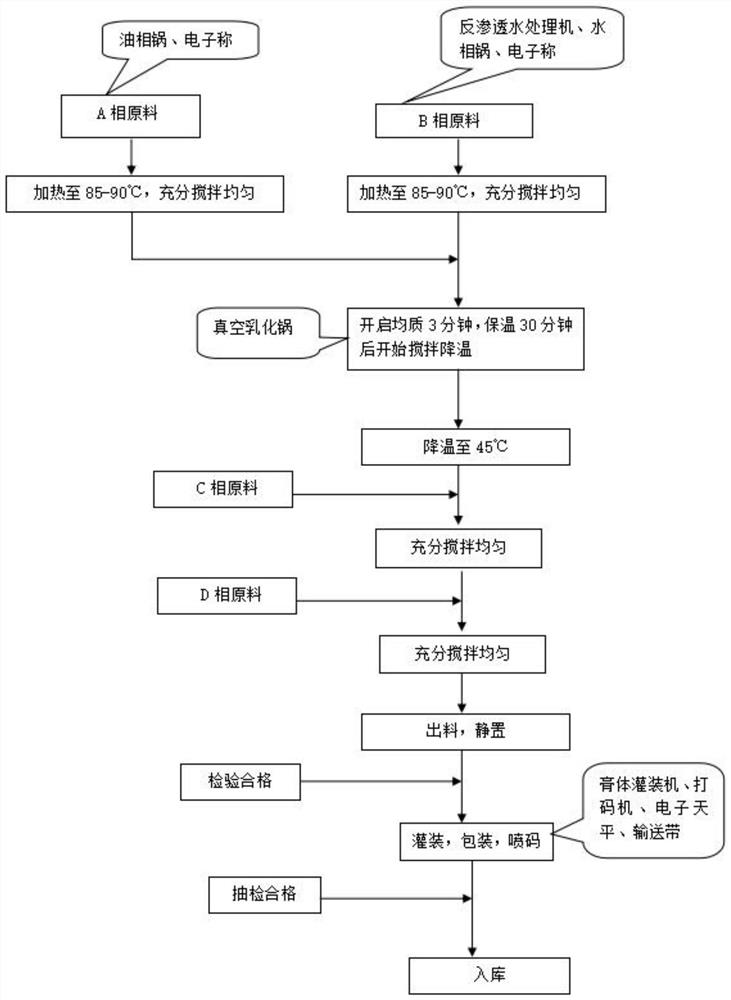
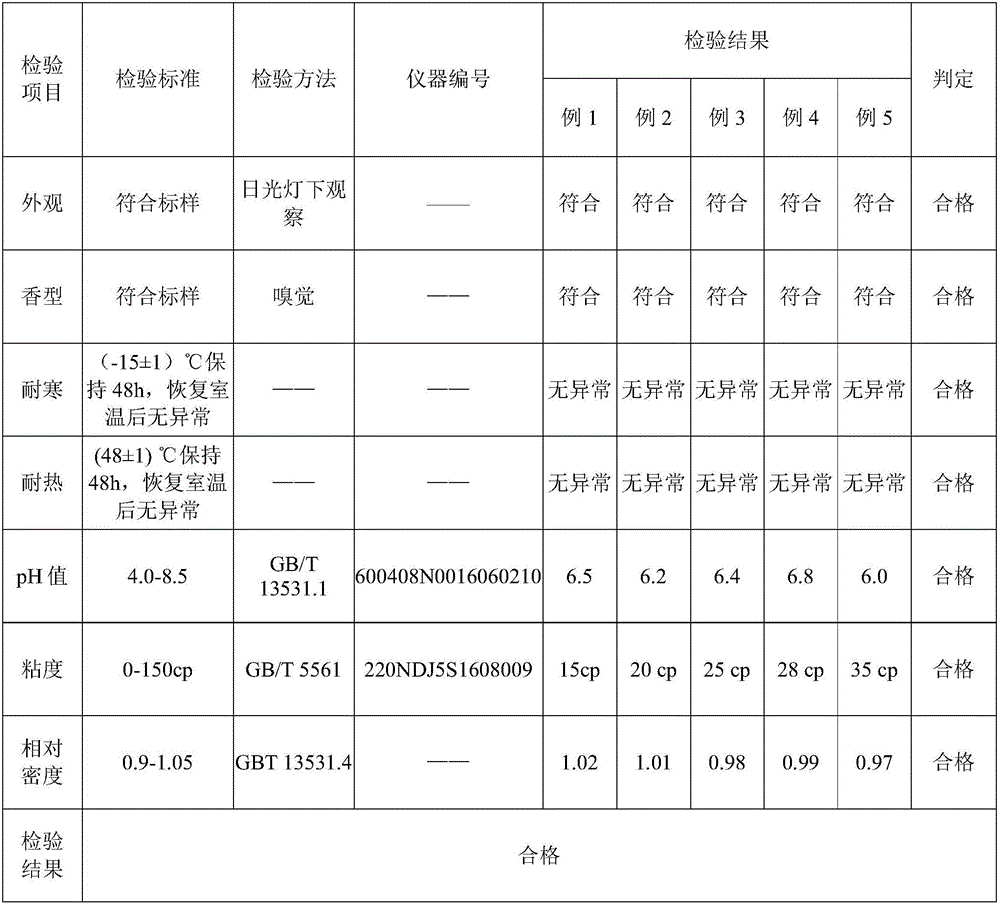
Patents
Literature
108 results about "Ceteareth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
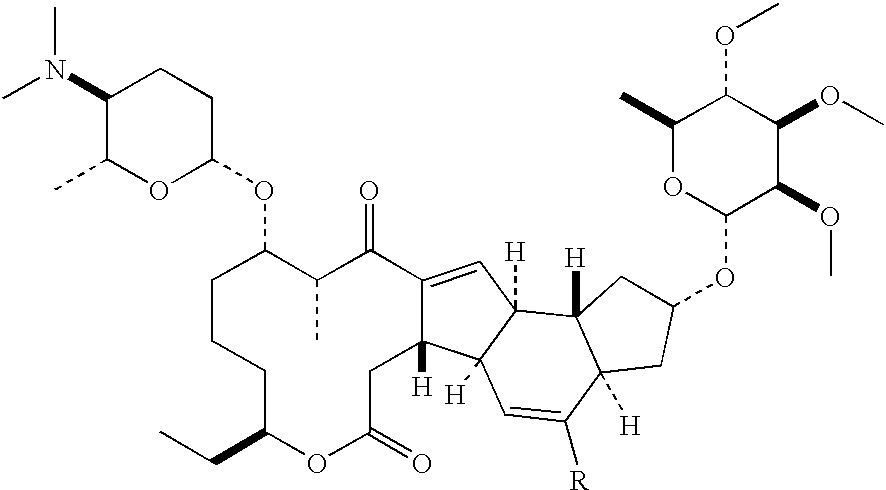
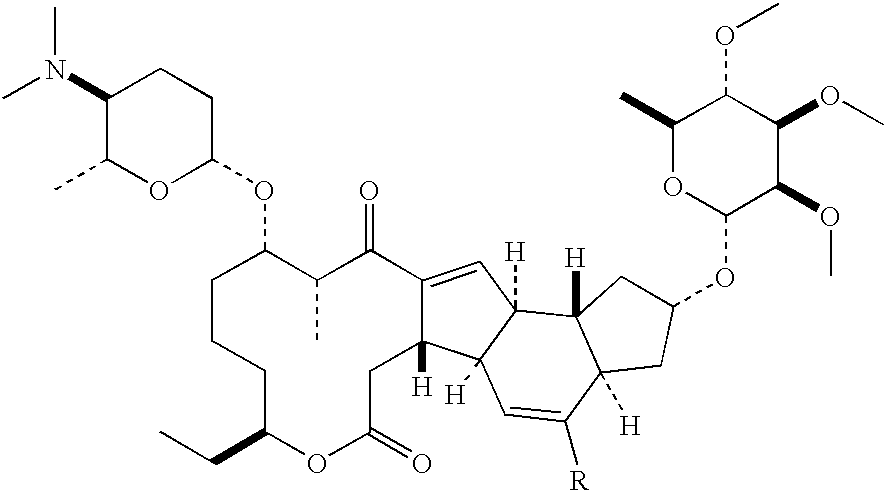
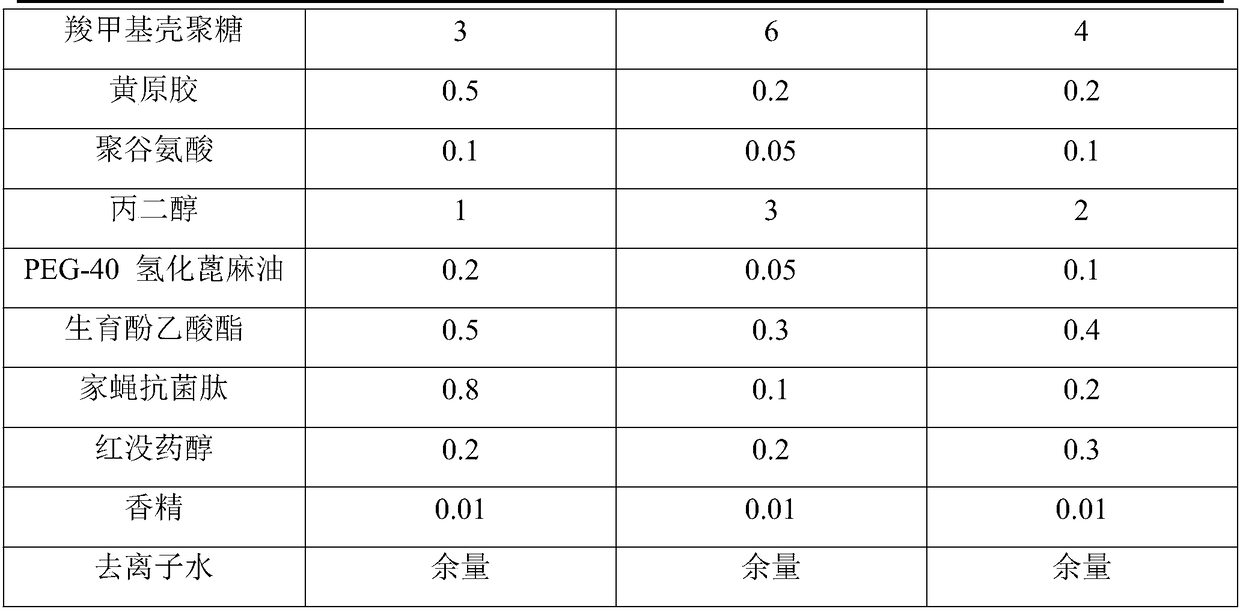
The INCI names ceteareth-n (where n is a number) refer to polyoxyethylene ethers of a mixture of high molecular mass saturated fatty alcohols, mainly cetyl alcohol (n = 15) and stearyl alcohol (m = 17). The number n indicates the average number of ethylene oxide residues in the polyoxyethylene chain.
