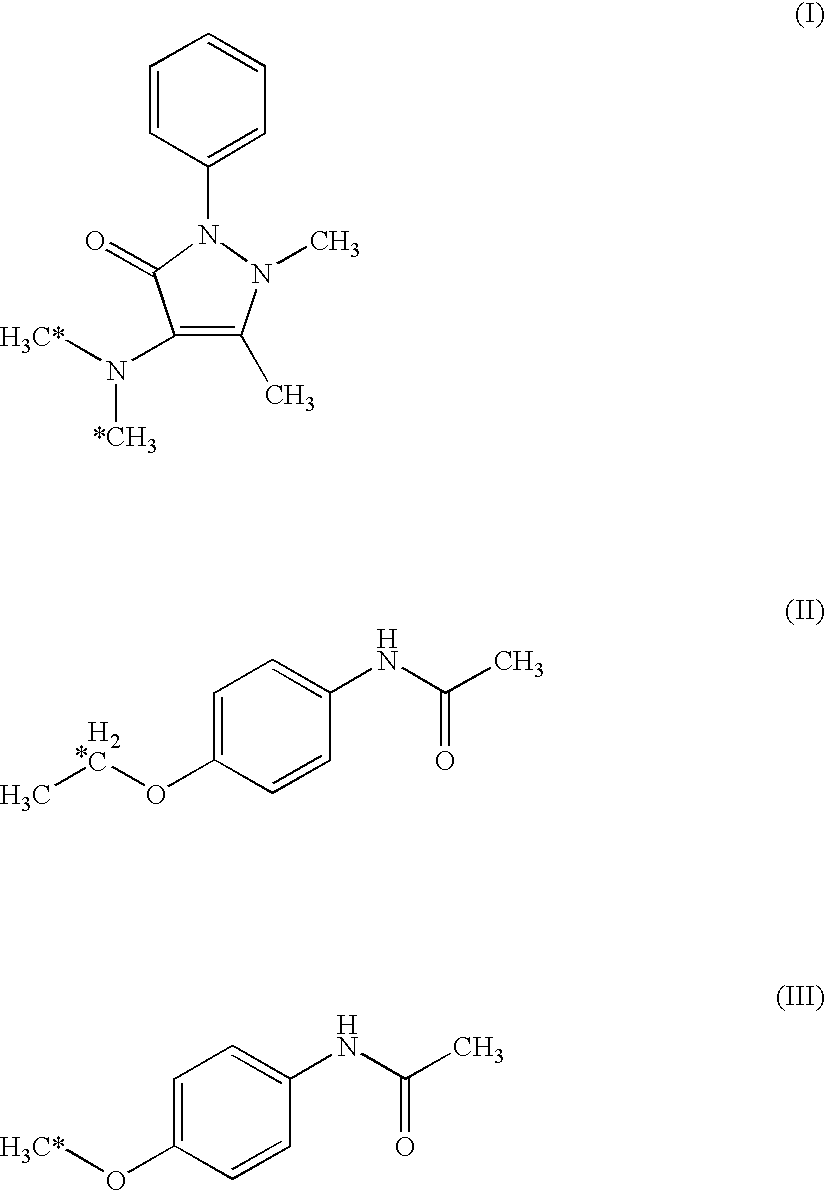
The invention relates to a specific probe substrate composition of cytochrome P450 (CYP450) enzyme and an application of the specific probe substrate composition in subtype enzyme activity detection of the cytochrome P450 enzyme. The composition comprises at least two of phenacetin, coumarin, amfebutamone, dextromethorphan, diclofenac and testosterone. The characteristics of multi-ion channels can be simultaneously detected by the specific probe substrate composition and a liquid chromatography-mass spectrometry technology, and the inhibition conditions of six CYP450 enzymes are determined simultaneously, so that the experiment flux efficiency is greatly improved, and the experiment cost is reduced.
Owner:广东中西达一新药开发有限公司
Diagnostic agent for diabetes
InactiveUS6509002B1Not impose heavy physical burdenEasy to useDispersion deliveryMetabolism disorderSide effectGlycerol
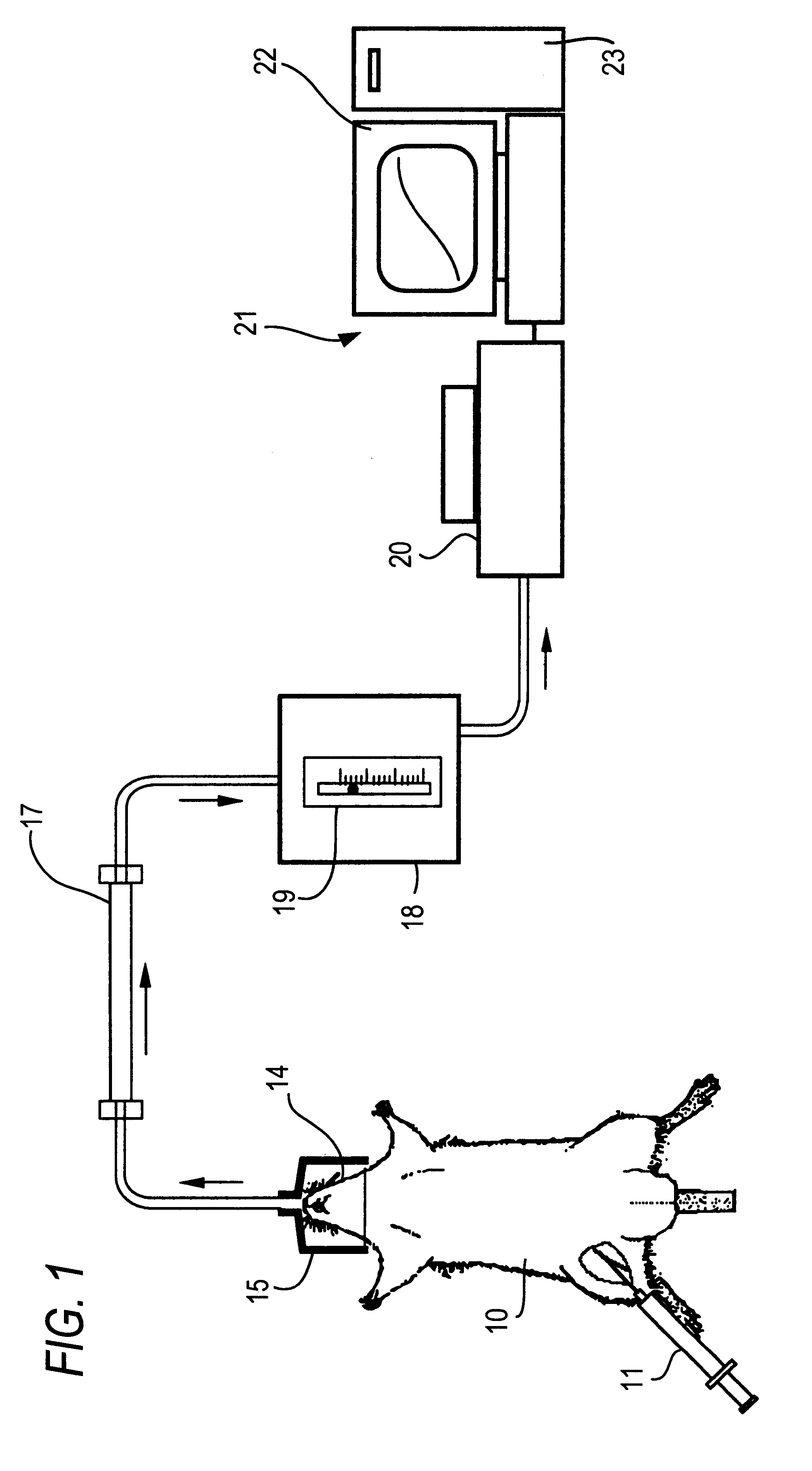
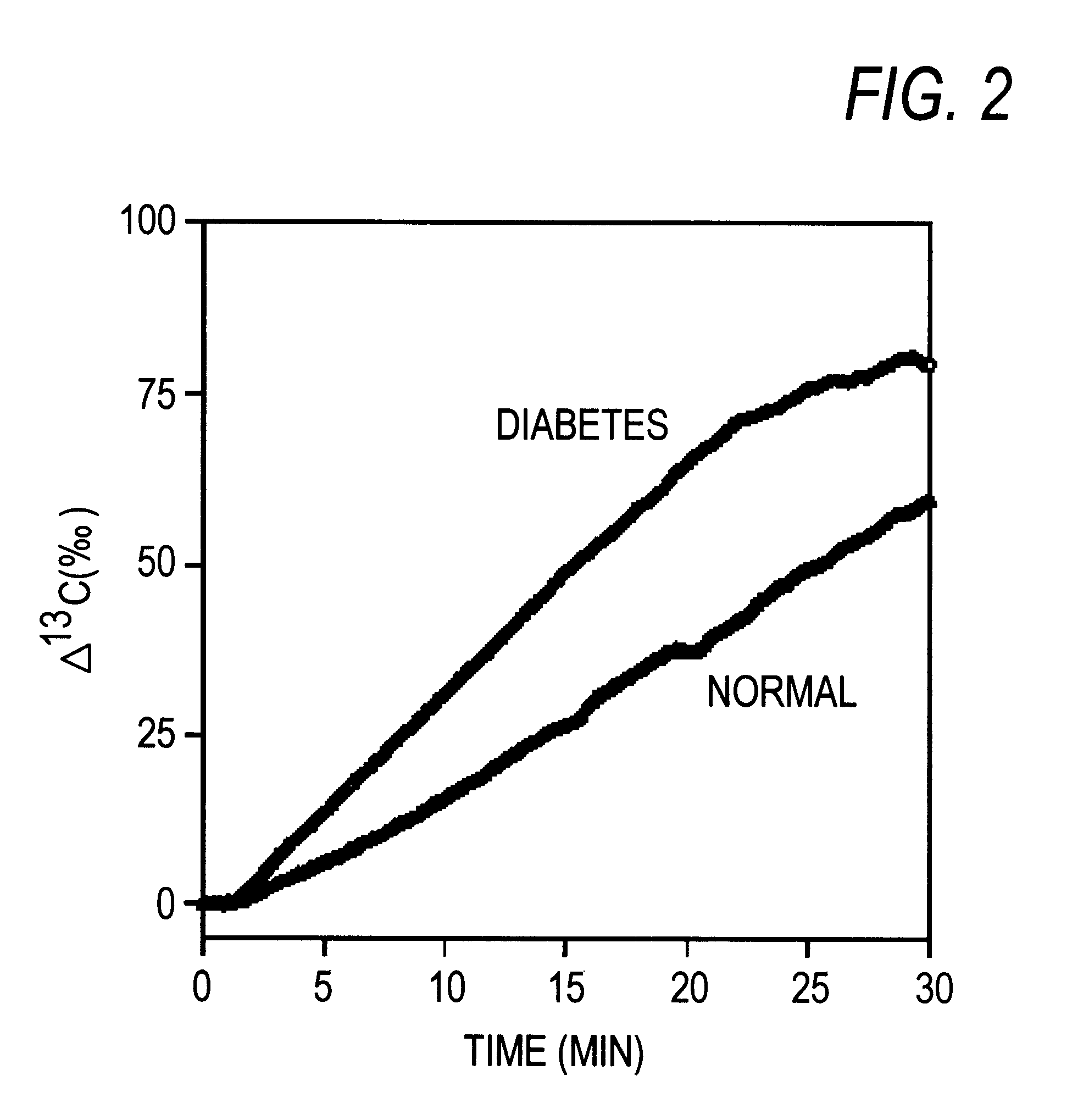
The present invention relates to a diagnostic agent for diabetes, comprising a compound labelled with 13C at least at one specific position selected from the group consisting of the following (a) to (g):(a) galactose, fructose or xylose labelled with 13C at least atone specific position, or a starch composed of glucose units labelled with 13C at least at one specific position;(b) an amino acid labelled with 13C at least at one specific position;(c) lactic acid or citric acid labelled with 13C at least at one specific position;(d) a fatty acid labelled with 13C at least at one specific position;(e) a glyceride labelled with 13C at least at one specific position;(f) glycerol labelled with 13C at least at one specific position; and(g) aminopyrin of which the dimethylamino group at position 4 is labelled with 13C, phenacetin of which the ethoxy group is labelled with 13C at position 1, or methacetin of which the methoxy group is labelled with 13C.According to the present invention, there is provided a diagnostic agent for diabetes which does not impose a heavy physical burden on a subject, can give accurate test results immediately and can be used safely without side effects. The diagnostic agent of the invention makes it possible to discriminate patients with diabetes from normal subjects even under circumstances where patients are easily missed.
