In-vitro evaluation method for drug lung metabolic characteristics
A lung and drug technology, applied in the field of biomedicine, can solve the problems of lack of perfect technical solutions, inability to correctly assess drug metabolism, incomplete application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of Animal Lung Subcellular Tissue
[0066] Animals were fasted overnight, anesthetized with isoflurane or 3% pentobarbital sodium aqueous solution 30mg / kg intraperitoneal injection, surgical scissors were used to open the abdominal cavity and thorax, the hemostatic forceps clamped the abdominal aorta, cut open the left ventricle of the heart, and inserted with normal saline Or a 50mL syringe of 0.15M potassium chloride solution (the solution is pre-cooled), quickly inject the solution to rinse until the lungs are milky white, and take the lung tissue in cold 0.15M potassium chloride (or 0.9% sodium chloride) solution In the beaker, soak and wash for 2-3 times according to the ratio of solid-liquid volume ratio of 2:3, and then wash twice with buffer A, then take the cleaned lung tissue and buffer A and transfer to centrifuge at the ratio of solid-liquid volume ratio of 2:3. tube, shredded, homogenized with a high-speed homogenizer, and collected th...
Embodiment 2
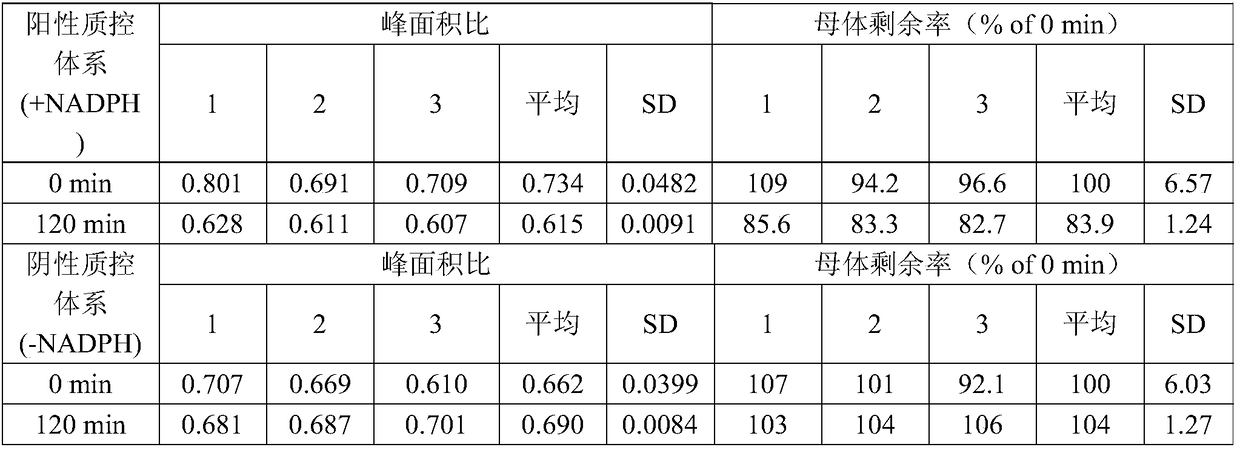
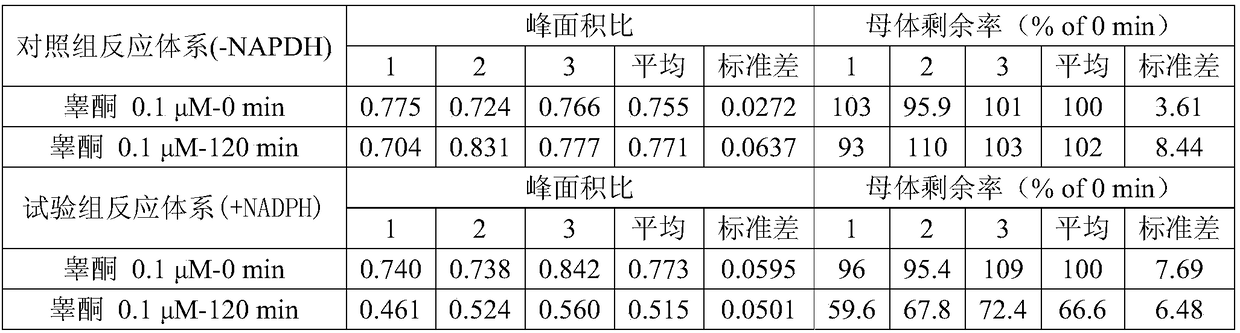
[0067] Example 2 Testosterone in vitro evaluation method of lung metabolic characteristics
[0068] (1) After thawing Beagle lung S9 in a water bath at 37°C, dilute lung S9 to 3 mg / mL with Tris buffer;
[0069](2) Prepare positive quality control system, negative quality control system, control group reaction system and test group reaction system respectively, wherein the positive quality control system includes mixing 2-aminofluorene and lung S9 and pre-incubating for 5 minutes, then adding NADPH coenzyme, According to the volumes of 2-aminofluorene, lung S9 and NADPH coenzyme being 25 μL, 25 μL and 50 μL, respectively, mix evenly, the final concentration of 2-aminofluorene is 0.05 μM, the final concentration of NADPH coenzyme is 1 mM, and the protein content of lung S9 is 3mg / mL; Negative quality control system includes mixing 2-aminofluorene and lung S9 and pre-incubating for 5min, then adding 0.1mM Tris buffer, according to 2-aminofluorene, lung S9 and Tris buffer respecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com