Synthetic method of (Z,Z,E)-7,11,13-hexadecatrienal
A technology of hexadecatrienal and synthesis method, which is applied in the field of citrus leafminer pheromone synthesis, can solve immaturity and other problems, and achieve the effects of sufficient reaction, cost reduction, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
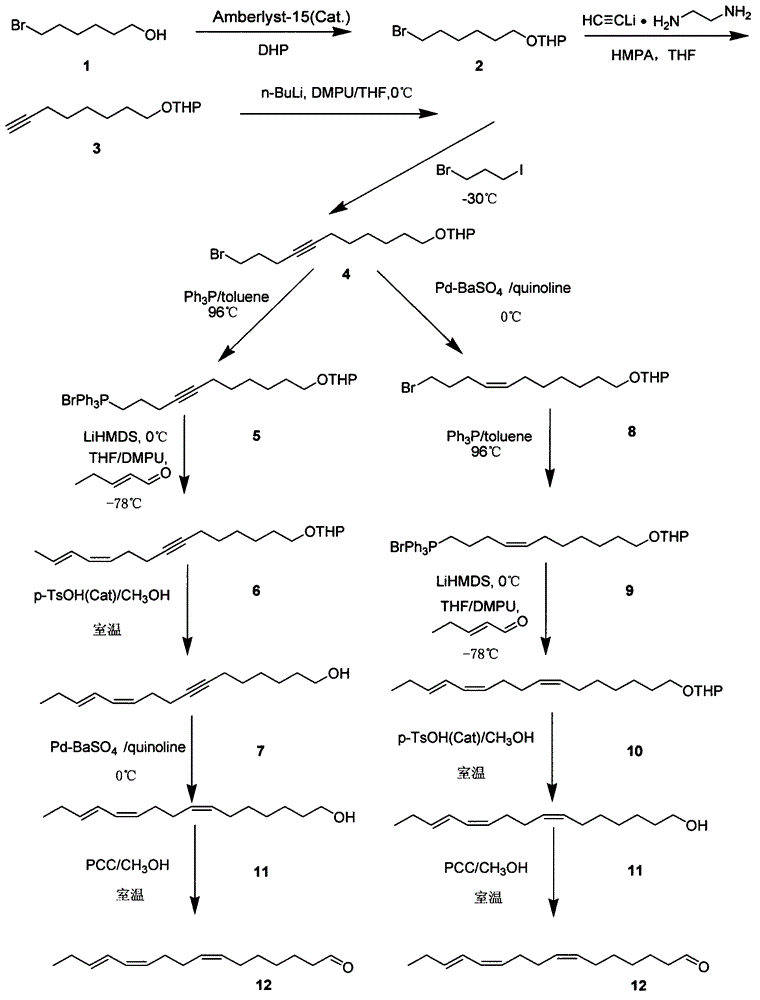
[0020]The synthetic method of a kind of (Z, Z, E)-7,11,13-hexadecatrienal provided by the present invention, concrete steps are: take 6-bromo-1-hexanol as starting reactant, by The protection reaction of the hydroxyl group and the lengthening of the carbon chain, the resulting product is then coupled with 1-bromo-3-iodopropane, and heated to form a salt with triphenylphosphine, and the obtained Wittig salt is reacted with trans-2 in the presence of an organic base (Z, Z, E)-7,11,13-hexadecatrienal was synthesized by Wittig reaction of -pentenal, followed by Lindlar catalytic hydrogenation, hydroxyl deprotection and oxidation. Wherein, the molecular structural formula of the present invention (Z, Z, E)-7,11,13-hexadecatrienal is as follows figure 1 Shown, the roadmap of synthetic method of the present invention is as figure 2 shown.
[0021] Synthesis of Compound 2: Under argon protection, 6-bromo-1-hexanol (39.82g, 0.22mol) was dissolved in dried dichloromethane (250mL), an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com