Owner:TOKYO GAS CO LTD
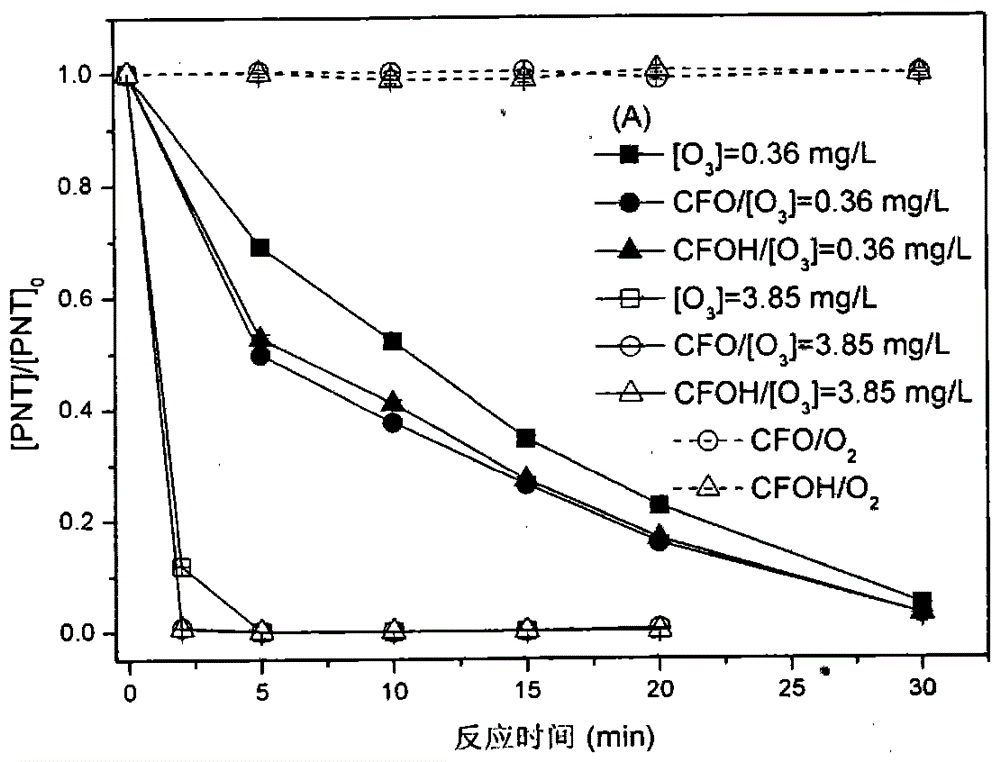
Preparation of magnetic nano copper-iron oxyhydroxide and application thereof to pollution removal by catalytic ozonation
ActiveCN104069860ARich reservesEasy to getWater contaminantsMetal/metal-oxides/metal-hydroxide catalystsIron saltsCuprate
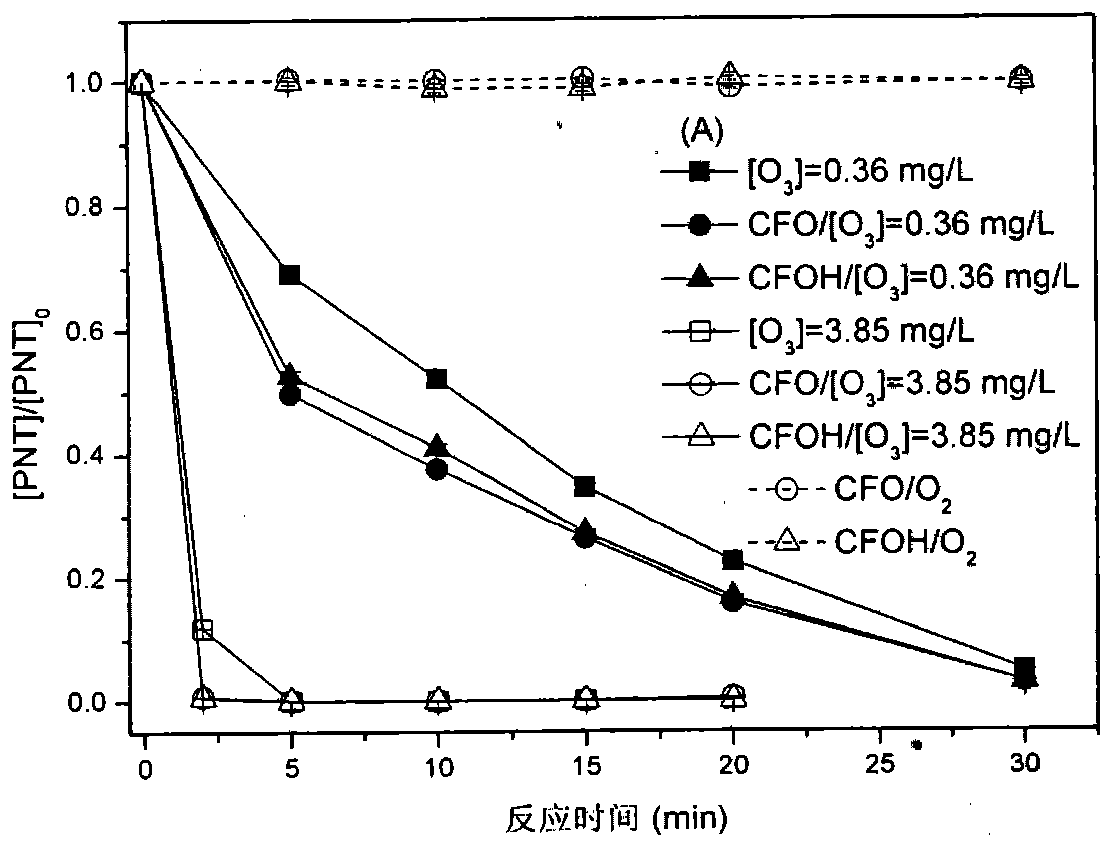
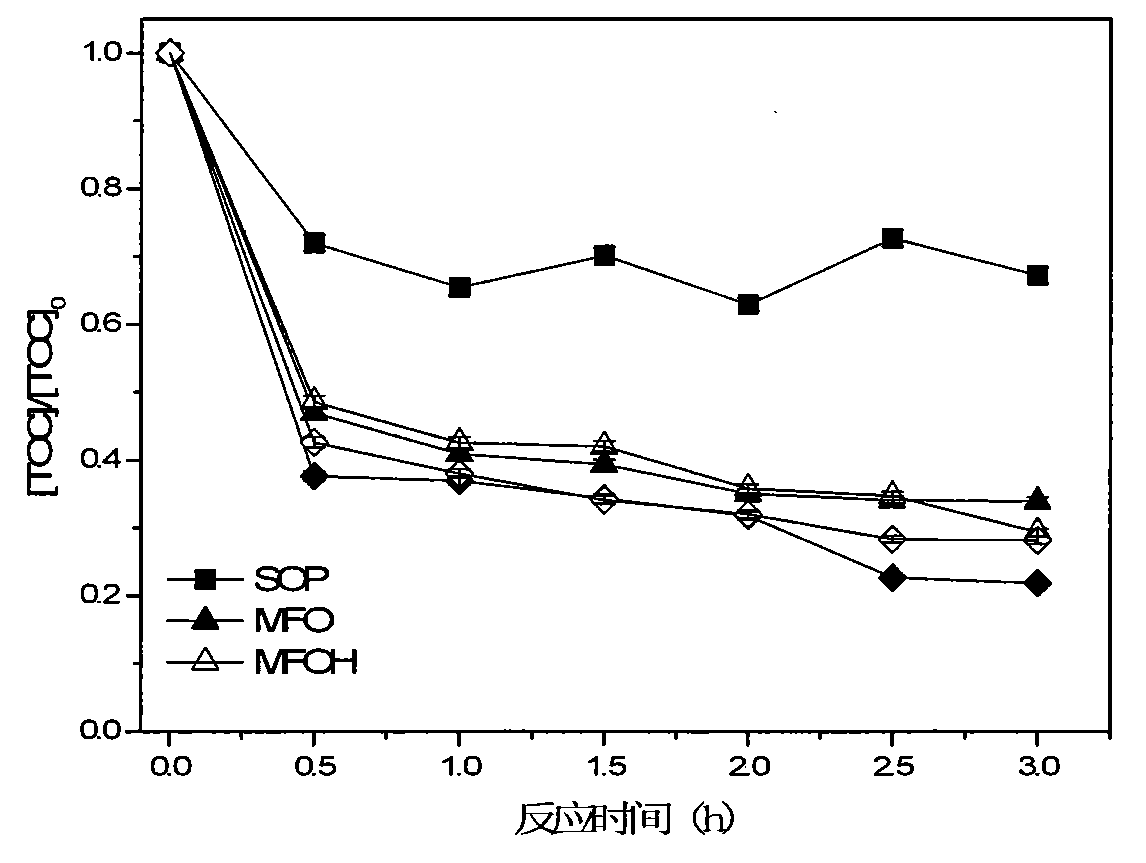
The invention provides a preparation method for a catalyst which is used for purifying a water body containing pharmaceutical and personal care products (PPCPs) including phenacetin and the like, aiming at the disadvantages and the defects of an existing water treatment technology for removing trace phenacetin in water. A magnetic nano copper-iron oxyhydroxide catalyst takes an iron salt and cuprate as key active components to integrate the surface characteristics of binary metal oxyhydroxides including Fex(OH)y and Cux(OH)y; the catalyst has a large specific surface area; meanwhile, the high surface hydroxyl density is the other important factor for providing the catalytic activity. More importantly, the magnetic nano copper-iron oxyhydroxide has the magnetism and the powdery catalyst is separated from a water phase by adopting a magnetic field. The catalyst can be applied to a catalytic ozonation technology so as to effectively improve the removing effects of the PPCPs including the phenacetin and the like in the water body; the phenacetin pollution removing effect is realized so as to obtain high-quality and stable output water; the catalyst has a wide application prospect.
