A kind of pretreatment method for detecting phenolic impurity content in samples
A detection method and impurity content technology, applied in the field of chemical analysis, can solve problems such as large deviation of results, large measurement results, and increased response value, and achieve low detection limit and quantification limit, good method durability, and the effect of inhibiting oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
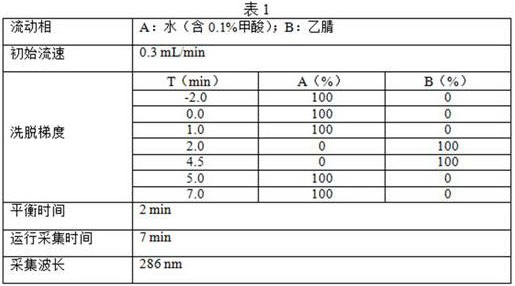
[0076] The determination of phenolic impurity content is carried out to 5 batches of commercially available paroxetine hydrochloride raw materials, comprising the following steps:
[0077] (1) Preparation of reference solution
[0078] Accurately weigh 10 mg of 1,2,4-glucinol reference substance, place it in a 10 mL volumetric flask, first add 0.5 mL of vitamin C (content 100 μg / ml) in 50% acetonitrile solution to dissolve the reference substance, then add vitamin C (content 100 μg / ml) / ml) was diluted with 0.1% formic acid solution, and the volume was adjusted to the mark to obtain phenolic impurity reference substance stock solution Ⅰ with a concentration of 1 mg / mL. The 0.1% formic acid solution dilution is prepared into the reference substance solution of different concentrations;
[0079] (2) Sample pretreatment
[0080] Test product: Accurately weigh 0.1g of raw drug powder, place it in a 10mL volumetric flask, first add 0.5mL of vitamin C (content 100μg / ml) in 50% ace...
Embodiment 2
[0102] Investigate the stability time of phenolic impurities in the determination process, including the following steps:
[0103] (1) The preparation of the reference substance solution is the same as in Example 1;
[0104] (2) Sample pretreatment is the same as in Example 1;
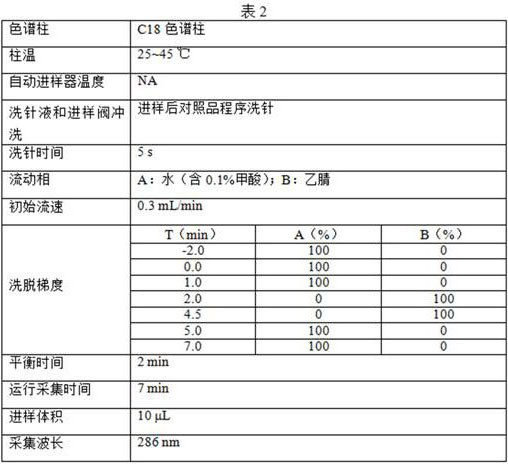
[0105] (3) Liquid chromatography analysis conditions are the same as in Example 1;
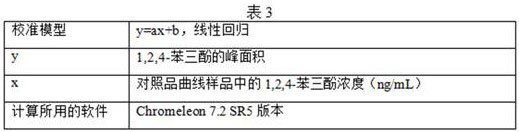
[0106] (4) Result analysis
[0107] After standing for different lengths of time, detect the concentration of phenolic impurities in the reference substance solution and the standard recovery solution, and the liquid chromatography analysis results are as follows: Figure 24-Figure 39 , as shown in Table 4 and Table 5:
[0108]
[0109] The concentration of the impurity reference substance solution was 197.10ng / ml at 0h, 177.73ng / ml at 30h, and the absolute value of the concentration change rate was 9.8%, and 157.80ng / ml at 40h, and the concentration change rate was absolute The value is 19.9%, more than 15%. It...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com