Anti-wrinkle face cream and preparation method thereof
ActiveCN105147591AAnti agingImprove youthCosmetic preparationsToilet preparationsEthylhexyl palmitateApple extract
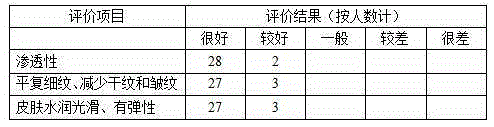
The invention belongs to the technical field of cosmetics, and in particular relates to an anti-wrinkle face cream and a preparation method thereof. The anti-wrinkle face cream comprises butanediol, ethylhexyl palmitate, caprylic / capric triglyceride, glycerol, polyglycerol-10, ethylhexyl isononanoate, polymethyl methacrylate, polydimethylsiloxane, ceteareth-21, cetostearyl alcohol, ceteareth-2, acrylic acid (ester) type / acrylamide copolymer, betaine, avocado fruit butter, a lactic acid bacillus / balausta fermentation product extract, an arctic rock chlamydomonas essence, a starfish essence, a ginseng extract, a lucid ganoderma extract, an apple extract, tocopheryl acetate and the like. The anti-wrinkle face cream provided by the invention is good in penetrability and easy to absorb, can effectively replenish water and preserve moisture, and can repair skin wrinkles and inhibit generation of the winkles, thereby delaying skin aging.
Owner:广州科玛生物科技股份有限公司
Oil-In-Water Nanoemulsion, A Cosmetic Composition And A Cosmetic Product Comprising It, A Process For Preparing Said Nanoemulsion
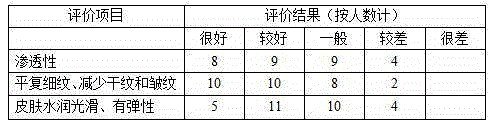
The present invention relates to an oil-in-water emulsion for cosmetic use, wherein the oil particles have an average diameter ranging from 50 to 200 nm (nanometers). The nanoemulsion comprises an emulsifying system having components such as ceteareth-20, ceteareth-12, glyceryl stearate, cetearyl alcohol and cetyl palmitate. This composition imparts to the nanoemulsion an opaque coloration, besides providing to the skin inherent properties of the nanoemulsion such as better absorption of the components by the skin, imparting softness, smoothness and moisturizing for 24 hours, and may be added to products indicated for hair care. The composition of the nanoemulsion without addition of preservatives imparts a bactericidal action. The nanoemulsion is preferably used for cosmetic applications 5 for the body, face and hair in the form of milk, lotions and gels. The present invention further relates to the cosmetic compositions and cosmetic products that comprise the nanoemulsions above, as well as to a process for preparing them.
Owner:NATURA COSMETICOS SA
Pediculicidal and ovacidal treatment compositions and methods for killing head lice and their eggs
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Wrinkle-free repair cream and preparation method thereof
InactiveCN105816377AIncrease elasticityTo promote metabolismCosmetic preparationsToilet preparationsWrinkle skinAdemetionine
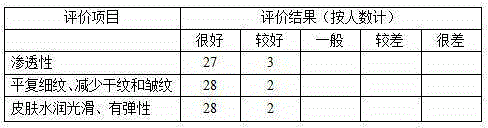
The invention relates to wrinkle-free repair cream. The wrinkle-free repair cream is prepared from the following raw materials in parts by weight: 40 to 80 parts of water, 2.5 to 4 parts of glycerol, 4 to 7 parts of butanediol, 0.8 to 1.2 parts of hydrolyzed collagen, 0.1 to 0.5 part of hyaluronic acid, 0.5 to 1 part of panthenol, 0.8 to 1.2 parts of evening primrose seed extracts, 0.8 to 1.2 parts of oligopeptide-1, 0.8 to 1.2 parts of oligopeptide-2, 1.6 to 2.4 parts of mycose, 4 to 6 parts of horse fat, 0.7 to 1.3 parts of centella asiatica root extracts, 1.3 to 1.8 parts of syringa vulgaris extracts, 1.7 to 2.3 parts of ceteareth-10, 1.3 to 1.8 parts of cetearyl alcohol, 1.7 to 2.3 parts of Shea butter oil and the like. According to the wrinkle-free repair cream and a preparation method of the wrinkle-free repair cream disclosed by the invention, the wrinkle-free repair cream is convenient to use, good in permeability, mild in skin and free of stimulation and harms to skin, and capable of stimulating growth factors to play a role based on the activity of resina draconis.
Owner:冯昱钦
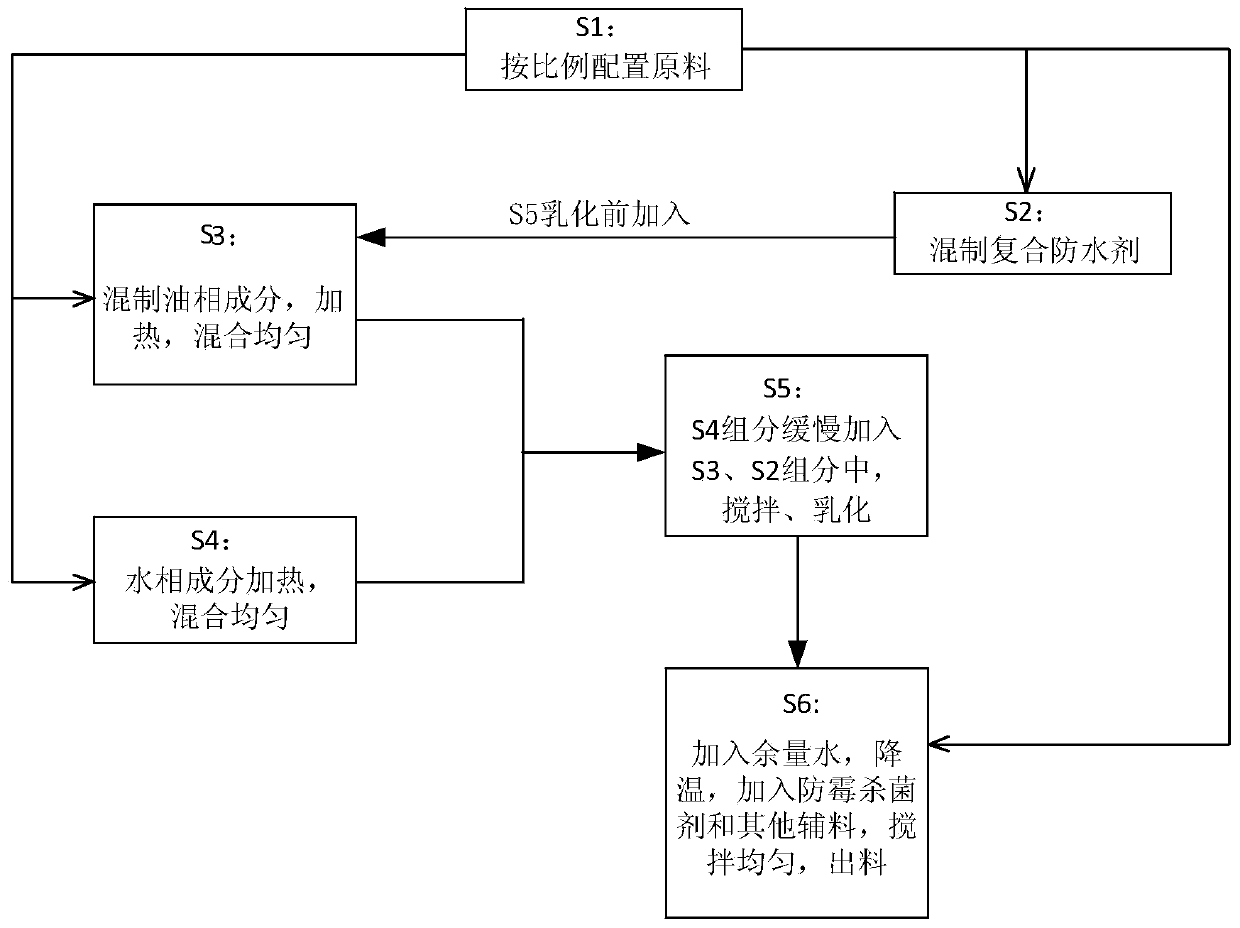
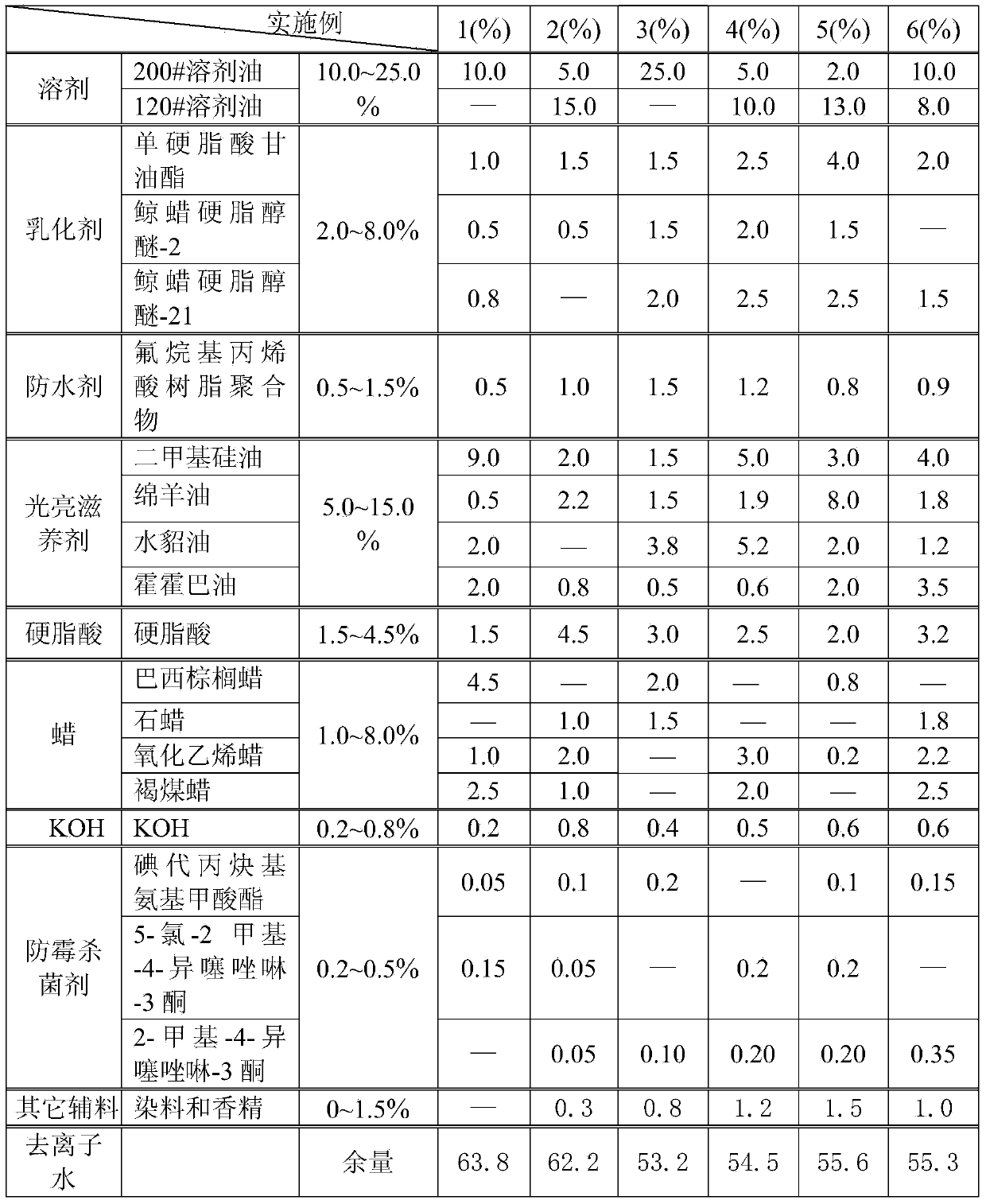
Leather water-proof curing agent and preparing method thereof