Owner:BEIJING FORESTRY UNIVERSITY
Preparation of magnetic nano ferromanganese oxyhydroxide and application of magnetic nano ferromanganese oxyhydroxide in catalytic ozonation to remove pollution
ActiveCN104043458AHigh selectivityImprove efficiencyMetal/metal-oxides/metal-hydroxide catalystsWater/sewage treatment by oxidationCatalytic oxidationVolumetric Mass Density
The invention discloses a preparation method of a catalyst for purifying a water body containing PPCPs (Pharmaceuticals and Personal Care products) such as phenacetin according to the shortcomings and the defects of the existing water treatment technology for removing PPCPs such as trace amount of phenacetin in water. The catalyst (magnetic nano ferromanganese oxyhydroxide) adopts manganese salts and ferrates as key active components, and integrates the surface characteristics of bimetal oxyhydroxides such as Fex(OH)y and Mnx(OH)y, not only is large specific surface area achieved, and simultaneously, high surface hydroxyl density is another important factor for providing the catalytic activity. More importantly, the magnetic nano ferromanganese oxyhydroxide is magnetic, and a magnetic field can be adopted for separating the catalyst in a powder state from an aqueous phase. The catalyst can be applied to ozone catalytic-oxidation technology, and has the advantages that the effect of removing PPCPs such as phenacetin in the water body can be effectively improved, the effect of removing pollution such as phenacetin is achieved, and discharged water with good quality and stability is obtained, so that the catalyst is wide in application prospect.
Owner:BEIJING FORESTRY UNIVERSITY
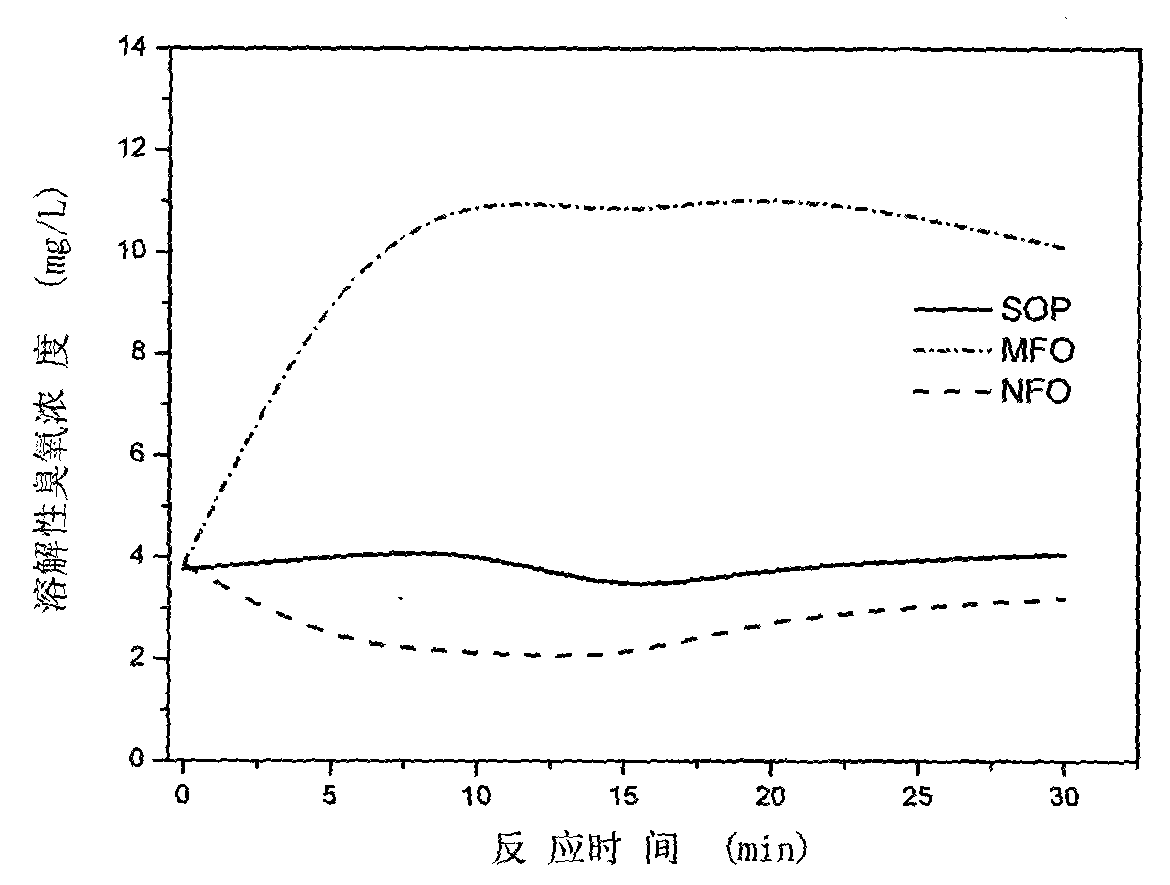
Multiphase catalytic ozone oxidation depollution technology based on enhanced ozone adsorption and application thereof
ActiveCN104028281AImprove solubilityImprove mass transfer efficiencyMetal/metal-oxides/metal-hydroxide catalystsWater/sewage treatment by oxidationSolubilityManganese oxide
The invention provides a preparation method of novel catalyst manganoferrogehnite (MnFe2O4, MFO), which aims at overcoming the weaknesses that the conventional powder catalyst is unlikely to separate in the water phase. By integrating the advantageous adsorption catalytic performance of different forms of ferrous oxides and manganese oxides such as MnOx, FexOy, the content of soluble ozone in water can be effectively increased, so that the manganoferrogehnite can be used as a catalyst to effectively improve the intensified removing effect of ozone on medicines such as phenacetin and pharmaceutical and personal care products in the water; more importantly, the manganoferrogehnite has magnetic property, the separation in the water can be completed through the magnetic field, and a novel method is provided for the washing and recycling of the catalyst. In addition, the manganese and iron sources in China are rich, and the high-efficient catalyst produced by adopting the manganese and ferrum as the raw materials has the advantages of low cost, easiness in acquisition and the like. The catalyst is prepared by a method for collectively depositing manganese salt, ferrates and alkaline liquid, the process is simple, and convenience in operation is realized; moreover, the preparation period of the catalyst is short, and the potential application prospect in the field of the treatment of drinking water or sewage containing PPCPs is promising.
Owner:BEIJING FORESTRY UNIVERSITY
Medicine for treating rheumatoid bone diseases and preparation method thereof
InactiveCN104840763AImprove production efficiencyQuality improvementOrganic active ingredientsAntipyreticBletilla striataDiclofenac Sodium
The invention discloses a medicine for treating rheumatoid bone diseases. The medicine is prepared by extracting and preparing traditional Chinese medicine composition and mixing the traditional Chinese medicine composition with a western medicine composition, wherein the traditional Chinese medicine composition comprises the components as follows: Chinese angelica, clematis root, cinnamon, common club moss herb, suberect spatholobus stem, Chinese taxillus twig, processed Sichuan aconite root, prepared kusnezoff monkshood root, processed semen strychni, pseudo-ginseng, jasmine, safflower, Chinese wolfberry, szechuan lovage rhizome, danshen, herba lycopi, cowherb seed, garden balsam, diverse wormwood herb, bletilla striata, Chinese arborvitae twig, folium artemisiae argyi, cattail pollen, red peanut coating, tamariskoid spikemoss herb, sophora flower, sophora fruit, camellia flower, sanguisorba officinalis, veronica undulata and herba orostachyos; the western medicine composition comprises the components as follows: diclofenac sodium, aspirin and phenacetin. According to the medicine for treating rheumatoid bone diseases, the rheumatoid bone disease symptoms can be quickly alleviated; the medicine is small in poor reaction, and has a good immunity regulating function and a good long-term curative effect.
