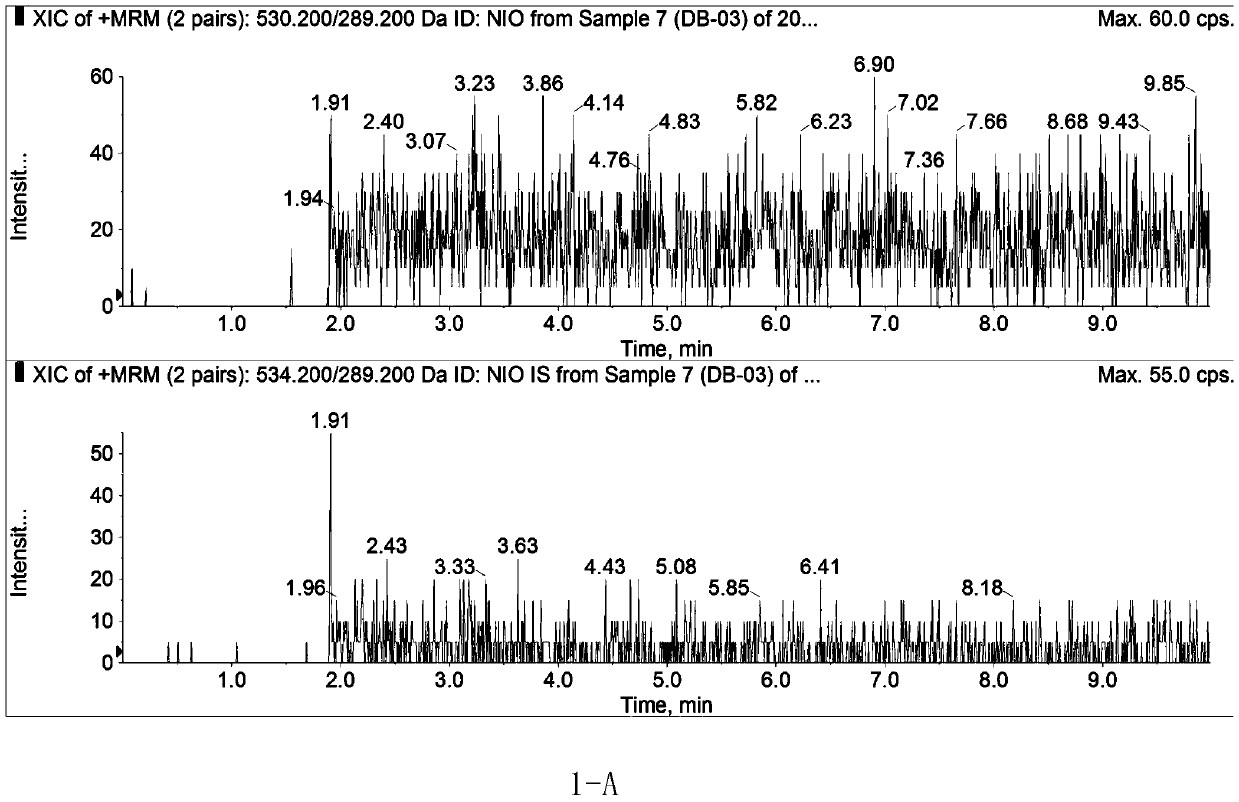
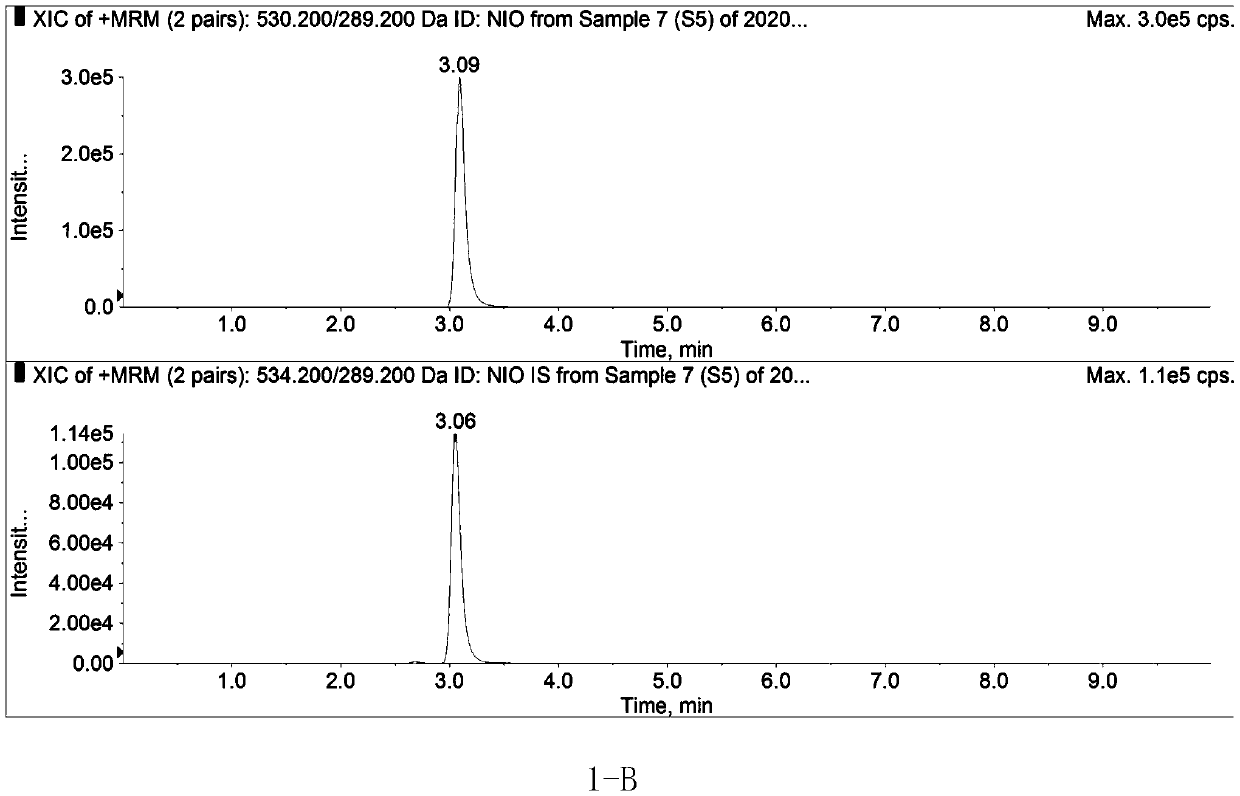
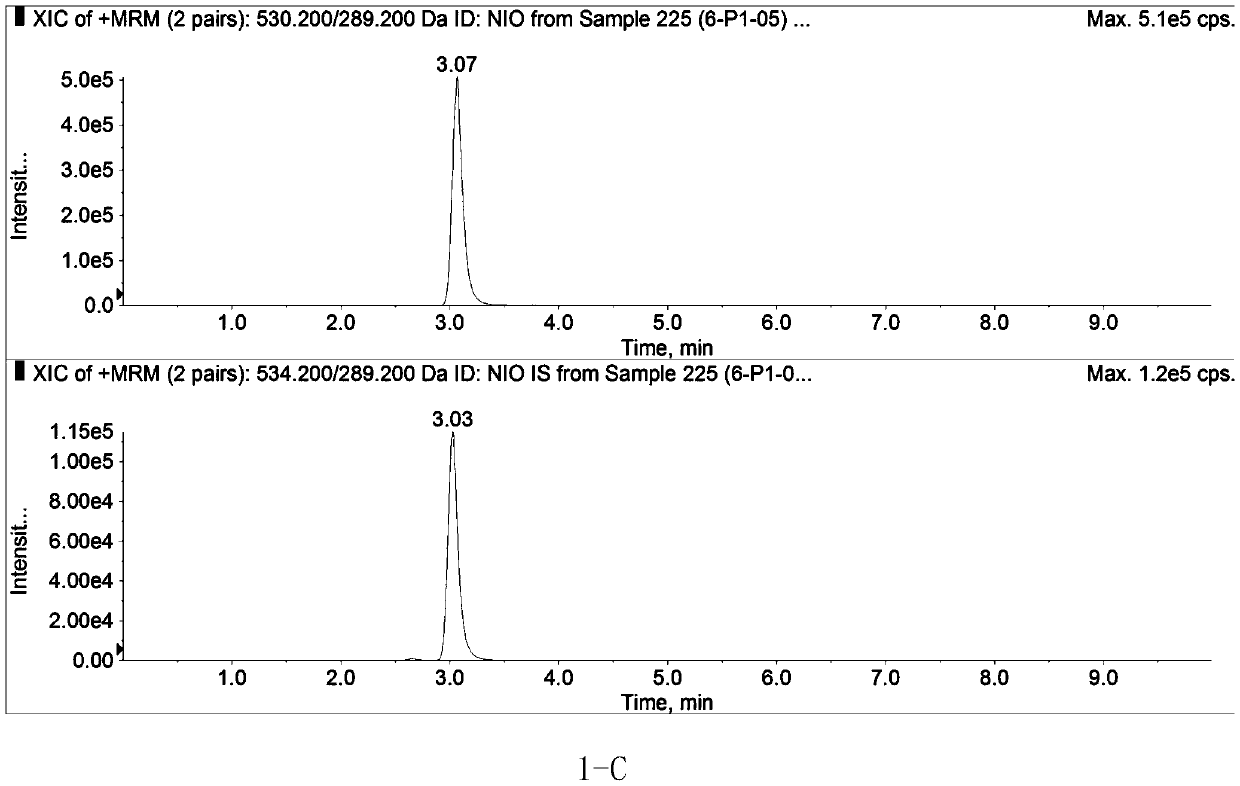
Method for determining concentration of nilotinib in blood plasma
A nilotinib and blood plasma technology, which is applied in the field of determination of nilotinib concentration in plasma, can solve the problems that the measured substance and the internal standard cannot change synchronously, affect the repeatability and measurement accuracy of the method, and achieve a simple pretreatment method , easy operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the mensuration of nilotinib concentration in human plasma;
[0028] 1. Experimental materials and instruments
[0029] Nilotinib reference substance: provided by Suzhou Terui Pharmaceutical Co., Ltd., batch number: b18018;
[0030] [ 13 C, 2 h 3 ]-Nilotinib reference substance: provided by Alsachim, batch number: DH-ALS-16-007-P1; Test water: ultrapure water; Methanol, formic acid: chromatographically pure (Merck Company); Ammonium acetate: analytically pure (Sinopharm Chemical Reagents Ltd.).
[0031] API4000 LC / MS / MS coupled instrument (AB Scisex, USA), chromatographic workstation:
[0032] Analyst 1.6; Mettler XS 105DU electronic balance (Mettler, Switzerland); Eppendorf Centrifuge 5424R high-speed low-temperature centrifuge (Eppendorf, Germany); KDC-2046 low-speed refrigerated centrifuge (Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd.); Millipore Drict-Q5 ultrapure water machine (French Millipore Company).
[0033] 2. Liquid conditions ...
Embodiment 2
[0058] Embodiment 2: the mensuration of nilotinib concentration in the plasma of female subjects;
[0059] Referring to Example 1, a healthy female subject took 200 mg of Nilotinib Capsules on an empty stomach, and took it with 240 mL of warm boiled water; 3 mL of peripheral venous blood was collected 3 hours after administration, injected into a heparin tube, centrifuged (4000 r min -1 , 5min), draw 200 μL of the subject’s plasma sample, and accurately add an internal standard of 500 ng·mL -1 [ 13 C, 2 h 3 ]-50 μL of Nilotinib solution, vortexed for 10 s, added 610 μL of methanol to precipitate protein, vortexed for 1 min, and centrifuged at 15000 rpm, 4°C for 10 min. Draw 100 μL of the supernatant into the injection bottle, add 700 μL of deionized water, and draw 20 μL of the mixed solution for injection. The results showed that 3 hours after oral administration of 200 mg nilotinib capsules on an empty stomach, the nilotinib content in the plasma of a healthy female subj...
Embodiment 3
[0060] Embodiment 3: the mensuration of nilotinib concentration in the blood plasma of experimenter 72h after administration;
[0061] Referring to Example 1, 5 healthy male subjects and 1 female subject took 200 mg of nilotinib capsules on an empty stomach, and took them with 240 mL of warm water; 72 hours after administration, 3 mL of peripheral venous blood was collected and injected into a heparin tube. Centrifugal (4000r·min -1 , 5min), draw 200 μL of subject’s plasma sample, and accurately add internal standard 500ng·mL -1 [ 13 C, 2 h 3 ]-Nilotinib solution 50 μL, vortex 10s, add 610 μL methanol to precipitate protein, vortex 1min, centrifuge at 15000rpm, 4℃ for 10min. Draw 100 μL of the supernatant into the injection bottle, add 700 μL of deionized water, draw 20 μL of the mixed solution for injection. The results showed that 72 hours after oral administration of Nilotinib Capsules 200 mg on an empty stomach, the contents of Nilotinib in the plasma of 5 healthy mal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com