ActiveCN103695578AReduce surface tensionImprove waterproof performancePolishing compositionsLeather surface finishingPotassium hydroxideKetone
The invention discloses a leather water-proof curing agent and a preparing method thereof. The water-proof curing agent comprises a solvent, emulsifiers, a water repellent, a brightness nourishing agent, stearic acid, wax, KOH (potassium hydroxide), a mould-proof bactericide, other auxiliary materials and deionized water, wherein the solvent is one or two of solvent oil 120# and solvent oil 200#; two or three of glycerin monostearate, ceteareth-2 and ceteareth-21 are used as the emulsifiers; one or several of the carnauba wax, lignite wax, ethylene oxide wax and paraffin are used as the wax; one or several of iodopropynyl butylcarbamate, 5-chloro-dimethyl-4- isothiazolin-3-ketone and dimethyl-4-isothiazolin-3-ketone are used as the mould-proof bactericide. A unique preparing technology is matched. The water-proof curing agent has efficient and lasting water-proof effect and can well resist oil stains and dust, and the comprehensive curing performance and the water-proof performance are obviously improved.
Owner:QINGHAO ENVIRONMENTAL PROTECTION SCI & TECH SHANGHAI
Sheet substrates impregnated with aromatic releasing compositions and a method of delivery of aromatic releasing compositions
An article of manufacture and a method directed to application of aromatic releasing compositions impregnated within substrates such as non-woven paper materials (e.g., wipes, paper towels) and dispensable cloth materials (e.g., gauze or a thin fabric of silk, linen, or cotton materials) for providing relief from cold, allergies, sinus and symptoms associated with respiratory disorders, the aromatic releasing compositions including the following: Menthol; Camphor; Eucalyptus oil; Cedarleaf Oil; Myristica Oil; Peppermint Oil; Lavender oil; Methyl Salicylate; Naproxen; Nutmeg Oil and Thymol; Beclometasone dipropionate; Benzethonium chloride with base solution consisting of Emollients, Emulsifiers and Moisturizer; Deionized Water; Vegetable Oil; Dicaprylyl Carbonate; Glyceryl Oleate; Polyglyceryl-2 Dipolyhydroxystearate; Cetearyl Isononanoate; Ceteareth-20; Cetearyl Alcohol; Glyceryl Stearate; Glycerin; Cetyl Palmitate; Ceteareth-12, Lauryl Glucose Carboxylate; Lauryl Glucoside; Sodium Citrate; Citric Acid; Benzethonium Chloride 0.05%; Ethylene diamine tetra acetic acid; Phenoxyethanol; Methylparaben; Propylparaben; 2-bromo-2-nitropropane-1,3-diol; and subcombinations thereof. In further embodiments, the compositions impregnated within substrate further include one or more topical actives, and are useful for providing relief from cold, allergies, sinus and symptoms associated with respiratory disorders, as well as repelling common virus and bacteria.
Owner:ADELAKUN OLUFEMI
Oil-in-water nanoemulsion, a cosmetic composition and a cosmetic product comprising it, a process for preparing said nanoemulsion
The present invention relates to an oil-in-water emulsion for cosmetic use, wherein the oil particles have an average diameter ranging from 50 to 200 nm (nanometers). The nanoemulsion comprises an emulsifying system having components such as ceteareth-20, ceteareth-12, glyceryl stearate, cetearyl alcohol and cetyl palmitate. This composition imparts to the nanoemulsion an opaque coloration, besides providing to the skin inherent properties of the nanoemulsion such as better absorption of the components by the skin, imparting softness, smoothness and moisturizing for 24 hours, and may be added to products indicated for hair care. The composition of the nanoemulsion without addition of preservatives imparts a bactericidal action. The nanoemulsion is preferably used for cosmetic applications 5 for the body, face and hair in the form of milk, lotions and gels. The present invention further relates to the cosmetic compositions and cosmetic products that comprise the nanoemulsions above, as well as to a process for preparing them.