Owner:申生林
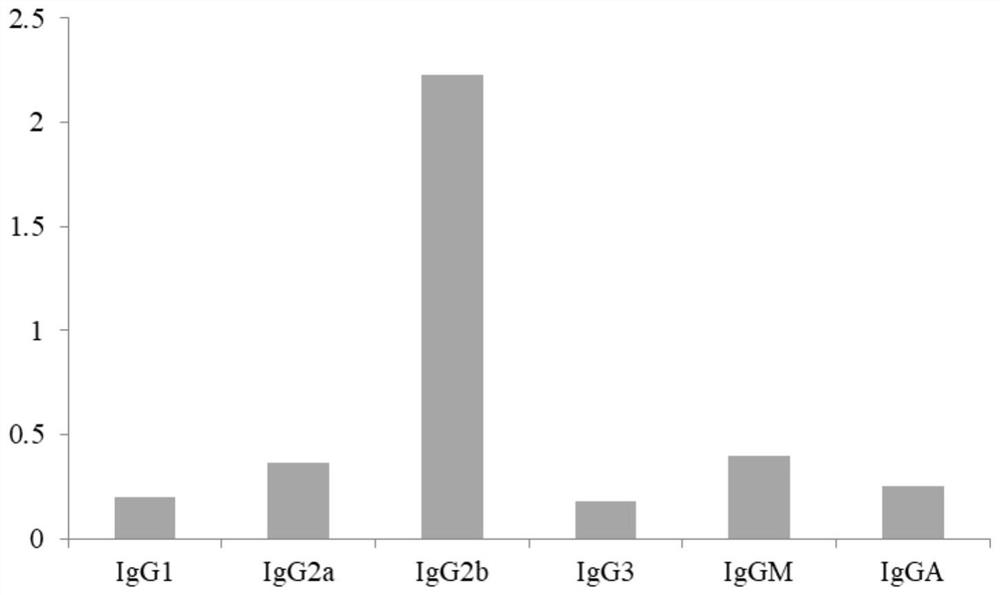
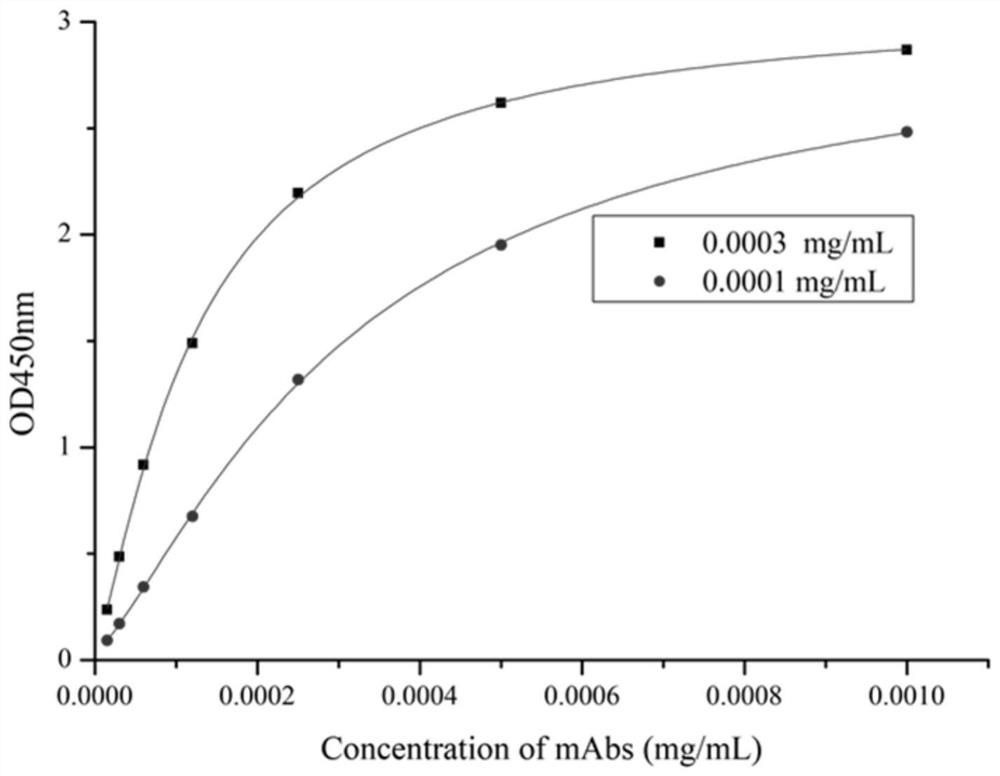
Anti-phenacetin monoclonal antibody hybridoma cell strain AD as well as preparation method and application thereof
ActiveCN112877296AImprove featuresHigh detection sensitivityOrganic compound preparationMicroorganism based processesAntiendomysial antibodiesMetabolite
The invention discloses an anti-phenacetin monoclonal antibody hybridoma cell strain AD as well as a preparation method and an application thereof, and relates to the technical field of food safety immunodetection, the monoclonal antibody hybridoma cell strain has a preservation name of monoclonal cell strain AD and a preservation number of CGMCC19681. A reaction product of phenacetin metabolite acetaminophen and ethyl 4-bromobutyrate is hydrolyzed to obtain Phe-BA as a hapten, and the hapten and carrier protein are coupled to prepare the immunogen Phe-BA-BSA. After a mouse is subjected to immunogen Phe-BA-BSA injection immunization, the mouse is fused with myeloma cells through a PEG method, and the hybridoma cell strain is obtained through screening and five times of subcloning of an indirect competitive enzyme-linked immunosorbent assay. The monoclonal antibody secreted by the cell strain can be prepared into a phenacetin detection kit, has good affinity and detection sensitivity to phenacetin, and can be used for immunodetection of phenacetin residual quantity in food.
Owner:JIANGNAN UNIV
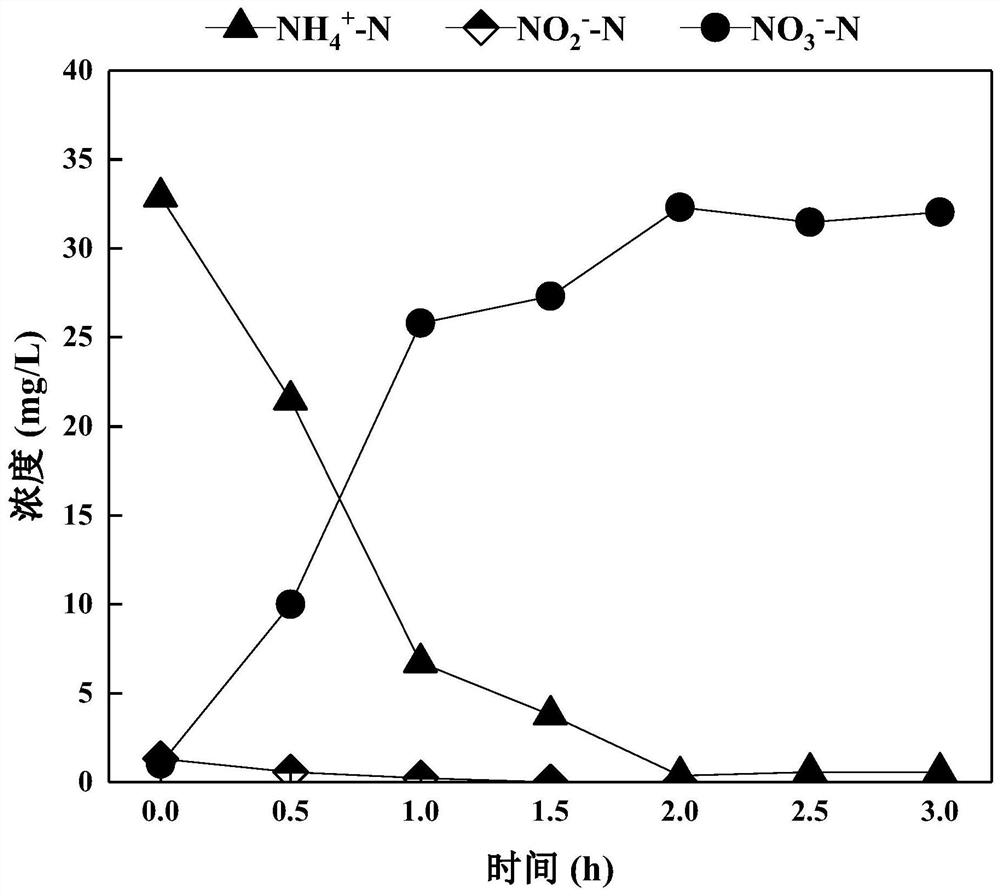
Method for rapidly starting and stably maintaining normal-temperature short-cut nitrification of municipal sewage by using phenacetin
PendingCN113104954ARapid start of short-cut nitrificationStabilized source of nitriteBiological treatment regulationWater contaminantsActivated sludgeAmmoniacal nitrogen
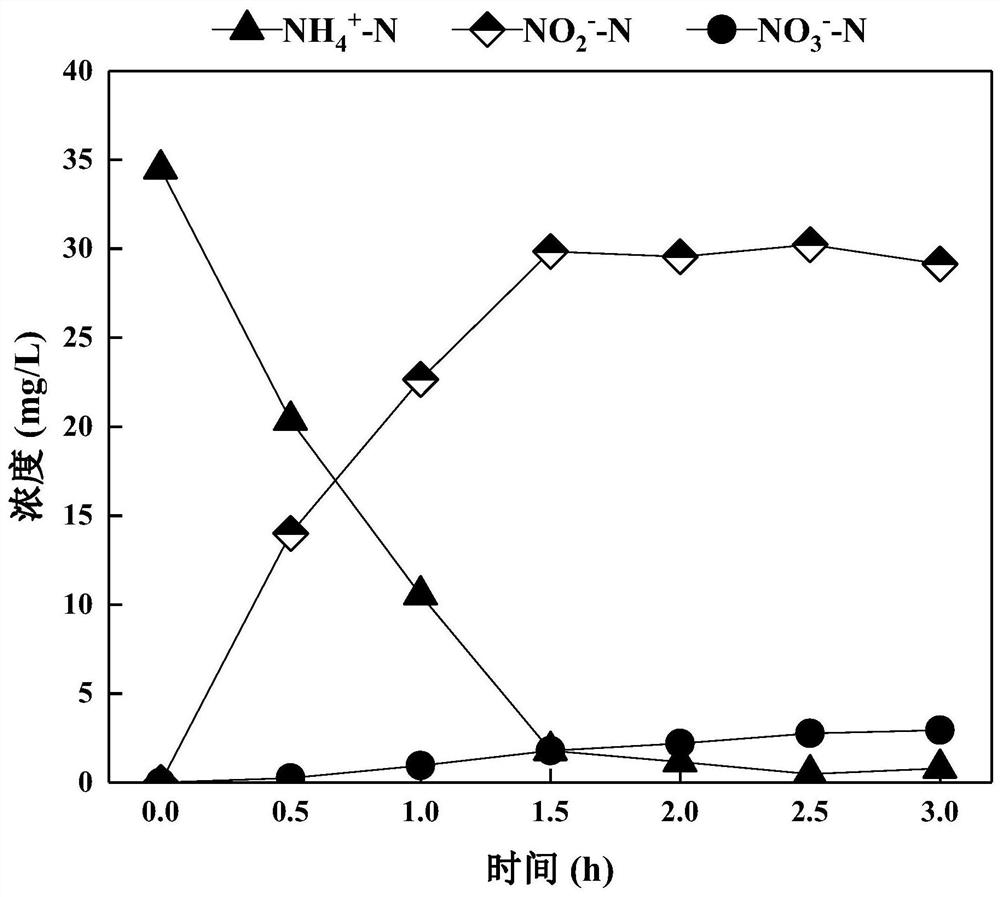
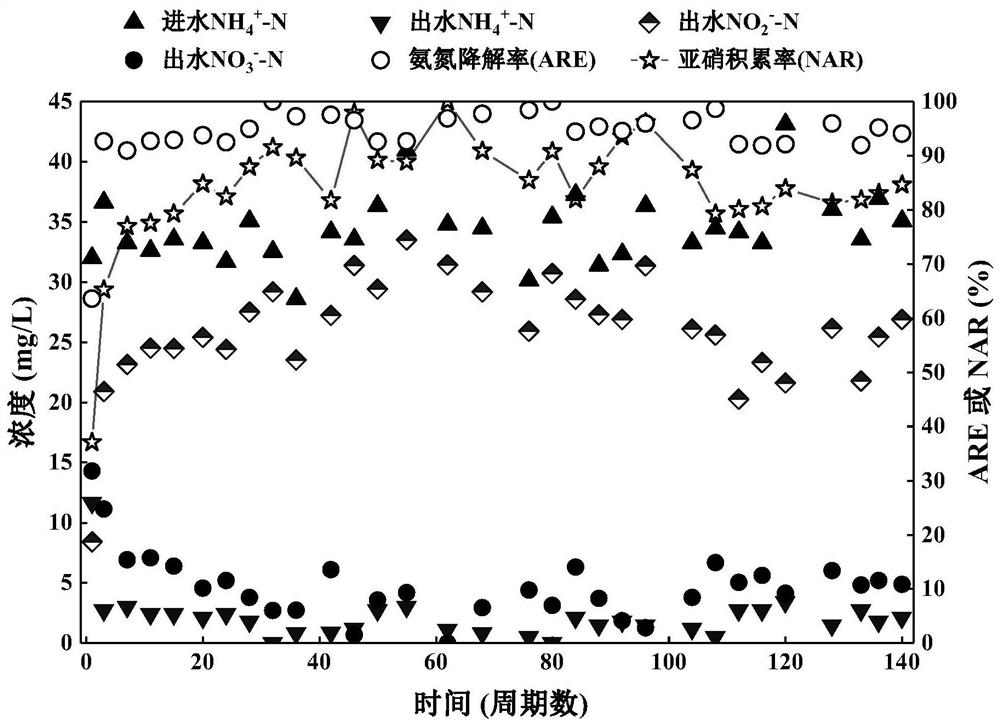
The invention discloses a method for rapidly starting and stably maintaining normal-temperature short-cut nitrification of municipal sewage by using phenacetin, and belongs to the technical field of biological sewage treatment. The method comprises the following steps: inoculating the whole-process nitrification activated sludge into a sequencing batch biochemical reactor, and soaking the activated sludge in the reactor with phenacetin with the concentration of 4-20 mg / L for 12-30 hours. The whole-process nitrification activated sludge is soaked in phenacetin, the ammonia oxidation activity of the reactor can be quickly recovered, the nitrite oxidation activity can be continuously inhibited, and when the ammonia nitrogen degradation rate (ARE) reaches 90% or above and the nitrite accumulation rate (NAR) reaches 80% or above, normal-temperature short-cut nitrification of the municipal sewage is formally started.