Owner:NATURA COSMETICOS SA
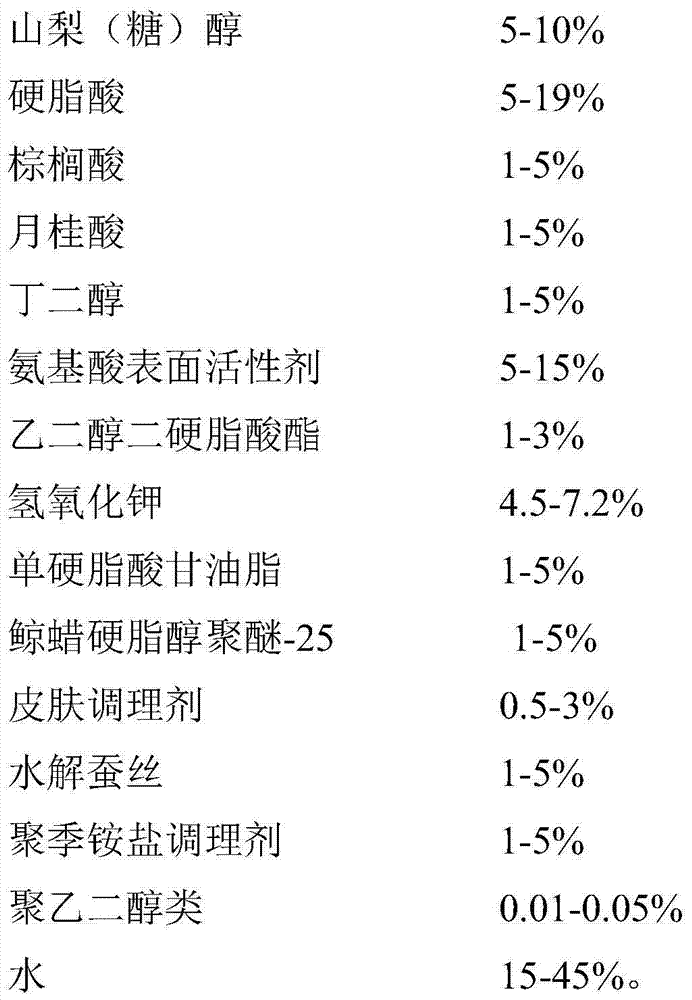
A hydrolyzed-silk face cleanser and a preparing method thereof
ActiveCN104758223ALess irritatingEasy to cleanCosmetic preparationsToilet preparationsIrritationPolyethylene glycol
The invention belongs to the field of cosmetics and particularly relates to a hydrolyzed-silk face cleanser and a preparing method thereof. The face cleanser comprises following raw materials: myristic acid, glycerol, sorbitol, stearic acid, palmitic acid, lauric acid, butanediol, an amino acid surfactant, glycol distearate, potassium hydroxide, glycerol monostearate, ceteareth-25, a skin conditioning agent, hydrolyzed silk, a polyquaternium conditioning agent, polyethylene glycols, and water. The face cleanser is rich, fine and smooth in foam, rich in elasticity, and good in wire drawing. The face cleanser is fine and smooth in pasty fluid, has high pearly luster, and can effectively remove dirt, fat granules, comedo, and the like in pores. During washing, a smooth sense is strong and skin feels comfort, and the skin after being washed is not dry, not tight and free of irritation. The face cleanser is suitable for oily skin, dry skin, neutral skin, and sensitive skin with skin damage.
Owner:上海彤颜实业有限公司
Cleansing gel and preparation method thereof
InactiveCN106902029AImprove makeup removal abilityGood moisturizing effectCosmetic preparationsMake-upAMINOMETHYL PROPANEDIOLPreservative
The invention discloses cleansing gel and a preparation method thereof. The cleansing gel is prepared from components in parts by weight as follows: 1.2-4.8 parts of grease, 3.2-11.4 parts of an emulsifier, 0.1-0.35 parts of an antibacterial preservative, 0-.01-0.05 parts of butylated hydroxytoluene, 0.1-0.6 parts of Carbomer, 0.1-0.6 parts of aminomethyl propanediol and 70-90 parts of deionized water, wherein the emulsifier is prepared from hydrogenated lecithin, PEG-7 glyceryl cocoate and PPG-4-ceteareth-20 in the weight ratio being (0.1-1):(3-10):(0.1-0.4). The cleansing gel has the advantages that the cleansing effect is good, makeup removal and face cleansing are realized simultaneously, and the moisturizing and skin repairing effects are remarkable.
Owner:SHANGHAI CHUANGYUAN COSMETICS
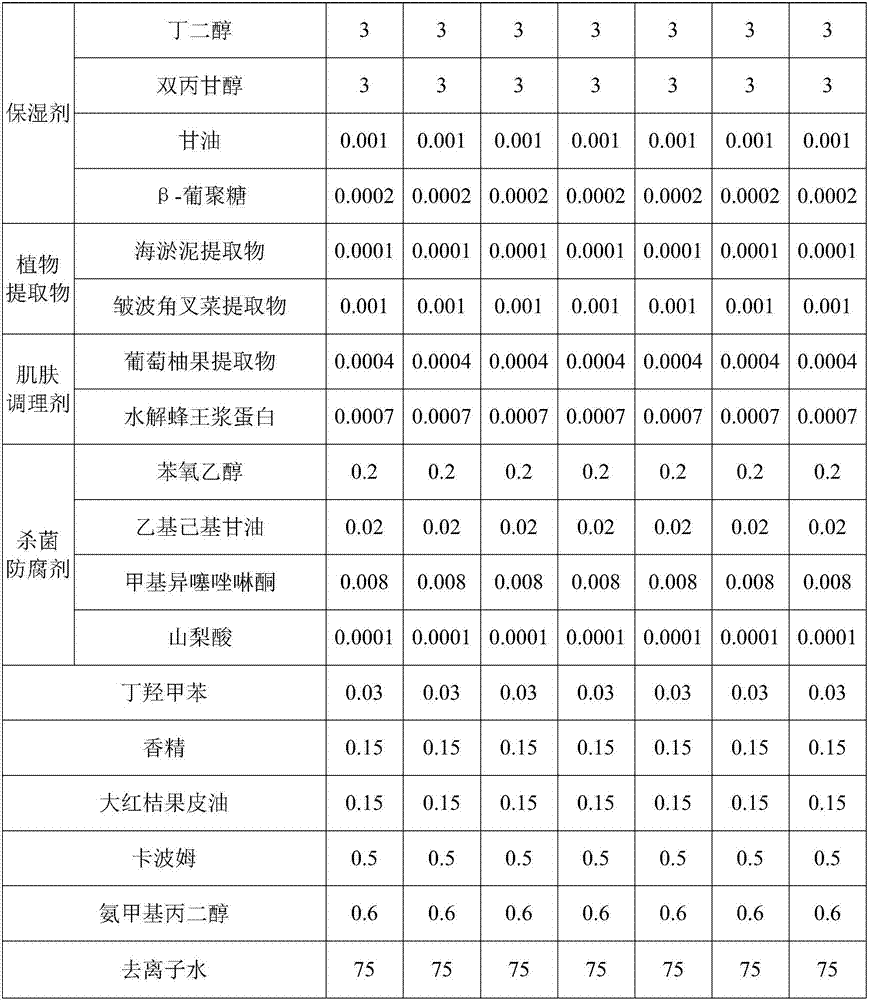
Sex pheromone composition for luring citrus leaf miner and luring core thereof
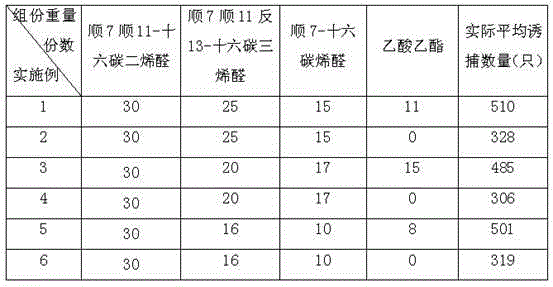
The invention relates to a sex pheromone composition for luring citrus leaf miner and a luring core thereof, which is characterized by comprising cis-7, cis-11, trans-13-hexadecatrienoic aldehyde, cis-7,cis-11-hexadecadienoic aldehyde, cis-7-hexadecene aldehyde according to mass proportion of 1-100:0-100:1-10 to produce the sex pheromone luring core. Each luring core within the dosage range of 40 micrograms to 1333 micrograms is effective, the general dosage for optimum trapping is 400 micrograms, the luring core is capable of maintaining for 45 days in field. A proper basin and a glued trap for grouping and trapping male moths of the citrus leaf miner are cooperated for solving the prevention and prediction of sexual luring insects such as the citrus leaf miner.
Owner:宁波纽康生物技术有限公司
Sunscreen compositions and methods of their use
Described herein are topical skin compositions useful for protecting the skin from the sun. The topical skin compositions comprise cosmetic ingredients such as homosalate, octisalate, oxybenzone, octocrylene, avobenzone, dimethicone, butylene glycol, styrene / acrylates copolymer, C12-15 alkyl benzoate, dicaprylyl carbonate, glycerin, ceteareth-25, dimethicone crosspolymer, magnesium aluminum silicate, acetyl dipeptide-1 cetyl ester, Crithmum maritimum extract, hydrolyzed algin, ascorbic acid, plankton extract, and Opuntia tuna fruit extract. The compositions may also comprise other cosmetic ingredients, examples of which include film formers, preservatives, emulsifiers, conditioning agents, and moisturizers. Also disclosed are methods of using the topical skin compositions.
Owner:MARY KAY INC
Hand and foot film and making method thereof
InactiveCN107126410AWith whiteningMoisturizingCosmetic preparationsToilet preparationsBetaineChamomile extract
The invention discloses a hand and foot film. The hand and foot film comprises a component A, a component B, a component C and a component D, and the component A comprises water, propylene glycol, glycerol, an acrylic acid (acrylate) / C10-30 alkanol acrylate crosslinked polymer, disodium EDTA, allantoin, nicotinamide and carbomer; the component B comprises cetyl ethylhexanoate, glyceryl stearate, cetearyl alcohol, polydimethylsiloxane, urea, petrolatum, betaine, ceteareth-25, methylparaben and propylparaben; the component C comprises panthenol, honey extract, di(hydroxymethyl)imidazolidinyl urea and an essence; and the component D comprises Sophora flavescens root extract, Leonurus sibiricus flower / leaf / stem extract, Atractylodes chinensis rhizome rhizome extract, Glycyrrhiza glabra leaf extract and chamomile extract. The hand and foot film has whitening, moisturizing, moistening, aging preventing, firming, cutin removing and crack preventing functions, and also has good skin care, high flexibility and excellent stability.
Owner:广州真润生物科技有限公司
Method for preparing whitening nanoparticle emulsion with solid phase core
ActiveCN103690380AExtended half-lifeImprove stabilityCosmetic preparationsToilet preparationsDAISY FLOWER EXTRACTOil phase
The invention relates to a method for preparing a whitening nanoparticle emulsion with a solid phase core. The method is characterized by adopting the following steps: A, weighing glyceryl stearate, ceteareth-20 and ceteareth-12, mixing and heating to 82-85 DEG C as an oil phase; B, weighing paeonol, daisy flower extract, ribes nigrum seed oil and mandelic acid, mixing to obtain a whitening active matter; C, weighing an oil phase and the whitening active matter, uniformly mixing at a temperature of 82-85 DEG C; D, adding deionized water, stirring at a rotating speed of 500rpm (revolutions per minute) by using a stirrer at a temperature of 82-85 DEG C for 10 minutes; E, homogenizing at a rotating speed of 15000-30000rpm by adopting a homogenizing machine, cooling to 70 DEG C at a speed of 0.5-1.5 DEG C per minute; F, cooling to a room temperature, adding a defined amount of anticorrosive agent to obtain the whitening nanoparticle emulsion with the solid phase core. The whitening nanoparticle emulsion with the solid phase core is capable of simultaneously loading the paeonol, the daisy flower extract, the ribes nigrum seed oil and the mandelic acid, is simple in preparation process, remarkable in whitening effect, and good in stability of the active matter.
Owner:PROYA COSMETICS
Composite silk textile material and preparation method thereof
InactiveCN105820588AAccelerated corrosionIncreased durabilityConjugated cellulose/protein artificial filamentsArtifical filament manufactureHollow fibreCeteareth
The invention discloses a composite silk textile material which comprises the following raw materials in parts by weight: 15-35 parts of hollow fiber, 15-35 parts of viscose, 15-35 parts of acetate fiber, 15-35 parts of carboxymethylcellulose, 20-50 parts of silk fiber, 5-15 parts of coconut fiber, 5-10 parts of xanthan gum, 5-10 parts of sorbitol, 5-10 parts of sodium alginate, 5-10 parts of ceteareth, 5-10 parts of 2-hydrochlorate, 5-10 parts of sulfur hexafluoride, 2-5 parts of 2-hydroxy-4-methoxy-5-benzophenone sulfonate, 2-5 parts of 4- methoxyphenol, 2-5 parts of sorbitan monolaurate, 5-10 parts of adhesive and 5-10 parts of stabilizer. The prepared textile material has good corrosion resistance, and the durability is remarkably improved. Meanwhile, the invention also discloses a corresponding preparation method.
Owner:WUJIANG FUHUIYUAN HOME TEXTILES CO LTD
Shampoo-hair conditioner composition with scalp anti-aging function and preparation method thereof
ActiveCN104887580AFeel goodReduce volatilityCosmetic preparationsHair cosmeticsChemical industryCocoyl glutamate
The invention belongs to the technical field of daily chemical industry, and discloses a shampoo-hair conditioner composition with a scalp anti-aging function and a preparation method thereof. The shampoo-hair conditioner composition consists of a shampoo and a hair conditioner, wherein the shampoo consists of cocamidopropyl betaine, a cocoyl glutamate TEA salt, glycerol, butanediol, a polyquaternary salt-10, hydrolyzed jojoba ester, ceteareth-60 myristyl glycol, glycol distearate, hydrolyzed collagen, a white willow extract, an essence and water; and the hair conditioner consists of polydimethylsiloxane, docosyl alcohol, hydroxyethyl cellulose, behentrimonium chloride, butanediol, glycerol, hydrolyzed jojoba ester, hydrolyzed collagen, oligopeptide-1, a white willow extract, an essence and water. The shampoo-hair conditioner composition disclosed by the invention has a good hair cleaning effect, can repair damaged hairs, and can ensure that the hairs are smooth, refreshing and convenient to comb; and meanwhile, the shampoo-hair conditioner composition can nurse the scalp, has a good scalp anti-aging effect, and is mild in formula.
Owner:GUANGDONG MARUBI BIOLOGICAL TECH CO LTD
Mild-type mite-killing anti-itching shower gel and preparation method thereof
ActiveCN111110577AEasy to rinseLess irritatingCosmetic preparationsToilet preparationsPropanoic acidShower gel
The invention discloses a mild-type mite-killing anti-itching shower gel and a preparation method thereof. The shower gel contains the following components in percentages by weight: 3-6% of sodium palmitoyl sarcosinate, 4-7% of sodium myristoyl methyl beta-alanine, 3-6% of sodium lauroyl aspartate, 3-6% of an emollient, 3-6% of ceteareth-60 myristyl glycol, 4-7% of a conditioning agent, 0.1-1% ofmagnesium nitrate, 0.5-1% of sodium chloride, 0.05-1% of a chelating agent, 0.005-0.1% of a preservative, 0.005-0.1% of essence, and the balance of deionized water. The shower gel provided by the invention has the advantages of mild nature, low irritation, fine foam, easy flushing and good cleaning power, and has the functions of bacteriostasis, mite killing and skin moisturizing, and enhances skin health while effectively nourishing skin.
Owner:广州玥颜化妆品有限公司
Conopomorpha sinensis bradley prevention and control composition
The invention discloses a conopomorpha sinensis bradley prevention and control composition including the following components by weight: 20-80 parts of trans-4, 6-cis-10-hexadecyl triene acetate, 5-35 parts of cis-9-hexadecenol acetate, 5-35 parts of trans-9-hexadecenol acetate and 10 parts of n-dodecyl alcohol. The conopomorpha sinensis bradley prevention and control composition is used for disturbing mating behavior of imago so as to reducing the amount of the larva. The conopomorpha sinensis bradley prevention and control composition is simple in formula and has good mating disruption effect, shows excellent control effect and greatly reduces the base number of the larva in fields during use in fruit tree pest insect conopomorpha sinensis bradley prevention and control, and has a huge advantage in the conopomorpha sinensis bradley prevention and control.
Owner:漳州市英格尔农业科技有限公司
Massage emulsion
ActiveCN101647760APromote absorptionNo cloggingCosmetic preparationsToilet preparationsCetearethUltraviolet
The invention belongs to the household chemicals field and particularly relates to a massage emulsion. The massage emulsion of the invention comprises emulsifier, preservative, metal ion chelator, humectant, thickener, lubricant, functional additive and nutritive materials, wherein the emulsifier is a mixture of ceteareth-2 and ceteareth-21, the thickener comprises xanthan gum and polyacrylamide / isoparaffin containing 13-14 carbon / dodecyl alcohol ether-7, and the humectant comprises 1,3-butanediol, Hispaqel oil and hyaluronic acid. The massage emulsion of the invention adopts new emulsifier,thickener and humectant, does not contain fat and oil products, does not have greasy feeling in use, has the advantage of good lubricating effect, low massage resistance and good absorptivity, are notneeded to wash, is simple to use, and has the functions of whitening skin, keeping moisture and replenishing water, replenishing nutritive materials, fixing ultraviolet ray damage and avoiding cutaneous anaphylaxis.
Owner:GUANGZHOU KENENG COSMETICS RES CO LTD +1
Plant extraction hair dyeing cream and preparation method thereof
InactiveCN113350240AAvoid damageStay hydratedCosmetic preparationsHair cosmeticsBiotechnologyHair Colorants
The invention discloses plant extraction hair dyeing cream and a preparation method thereof, and belongs to the technical field of hair dye cream. The plant extraction hair dyeing cream comprises a hair dye and an oxidizing agent; the hair dye is prepared from the following components in percentage by weight of cetearyl alcohol, ceteareth, a stearyl trimethyl ammonium chloride solution, polydimethylsiloxane, liquid paraffin, ammonia-terminated polydimethylsiloxane, a chrysanthellum indicum extract, hydrolyzed keratin, p-phenylenediamine, resorcinol, phenyl methyl pyrazolone, m-aminophenol, 2, 4-diaminophenoxy ethanol HCl, an antioxidant, a chelating agent, a PH regulator, a humectant and an aromatic; and the oxidizing agent comprises the following components in percentage by weight of a hydrogen peroxide aqueous solution, cetearyl alcohol, sodium lauryl sulfate, ceteareth-25, a chelating agent, a stabilizer, a pH regulator, an aromatic and the balance of solvent water. According to the plant extraction hair dyeing cream and the preparation method thereof, the problems that some existing hair dyeing cream is poor in hair dyeing effect, causes harm to human bodies after being used and the like are solved.