Owner:BEIJING UNIV OF TECH
Method for detecting forbidden drugs with internal modified molecular tube as chemical sensor
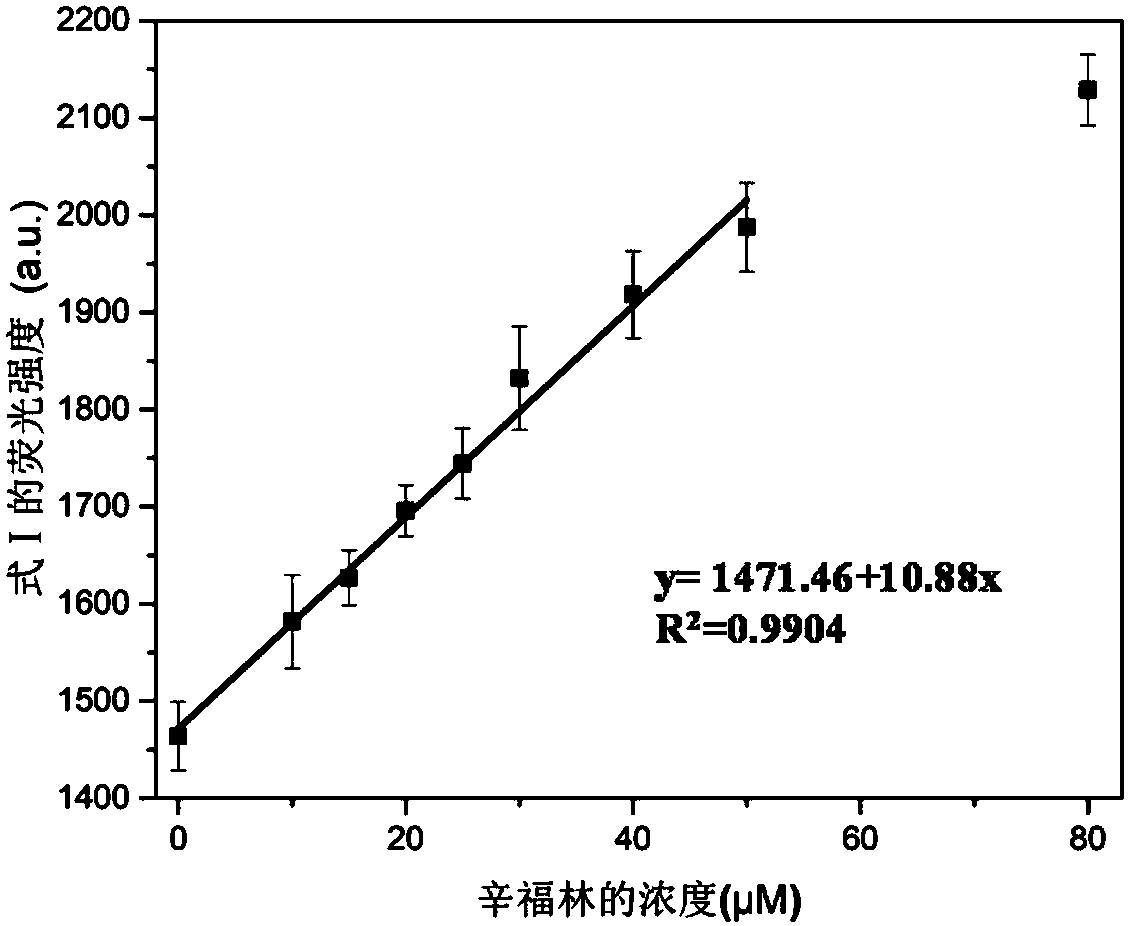
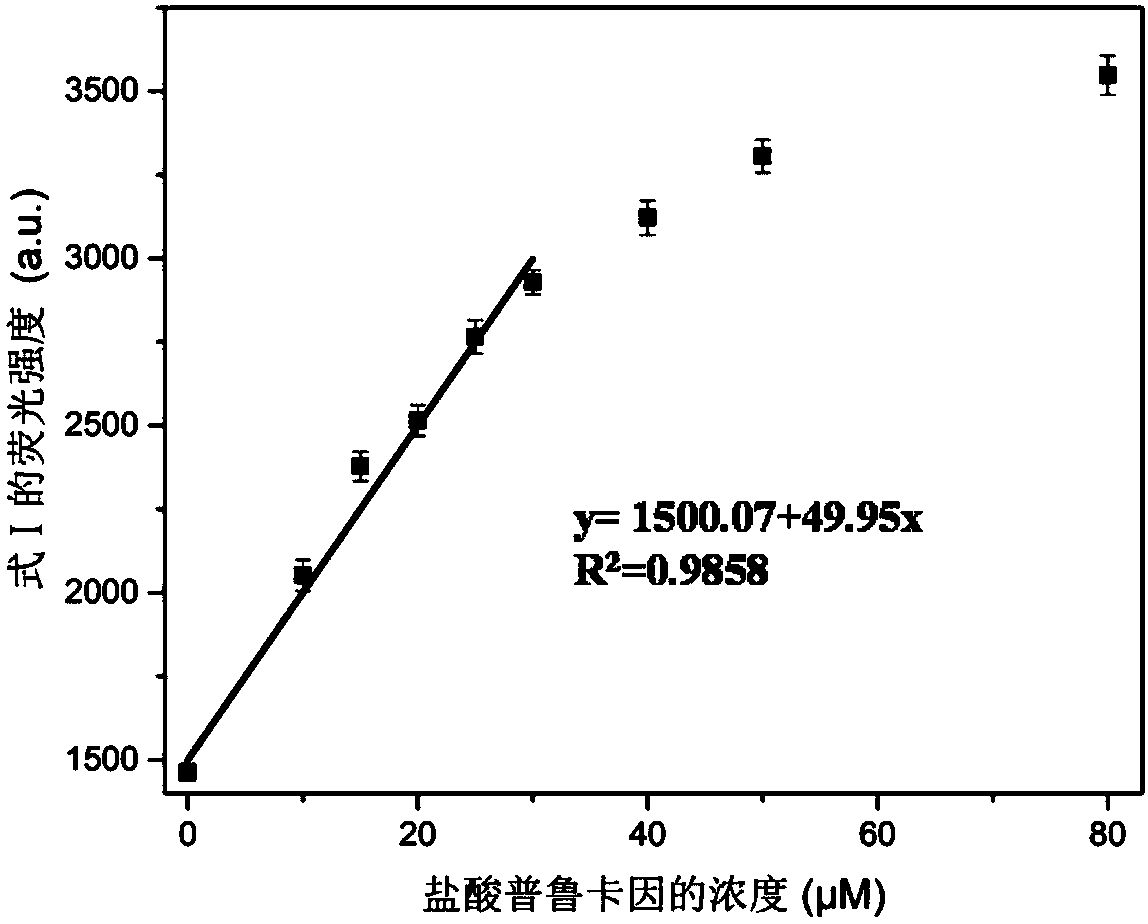
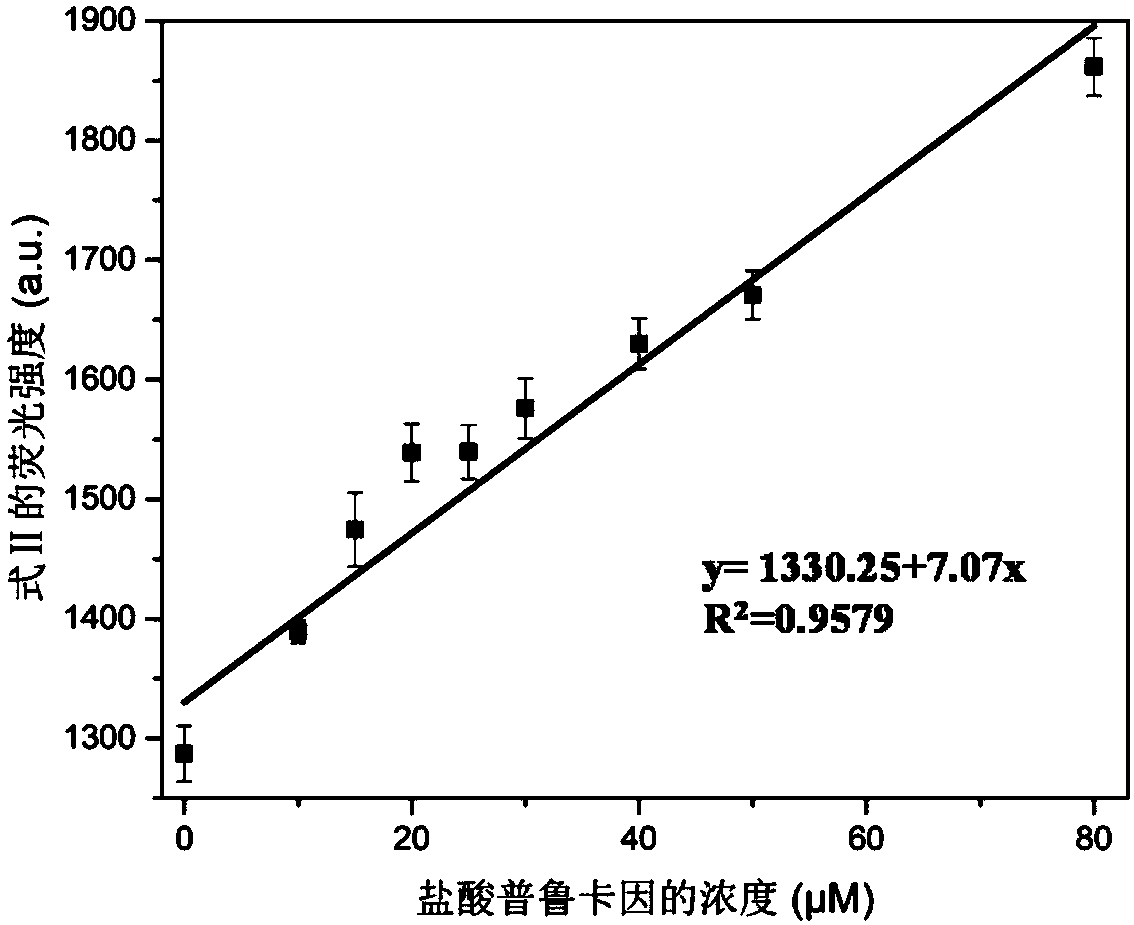
The invention provides a method for detecting forbidden drugs with an internal modified molecular tube as a chemical sensor. According to the method, the internal modified molecular tube is taken as the chemical sensor, the chemical sensor and a detected sample are mixed, a mixed solution is obtained, and the fluorescence intensity of the mixed solution is tested. The method can rapidly, simply and sensitively detect four forbidden drugs including ephedrine, synephrine, phenacetin and procaine in a water-phase system. The method has very important application prospects in detection and judicial fields of stimulants in sports events.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Medicine for treating toothache
A medicine for treating toothache, dental marrow inflammation and periodontitis is prepared from VB1, VC, aminopyrine, phenacetin, coffeine, austrominal, prednisone acetate, and safflower.
Owner:王明贤
Acetylsalicylic acid-phenacetin-caffeine sheet composition and preparation technology thereof
ActiveCN103191135AImprove stabilityGuaranteed stabilityAntipyreticAnalgesicsMicrosphereSalicylic acid
The invention discloses an acetylsalicylic acid-phenacetin-caffeine sheet composition and a preparation technology thereof. The preparation technology comprises the following steps of 1, screening acetylsalicylic acid, phenacetin, caffeine, citric acid, microcrystalline cellulose, starch, sodium dodecyl sulfate, hydroxypropyl methylcellulose and polyvinylpyrrolidone K30 by a sieve of 80 meshes for next use, 2, by a fluidized drying granulator, preparing acetylsalicylic acid single-phase microsphere particles from the acetylsalicylic acid, a part of the starch and a part of the sodium dodecyl sulfate screened by the sieve of 80 meshes, 3, by the fluidized drying granulator, preparing phenacetin-caffeine mixed microsphere particles from the phenacetin, the caffeine, a part of the starch and a part of the sodium dodecyl sulfate screened by the sieve of 80 meshes, 4, uniformly mixing the acetylsalicylic acid single-phase microsphere particles, the phenacetin-caffeine mixed microsphere particles and the microcrystalline cellulose, and carrying out tabletting. The preparation technology improves stability of the drugs in the acetylsalicylic acid-phenacetin-caffeine sheet composition, reduces free salicylic acid content of the acetylsalicylic acid-phenacetin-caffeine sheet composition, and guarantees product quality stability.
Owner:GUANGDONG JIUMING PHARMA +1
Medicine for treating toothache
InactiveCN100484535CQuick cureDigestive systemHeterocyclic compound active ingredientsAminophenazonPharmaceutical drug
Owner:王明贤
Method for simultaneous determination of rabeprazole sodium and metabolites thereof in Beagle dog plasma
InactiveCN107703221AThe pretreatment method is simpleSuitable for routine testingComponent separationMetaboliteLiquid chromatography mass spectroscopy
The invention relates to a method for simultaneous determination of levorabeprazole sodium, dexrabeprazole sodium and rabeprazole sodium metabolites comprising demethylated rabeprazole sodium, thioether rabeprazole sodium and rabeprazole sodium sulphone in Beagle dog plasma by using LC / MS / MS, and belongs to the field of biological detection. In experiments, phenacetin is used as an internal standard, and after ethyl acetate extraction pretreatment is performed, a high performance liquid chromatography-tandem mass spectrometry is adopted for simultaneous determination of the blood drug concentrations of levorabeprazole sodium, dexrabeprazole sodium and three metabolites of rabeprazole sodium in the dog plasma. The method has the advantages of high specificity, high sensitivity and simple operation, and overcomes the limitation of batch test by using two instruments, and is successfully applied to analysis and study of optical isomers of rabeprazole sodium and the metabolites thereof inthe Beagle dog plasma.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
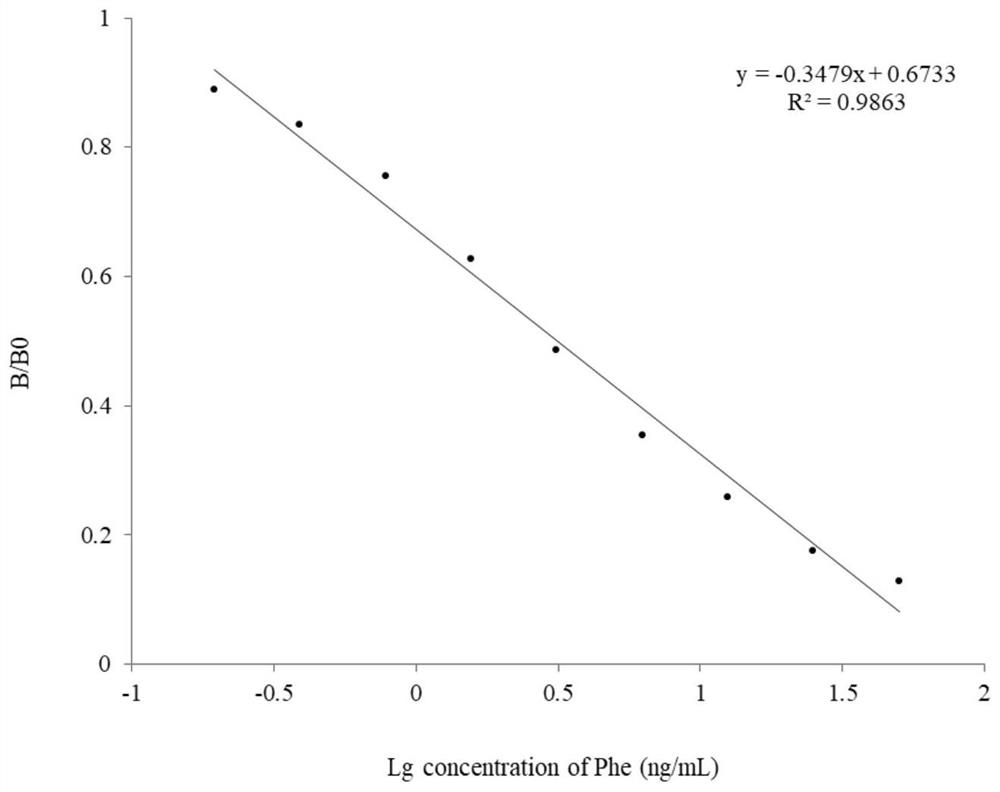
Hapten, artificial antigens and antibody for detecting phenacetin as well as preparation method and application of hapten, artificial antigens and antibody
ActiveCN113248398AStrong specificityHigh sensitivitySerum immunoglobulinsOvalbuminImmune profilingBiochemistry
The invention provides a hapten, artificial antigens and an antibody for detecting phenacetin as well as a preparation method and application of the hapten, the artificial antigens and the antibody. A hapten 1, an artificial antigen 1 and an artificial antigen 2 are prepared, the artificial antigen 2 is used for preparing the specific antibody for detecting phenacetin, and the artificial antigen 1 is used as a coating antigen. The antibody has high-sensitivity and high-specificity recognition capability to phenacetin. The detection limit is 2ng / mL, and the cross reaction rate to structural analogues is lower than 3%. An immunoassay method of phenacetin is established, and the purpose of rapidly and accurately detecting phenacetin in herbal tea and other food is achieved.