Owner:张勇
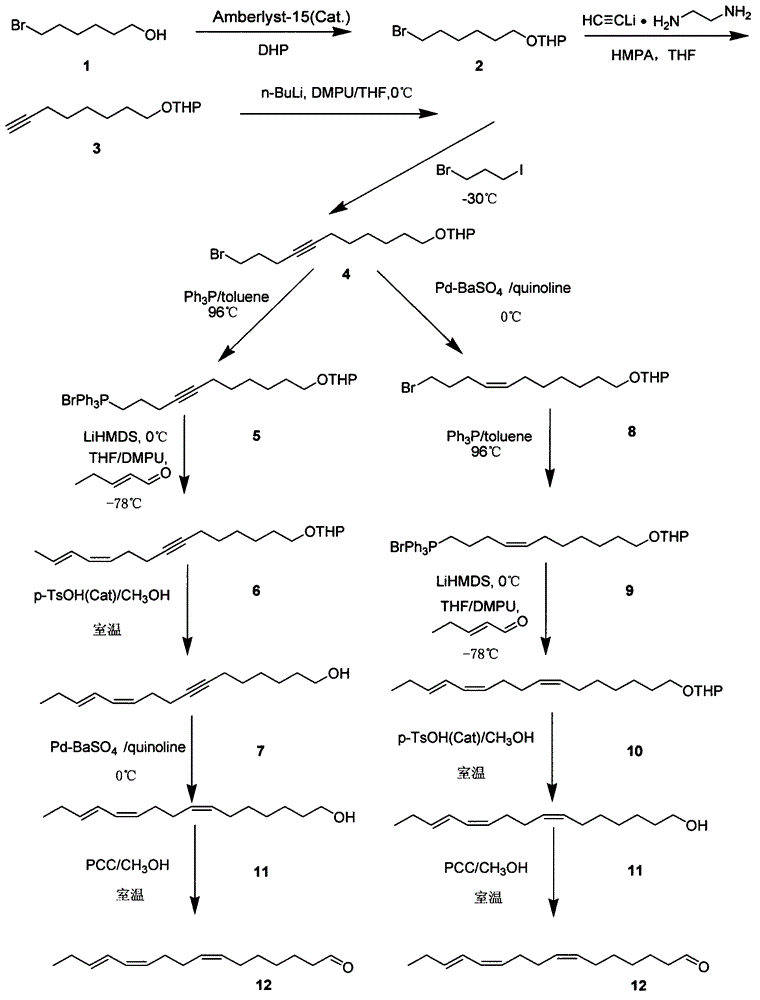
Synthetic method of (Z,Z,E)-7,11,13-hexadecatrienal
InactiveCN104829439AIncrease profitLow costOrganic compound preparationPreparation by hydrogenationWittig reactionCeteareth
The invention relates to an improved synthetic method of (Z,Z,E)-7,11,13-hexadecatrienal, wherein the (Z,Z,E)-7,11,13-hexadecatrienal is an important component in a sex pheromone compound of phyllocnistis citrella. The improved synthetic method is carried out with 6-bromo-1-hexanol as an initial raw material and through a coupled reaction, a Wittig reaction and other reactions to synthesize the (Z,Z,E)-7,11,13-hexadecatrienal. The improved synthetic method is advantaged in that the raw material is low in cost and easy to get, and the reactions are safe and convenient in operations and is short in step periods. The improved synthetic method is high in product yield, is free of environmental pollution and can achieve better economic benefit.
Owner:宁波纽康生物技术有限公司
Micro emulsion mist spray containing butyrospermum parkii shea butter and preparation method thereof
InactiveCN107174548APromote absorptionRich in non-saponifiable ingredientsCosmetic preparationsToilet preparationsDicaprylyl carbonateButyrospermum parkii
The invention discloses micro emulsion mist spray containing butyrospermum parkii shea butter and a preparation method thereof. The micro emulsion mist spray is prepared from the butyrospermum parkii shea butter, a hygroscopic agent, cetostearyl alcohol, ceteareth-25, EDTA (Ethylene Diamine Tetraacetic Acid) sodium salt, PEG-20 hydrogenated castor oil, tridecyl stearate, a skin natural moisturizing factor, a skin repairing factor, dipotassium glycyrrhizinate, allantoin, glyceryl monostearate, hexylene glycol, methylparaben, ethylparaben, phenoxyethanol, dicaprylyl carbonate and deionized water. The micro emulsion mist spray contains the butyrospermum parkii shea butter which is most approximate to various indexes of human sebum secretion grease, is rich in non-saponifiable components, and can be absorbed by human body easily; by adopting the mist spray added with the butyrospermum parkii shea butter, the skin is kept young, aging is prevented, and the skin elasticity is enhanced.
Owner:DOCTOR PLANT GUANGDONG BIOTECHNOLOGY CO LTD
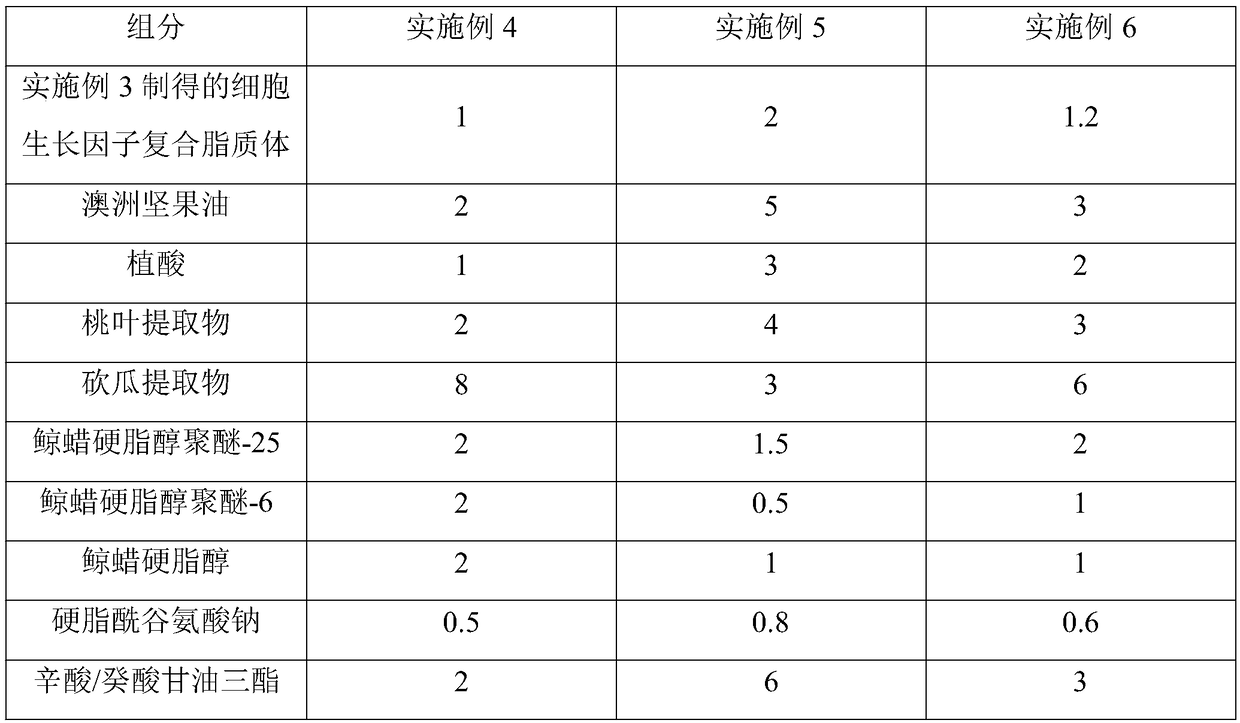
Acne-removing repair cream containing cell growth factors and preparation method thereof
InactiveCN108261361AAvoid damageImprove nutrient metabolismCosmetic preparationsToilet preparationsPhytic acidLiposome
Belonging to the technical field of cosmetics, the invention relates to an acne-removing repair cream containing cell growth factors. The acne-removing repair cream containing cell growth factors comprises the following components: a cell growth factor compound liposome, macadamia oil, phytic acid, a peach leaf extract, a Kangua extract, ceteareth-25, ceteareth-6, cetearyl alcohol, sodium stearoylglutamate, caprylic / capric triglyceride, carboxymethyl chitosan, xanthan gum, polyglutamic acid, propylene glycol, PEG-40 hydrogenated castor oil, tocopheryl acetate, bisabolol, a preservative, essence and deionized water. Through the synergistic effect of all the components, the acne-removing repair cream containing cell growth factors provided by the invention can effectively maintain the activity of cell growth factors and improve the bioavailability of cell growth factors, has significant acne removing and inhibition effects, and has obvious prevention and repair effects on dark acne marks, sunken or hyperplastic scars left after healing of acnes.
Owner:GUANGDONG COOWAY BIOTECH CO LTD
Method and composition for treating burned skin
The present invention relates to a method and composition for treating sunburned skin. The present invention provides a method and composition for applying a mixture of indomethacin and moisturizing lotion topically to sunburned skin. The composition includes a mixture having substantially 100 milligrams of indomethacin per 30 cc of moisturizing lotion. The moisturizing lotion is marketed under the trade name Cetaphil® and includes the following ingredients: purified water, glycerin, hydrogenated polyisobutene, cetearyl alcohol (and) ceteareth-20, macadamia nut oil, dimethicone, tocopheryl acetate, stearoxytrimethylsilane (and) stearyl alcohol, panthenol, famesol, benzyl alcohol, phenoxyethanol, acrylates / C10-30 alkyl acrylate crosspolymer, sodium hydroxide, and citric acid. It is theorized that the Cetaphil® provides certain pH and viscosity levels which allow for the stabilization and solubilation of the indomethacin within the Cetaphil®. The present invention may also be utilized for treating skin burns caused by radiation therapy and excessive heat.