Owner:SOUTH CHINA AGRI UNIV
Novel antifogging agent for vehicles, and process method thereof
InactiveCN108276959AStrong adhesionImprove adhesionOther chemical processesFatty alcoholDiethanolamide
The invention belongs to the technical field of production of antifogging agents, and discloses a novel antifogging agent for vehicles, wherein the novel antifogging agent composition comprises, by weight, 1-2 parts of brominated acetylcholine, 1-2 parts of phenacetin, 1-3 parts of sodium lauryl sulfate, 1-3 parts of sodium secondary alkylsulfonate, 1-3 parts of fatty alcohol sodium isethionate, 0.5-2 parts of coconut oil fatty acid diethanolamide, 0.5-2 parts of hydroxy synthetic alcohol polyoxyethylene, 0.5-2 parts of alkyl glycoside, 0.2-1 part of sodium citrate, 2-7 parts of glacial aceticacid, 60-75 parts of anhydrous ethanol, 8-15 parts of hard water softener, 0.2-1 part of preservative, and 16-32 parts of water. The process of the novel antifogging agent comprises selecting materials, stirring, mixing the raw materials, filtering, and preparing the finished product. According to the present invention, the novel antifogging agent can rapidly remove mist, and has substantially improved durability and substantially improved low-temperature resistance.
Owner:四川中科领超科技有限公司
Preparation and crystallization method of phenacetin
ActiveCN105237431AHigh yieldImprove stabilityOrganic compound preparationCarboxylic acid amide separation/purificationChemical industryOrganic solvent
The invention discloses a preparation and crystallization method of phenacetin, and belongs to the field of chemical industry. According to the preparation and crystallization method, p-phenetidine is reacted with an acid in water so as to obtain a p-phenetidine salt; the p-phenetidine salt is reacted with an anhydride in a buffer solution system; after reaction, a mixed solution of water and an organic solvent is added into an obtained reaction solution, and phenacetin crystals are obtained via stirring. Product yield is high; two-step reaction total yield is higher than 92%; the obtained products are crystalline solids; stability is high; purity is high, and is higher than 99%; reaction time is short; reaction conditions are mild; product separation operation is simple; direct filtering separation can be carried out after reaction; and batch production period is shortened greatly.
Owner:济南鼎皓医药科技有限公司
In-vitro evaluation method for drug lung metabolic characteristics
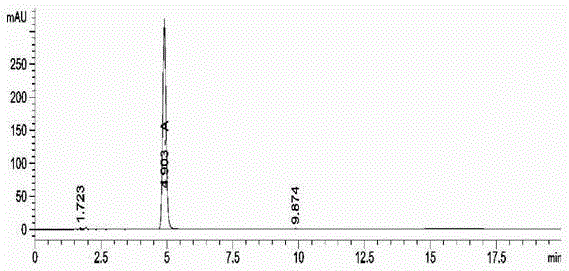
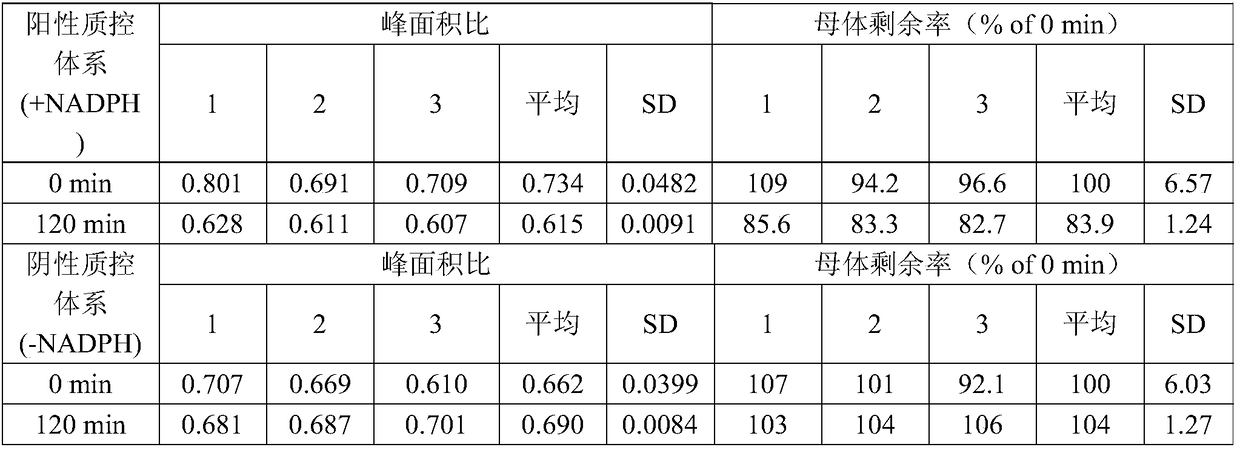
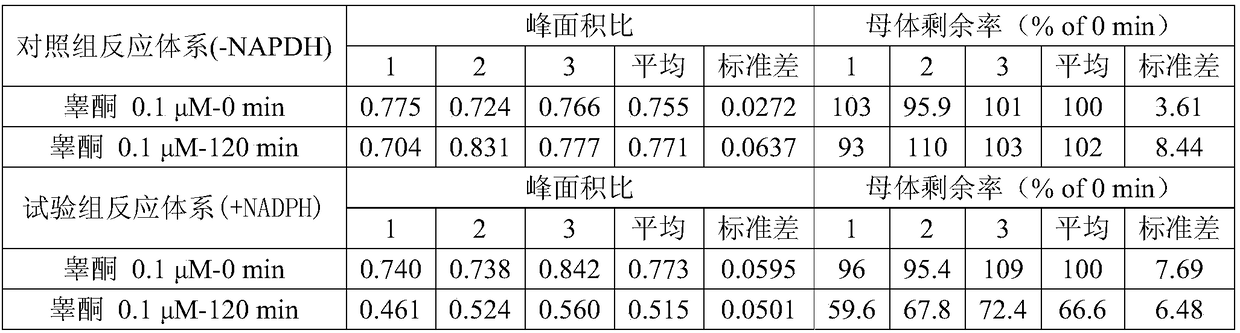
ActiveCN109298102AGood effectReduce false negative rateComponent separationDrug interactionControl system
The invention provides an in-vitro evaluation method for drug lung metabolic characteristics. The method comprises the following steps of (1) preparing lung sub-cell tissues; (2) adopting the lung sub-cell tissues obtained in the step (1), and preparing a positive property control system and a negative property control system, a control group reaction system and a test group reaction system separately, performing incubating and culturing, and centrifuging to obtain supernate; and (3) detecting the parent content of the positive substrate in the supernate of the positive property control systemand the negative property control system separately, and the parent content of the substance to be detected in the supernate of the control group reaction system and the test group reaction system separately, wherein the positive substrate in the positive property control system comprises any one or a combination of any two kinds of 2-anlinofluorene, 4-methoxy-1, 8-naphthalimide, phenacetin or 4-Ipomeanol. The evaluation method provided by the invention is stable in effect and is a perfect evaluation method for pulmonary drug delivery, and provides technical support for research and development of new drugs and drug interaction.
Owner:瑞德肝脏疾病研究(上海)有限公司
Preparation of magnetic nano-copper-iron oxyhydroxides and its application in catalytic ozone oxidation for pollution removal
ActiveCN104069860BHigh densityLarge specific surface areaWater contaminantsMetal/metal-oxides/metal-hydroxide catalystsIron saltsCatalytic oxidation
The invention aims at the deficiencies and defects of existing water treatment technologies for removing trace amounts of phenacetin in water, and proposes a preparation method of a catalyst for water purification containing phenacetin and other medicines and personal care products (PPCPs). . The magnetic nano-copper iron oxyhydroxide catalyst is based on iron salts and cuprates as the key active components, and integrates Fe x (OH) y , Cu x (OH) y The surface characteristics of binary metal oxyhydroxides not only have large specific surface area, but also high surface hydroxyl density is another important factor to provide their catalytic activity. More importantly, the magnetic nano-copper iron oxyhydroxide is magnetic, and a magnetic field can be used to separate the catalyst in powder state from the aqueous phase. The catalyst can be applied to ozone catalytic oxidation technology to effectively improve the removal effect of PPCPs such as phenacetin in water, and play a role in decontamination of phenacetin to obtain better and stable effluent. A catalyst with broad application prospects.
Owner:BEIJING FORESTRY UNIVERSITY
Artificial antigen and antibody capable of simultaneously detecting acetaminophen and phenacetin and preparation method and application of artificial antigen and antibody
ActiveCN113248596AStrong specificityHigh sensitivityTransferrinsSerum immunoglobulinsImmune profilingAntiendomysial antibodies
The invention discloses an artificial antigen and antibody capable of simultaneously detecting acetaminophen and phenacetin and a preparation method and application of the artificial antigen and antibody. An artificial antigen 1 and an artificial antigen 2 are prepared, the artificial antigen 1 is used for preparing a bispecific antibody for simultaneously detecting acetaminophen and phenacetin, the artificial antigen 2 is used as an artificial coating antigen, the antibody has high-sensitivity and high-specificity recognition capability on acetaminophen and phenacetin, the lowest detection limits are 2.8 ng / mL and 2.1 ng / mL respectively, the cross reaction rate to structural analogues is lower than 0.1%, an immunoassay method of the acetaminophen and the phenacetin is established, and the purpose of rapidly and accurately detecting the acetaminophen and the phenacetin in food is achieved.
Owner:SOUTH CHINA AGRI UNIV
Lachnum melanin hair dye and preparation method thereof
InactiveCN102166180APlay melanin anti-oxidationAntioxidantCosmetic preparationsHair cosmeticsSodium bicarbonateEthylenediamine
The invention discloses a lachnum melanin hair dye and a preparation method thereof. The preparation method is characterized in that: an agent A is prepared by dissolving para phenylene diamine, lachnum melanin, sodium sulfite, sodium bicarbonate, ascorbic acid, palmitic acid and ethylene diamine tetraacetic acid disodium in water and ammonia water to form an aqueous phase; adding into an oleic phase which is formed by heating to melt sodium carboxymethyl cellulose, nonylphenol polyoxyethylene ether and isopropanol or ethanol under stirring; and regulating the pH to be between 9.0 and 9.5 with the ammonia water; an agent B is prepared by adding the sodium carboxymethyl cellulose into water, aqueous solution of hydrogen peroxide and phenacetin, and regulating the pH to be between 3.4 and 4.0 by using phosphoric acid; and uniformly mixing the agent A with the agent B in the volume ratio of 1:(1-2) for use. By a hair-care dye with high safety, high efficiency and low toxicity provided by the invention, the content of the para phenylene diamine in the hair dye is reduced, the antioxidation, the antiradiation, the light protectiveness and the safety of the lachnum melanin are exerted, and natural and lustrous dying effects can be kept.
Owner:HEFEI UNIV OF TECH
Preparation method of medicinal liquor for treating rheumatic pain
InactiveCN106620094AEasy to useNo side effectsHydroxy compound active ingredientsAntipyreticCentipedeSide effect
The invention discloses a preparation method of medicinal liquor for treating rheumatic pain. The preparation method includes steps of (1), weighing components by mass: 5-10 parts of corn cervi pantotrichum, 10-15 parts of rhodiola rosea, 5-10 parts of pepper, 10-15 parts of safflower carthamus, 8-12 parts of rhizoma corydalis, 8-10 parts of fraxinus bungeana, 12-15 parts of jasmine, 8-10 parts of scorpio, 10-15 parts of borneol, 5-8 parts of musk, 5-10 parts of Chinese lizardtail rhizome, 8-10 parts of centipede, 8-12 parts of arisaema rhizomatum, and 12-15 parts of folium artemisiae argyi; (2), smashing and mixing, immersing and cooking components by millet wine; (3), extracting the filtering slag by ethanol; (4), combining the extracting agent and the decocta; mixing the extracting agent with ethanol with volume ratio of 1: 1-1.5; (5), adding 1-1.2% of methyl salicylate, 1-1.5% of turpentine oil, 0.2-0.3% of aminopyrine, and 0.2-0.3% of phenacetin in the raw fluid of the medicinal liquor. The drug can treat both symptoms and root causes, convenient to use, free from toxic and side effects, fast in effect and not easy to relapse.
Owner:贺文娟
Lingjingchuandu compound preparation for treating non-typical pneumonia
The invention provides an antelope horn-honeysuckle-andrographtiis poisonous compound preparation for curing SARS, its character: it comprises the following medicinal materials according to weight share: antelope horn 2g, honeysuckle flower 16g, andrographtiis 15g, burdock seeds 10g, weeping forsythia 10g, aspirin 0.4g, phenacetin 0.4g and caffeine 0.06g, able to substitute feijincao 8g and angleaniseed 8g for honeysuckle flower 16g, and add in menthol crystal 5g and morphine 0.04-0.4g, but the use dose of morphine must be control to addiction.
Owner:陈聪慧
Medicinal composition for relieving fever and easing pain
The invention discloses a medicinal composition for relieving fever and easing pain. The medicinal composition is characterized by being composed of aminopyrine, phenacetin, caffeine, phenobarbital and medicinal auxiliary materials. The aminopyrine and the phenacetin are capable of inhibiting synthesis and release of thalamus opticus prostaglandin and recovering orthocrasia of heat-regulating center receptive neuron and play a role of fever relieving; meanwhile a function of pain easing is also achieved by inhibiting synthesis of prostaglandin and the like; the aminopyrine is capable of inhibiting synthesis and release of aminopyrine in partial inflammation tissue, stabilizing lysosomal enzyme and influencing phagocytosis of phagocyte and plays a role of antiinflammatory action; the caffeine is a central nervous stimulant which is capable of stimulating cerebral cortex and improving the irritability to the external world and has the effects of shrinking brain blood vessels and enhancing the effects of the two former medicines in relieving headache; the phenobarbital has functions of calming, hypnosis and anticonvulsion, the calming functions of aminopyrine and phenacetin are enhanced, and convulsions caused by fever can be also prevented.
Owner:KAMP PHARMA
Ointment for treating sweety feet and body odor and its preparation
InactiveCN1121222CReduce secretionNo side effectsSalicyclic acid active ingredientsAerosol deliverySide effectSalicylic acid
The invention discloses an ointment for treating sweaty feet and body odor, which is composed of stearic acid, potassium stearate, glycerin, stearyl alcohol, salicylic acid, phenacetin, caffeine and water, and a preparation method thereof. The ointment has the advantages of obvious curative effect, no side effects and high cure rate.
Owner:刘志义
Coupling agent of light-colored hair dye
InactiveCN103239364ABroaden your optionsGood hair dyeing effectCosmetic preparationsHair cosmeticsMonoglycerideSulfite salt
The invention relates to a coupling agent and formula of a light-colored hair dye. The coupling agent of the light-colored hair dye, provided by the invention, is 6-amino-m-cresol; and an agent A and an agent B are contained in the formula of the hair dye, wherein the agent A comprises the following substances in percentage by mass: 8-12% of stearyl alcohol, 8-12% of propylene glycol, 5-8% of stearic acid, 2-4% of monoglyceride, 2-4% of ceteareth-20, 0.5-2% of dimethicone, ethanolamine for adjusting the pH value to 9-10, 0.5-1% of sodium sulfite, 0.1-0.5% of ascorbic acid, 0.05-0.1% of EDTA (Ethylene Diamine Tetraacetic Acid) disodium, 0.5-1.5% of N,N-bi-(beta-ethoxyl)-p-phenylenediamine sulfate or 0.5-2% of 2,5-diaminophenyl-ethanol sulfate, 0.03125-0.5% of 2-amino-5-methylphenol and the balance of water up to 100%; and the agent B comprises the following substances in percentage by mass: 4-6% of hydrogen peroxide, 8-12% of propylene glycol, 5-8% of stearic acid, 8-12% of stearyl alcohol, 2-4% of monoglyceride, 2-4% of ceteareth-20, 0.5-2% of dimethicone, phosphoric acid for adjusting the pH value to 2-5, 0.05-0.1% of EDTA disodium, a proper quantity of phenacetin and the balance of water up to 100%. The coupling agent and formula of the light-colored hair dye, provided by the invention, have the advantages of durable and stable dyeing effect, good fastness to washing and higher safety.
Owner:JIANGNAN UNIV
Drug for curing dermatophytosis and preparation method thereof
The invention discloses a drug for curing the dermatophytosis. The drug for curing the dermatophytosis uses analgin, aminopyrine, phenacetin, caffeine, Phenobarbital and auxiliary materials. The drug for curing the dermatophytosis can effectively reduce the generation of foot fungus, eliminates the fungal infection, relieves pain and itching, eliminates the foot odor, and is fast in effect, high in cure rate, hard for repeated attack and free of side effects.
Owner:重庆市潼南区中药研究院有限公司
Preparation method of phenacetin micropowder
InactiveCN106478445AHigh yieldMild conditionsCarboxylic acid amide separation/purificationBioavailabilityPurified water
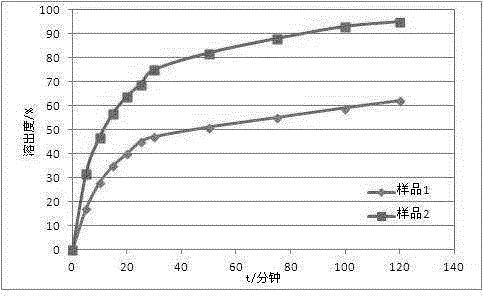
The invention discloses a preparation method of phenacetin micropowder. The preparation method comprises steps as follows: (1) ethanol is added to a raw material of phenacetin, and the solution is heated to 50-78 DEG C, kept at the constant temperature and stirred to be dissolved; (2) the stirring rate is controlled between 250 rpm and 350 rpm, the phenacetin ethanol solution is added to purified water at 25-40 DEG C while hot; (3) after addition, the solution is continuously stirred for 30 min, then cooled to 15-30 DEG C and stirred for crystallization for 2-4 h, and the phenacetin micropowder is obtained after filtering and drying. Compared with the prior art, the preparation method has the advantages as follows: 1) the micropowder is uniform in particle and smaller in particle size, and 95% or higher of the micropowder has the particle size of 120 meshes or below; 2) the dissolution rate of the micropowder is obviously increased, the dissolution rate of phenacetin is remarkable increased, and the bioavailability of phenacetin is improved; 3) the preparation method is high in yield, adopts a mild condition, a simple production technology and an easily controllable process and is safe to operate, low in cost and suitable for industrial production.
Owner:HUAZHONG PHARMA
Preparation of magnetic nano-manganese iron oxyhydroxides and its application in catalytic ozone oxidation for pollution removal
ActiveCN104043458BHigh selectivityImprove efficiencyMetal/metal-oxides/metal-hydroxide catalystsWater/sewage treatment by oxidationNano catalystCatalytic oxidation
Owner:BEIJING FORESTRY UNIVERSITY
Product for deodorizing human body and objects
InactiveCN103933117ACan treat oral inflammationOdorlessAntimycoticsHydroxy compound active ingredientsBrush toothBody odors
Owner:邓发强
A kind of preparation and crystallization method of phenacetin
ActiveCN105237431BHigh yieldImprove stabilityOrganic compound preparationCarboxylic acid amide separation/purificationChemical industryOrganic solvent
The invention discloses a preparation and crystallization method of phenacetin, and belongs to the field of chemical industry. According to the preparation and crystallization method, p-phenetidine is reacted with an acid in water so as to obtain a p-phenetidine salt; the p-phenetidine salt is reacted with an anhydride in a buffer solution system; after reaction, a mixed solution of water and an organic solvent is added into an obtained reaction solution, and phenacetin crystals are obtained via stirring. Product yield is high; two-step reaction total yield is higher than 92%; the obtained products are crystalline solids; stability is high; purity is high, and is higher than 99%; reaction time is short; reaction conditions are mild; product separation operation is simple; direct filtering separation can be carried out after reaction; and batch production period is shortened greatly.
Owner:济南鼎皓医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com