Owner:LIL BRAT PHARMA
Artificially synthesized phyllocnistis citrella stainton gyplure
The invention discloses an artificially synthesized phyllocnistis citrella stainton gyplure. The gyplure is prepared by the following steps: mixing cis7 cis11-hexadecadienal, cis7 cis11 trans13- hexadecatrienal, cis7-hexedecanal and ethyl acetate in proportion by weight parts of 30:(1-30):(0.1-20):(0.1-20); adding 0.1 to 1 weight percent of vitamin E, TBHQ (tert-butyl hydroquinone), BHT (butylated hydroxytoluene) or BHA (butylated hydroxyanisole) as an antioxidant; and adding 0.1 to 1 weight percent of benzophenone, benzotriazole or hindered amine and mixture as an optical stabilizer. The artificially synthesized phyllocnistis citrella stainton gyplure has the following advantages: phyllocnistis citrella stainton diseases can be prevented and monitored, the trapping effect can be intensified and cost can be reduced, industrial efficiency improvement and orchard worker income increase can be facilitated, orange pesticide residue does not exist, the environment is not polluted, ecological balance is not destroyed, and sustainable development of orange industry is facilitated.
Owner:漳州市英格尔农业科技有限公司
Essence for improving skin aging and damage and preparation method thereof
InactiveCN113274312AAccelerated agingEasily damagedCosmetic preparationsToilet preparationsBenzoic acidArginine
The invention discloses essence for improving skin aging and damage, and belongs to the technical field of skin care product processing. The essence is prepared from the following ingredients including 1, 3-propanediol, glycerol, isononanoate cetearyl stearate, cetostearyl alcohol, ceteareth-20, glyceryl stearate, ceteareth-12, cetyl palmitate, serum protein, acetyl hexapeptide-8, hydrolyzed sodium hyaluronate, glyceryl acrylate / acrylic acid copolymers, propanediol, PVM / MA copolymers, sodium hyaluronate, lactose, milk protein, oligopeptide-1, arginine / lysine polypeptide, polyacrylate cross-linked polymers-6, tert-butyl alcohol, 1, 2-hexanediol, caprylyl glycol, benzoic acid, ethylhexylglycerin, p-hydroxyacetophenone and water. A preparation method of the essence comprises the following steps of (1) raw material weighing; (2) stirring treatment; (3) cold plasma-ultraviolet light circulation alternate treatment; (4) micro jet high-pressure homogenizing treatment; and (5) quality inspection and packaging. The finally prepared essence has the obvious anti-aging effect.
Owner:上海活彩生物科技有限公司
Massage cream containing cannabidiol nanoliposomes and preparation method thereof
InactiveCN110559218AGood water solubilityImprove stabilityCosmetic preparationsToilet preparationsSolubilityEthylhexyl palmitate
The invention discloses a massage cream containing cannabidiol nanoliposomes and a preparation method thereof. The massage cream comprises the following raw materials in percentage by mass: 0.1-1% ofcannabidiol nanoliposome, 5-10% of beeswax, 5-10% of glycerol, 2-5% of glyceryl monostearate, 1-5% of cetyl alcohol, 1-5% of isopropyl myristate, 1-5% of ethylhexyl palmitate, 0.5-2% of ceteareth-20,0.5-1% of essential oil, 0.1-1% of carbomer, 0.05-0.1% of triethanolamine, 0.01-0.1% of phenoxyethanol, and the balance deionized water. According to the invention, nano-scale cannabidiol (CBD) is prepared by adopting a liposome technology, so that the water solubility and stability of the CBD are improved, and meanwhile, the transdermal absorption effect of the CBD is promoted, so that the CBD can quickly and effectively penetrate through the surface layer of the skin and directly reach the deep layer of the skin, the CBD is slowly released, and the effect is more durable. The massage cream containing the cannabidiol nanoliposomes can effectively remove free radicals, and has good effects of resisting inflammation, resisting oxidation and delaying senescence.
Owner:YUNNAN LVXIN BIOLOGICAL PHARMA CO LTD
Nourishing care foot mask and preparation method thereof
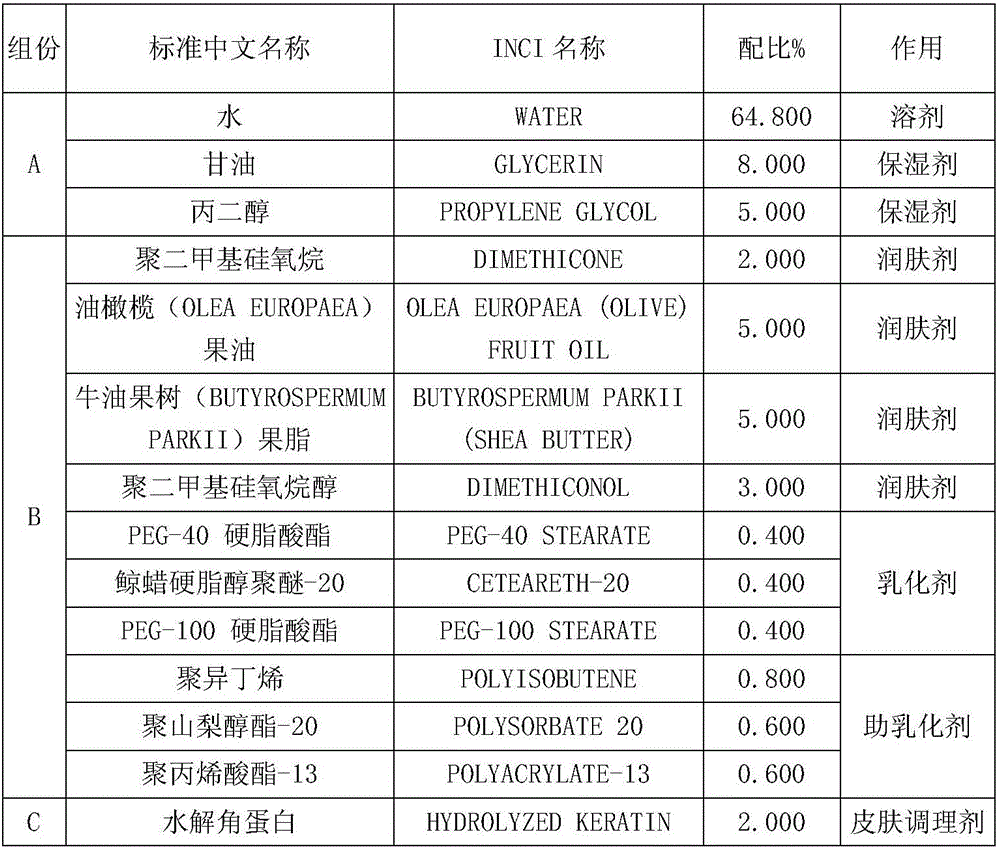
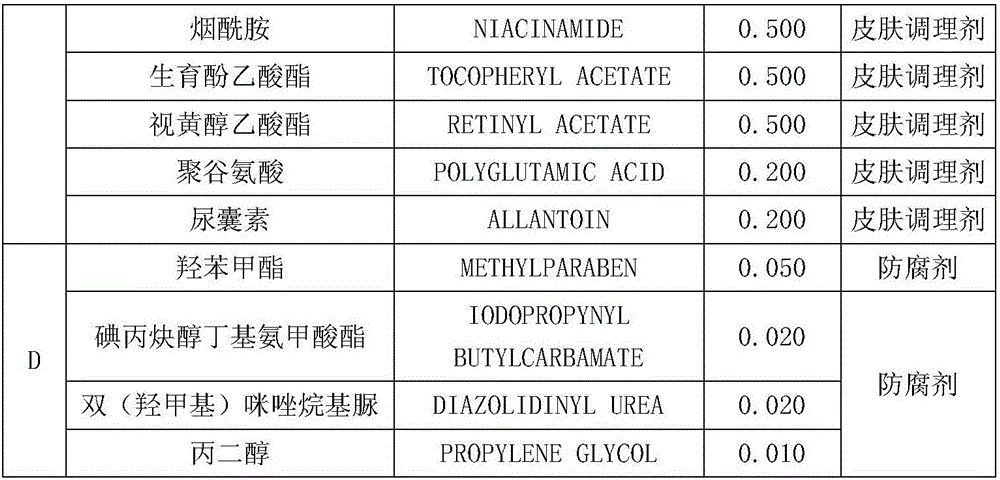
The invention discloses a nourishing care foot mask which comprises the following formula components: phase A: 64.8% of water, 8% of glycerin and 5% of propylene glycol; phase B: 2% of polydimethylsiloxane, 5% of olive-pomace oil, 5% of butyrspermum parkii butter, 3% of dimethiconol, 0.4% of PEG-40 stearate, 0.4% of ceteareth-20, 0.4% of PEG-100 stearate, 0.8% of polyisobutene, 0.6% of polysorbate-20 and 0.6% of polyacrylate-13; phase C: 2% of hydrolytic keratin, 0.5% of nicotinamide, 0.5% of tocopheryl acetate, 0.5% of retinyl acetate, 0.2% of polyglutamic acid and 0.2% of allantoin; and phase D: 0.05% of methylparaben, 0.02% of iodopropynyl butylcarbamate, 0.02% of bis(hydroxymethyl) imidazolidinyl urea and 0.01% of propylene glycol. The nourishing care foot mask disclosed by the invention is capable of nourishing foot skin, so that the problems of both feet, such as roughness, dryness, hangnails, cracking and the like can be solved.
Owner:FABLED ENVIRONMENTAL PROTECTION TECH SUZHOU CO LTD
Acne-removing aloe gel and preparation method thereof
InactiveCN112472653AHas anti-acne effectHigh activityCosmetic preparationsToilet preparationsCelluloseSalicylic acid
The invention provides acne-removing aloe gel and a preparation method thereof. The acne-removing aloe gel comprises the following components in percentage by weight: 1% of carbomer; 0.5% of hydroxyethyl cellulose; 3% of ceteareth-25; 1% of SENSANOV WR; 4% of propylene glycol; 0.05% of sodium hyaluronate; 0.2% of methyl paraben; 0.2% of a salicylic acid inclusion compound; 1.2% of triethanolamine;0.05% of PEG-35 castor oil; 0.01% of an essence; 2% of an aloe extract; 1% of a purslane extract; 1% of an oil control compound; 0.005% of a quaternary ammonium salt-73; 2% of a skin care product; 2%of SELASTIN, and the balance of deionized water; according to an acne forming mechanism, natural plant extracts and safe and effective acne treatment raw materials are reasonably matched, all the components in the formula have a synergistic reaction, the effect is brought into full play, the excellent acne removal effect is achieved, meanwhile, the invention further discloses a preparation methodof the acne-removing aloe gel, the raw material source is wide, and manufacturing and processing are convenient.
Owner:广州澳梓美生物科技有限公司 +1
Anti-freckle cream and preparation method thereof
ActiveCN103566300ALow costSignificant effectAnthropod material medical ingredientsDermatological disorderVitamin CGLYCYRRHIZA EXTRACT
The invention discloses an anti-freckle cream prepared from the following raw materials: isohexadecane, olive fruit oil, stearyl alcohol, polydimethylsiloxane, glycerin stearate, cetearyl alcohol polyether-6, cetearyl alcohol polyether-25, propyl hydroxybenzoate, butanediol, 2-bromo-2-nitropropane-1, 3-diol, imidazolidinyl urea, deionized water, functional components of traditional Chinese medicines and vitamin C, wherein the functional components of traditional Chinese medicines include the following constituents: silkworm larva, olibanum, savia miltiorrhiza, panax pseudoginseng, liquorice, cortex fraxini, turmeric and sambucus williamsii hance. The functional components of the traditional Chinese medicines in the anti-freckle cream are screened by adopting the syndrome differentiation theory of the traditional Chinese medicine and combining many-year clinical experience according to a freckle forming principle. The anti-freckle cream disclosed by the invention is low in cost, remarkable in curative effect and capable of achieving the aims of regulating qi and blood, dredging channels, activating blood and dissolving stasis as well as nourishing qi and blood, so that freckles are fundamentally treated.
Owner:TIANJIN JIASHITANG SCI & TECH
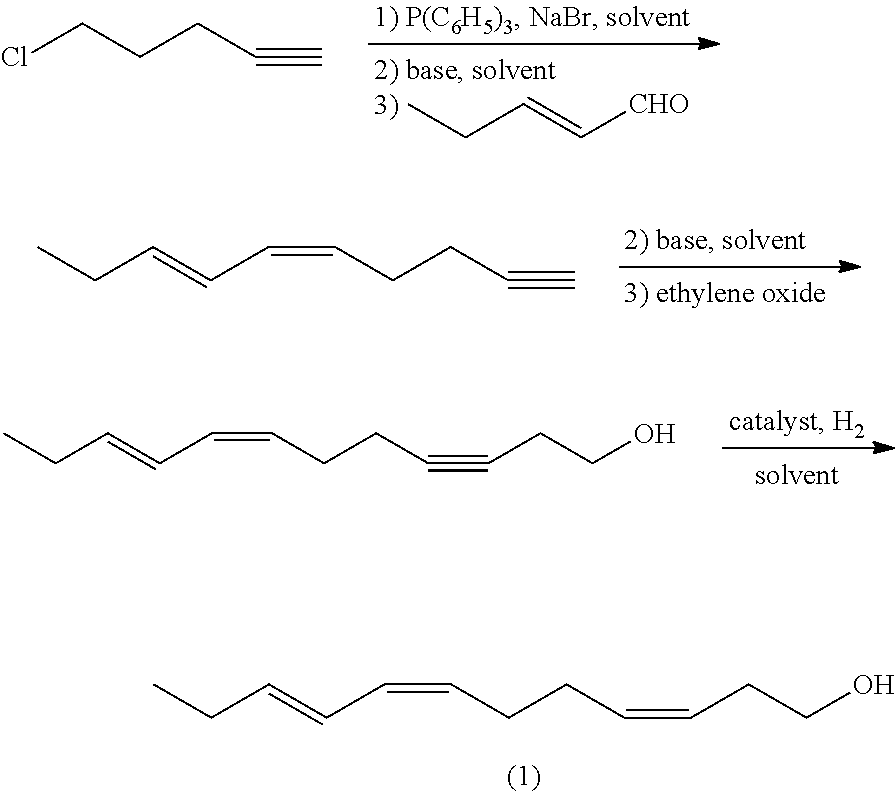
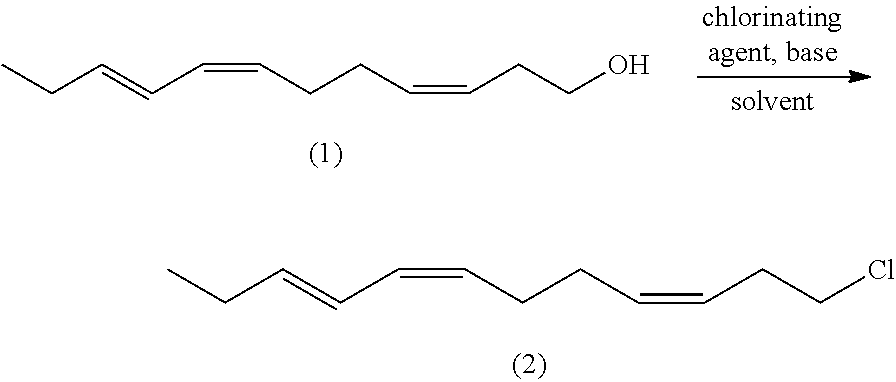
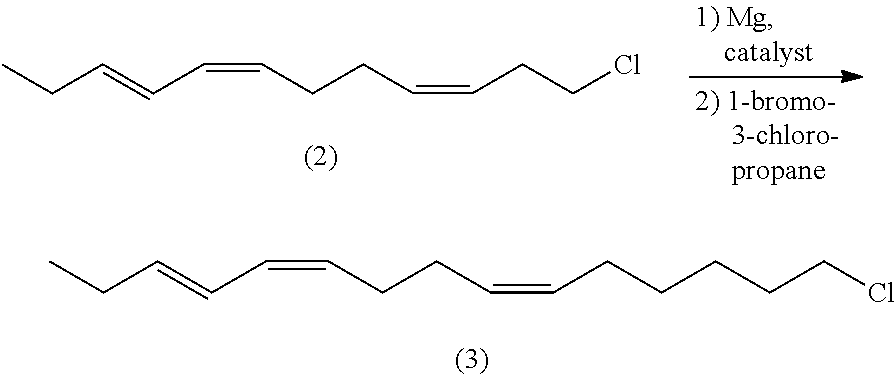
(z,z,e)-1-chloro-6,10,12-pentadecatriene and method for preparing (z,z,e)-7,11,13-hexadecatrienal by using same
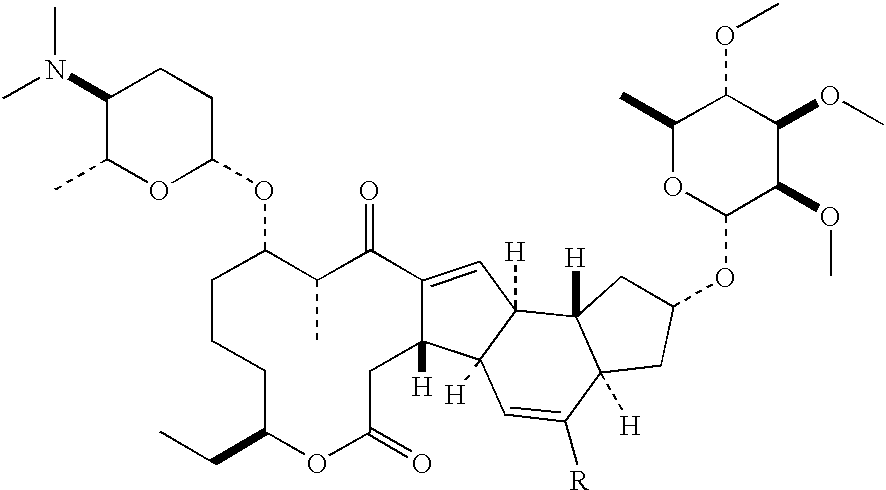
ActiveUS20140275656A1Low costHigh purityOrganic compound preparationCarbonyl compound preparationCetearethEthyl ester
Owner:SHIN ETSU CHEM IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com