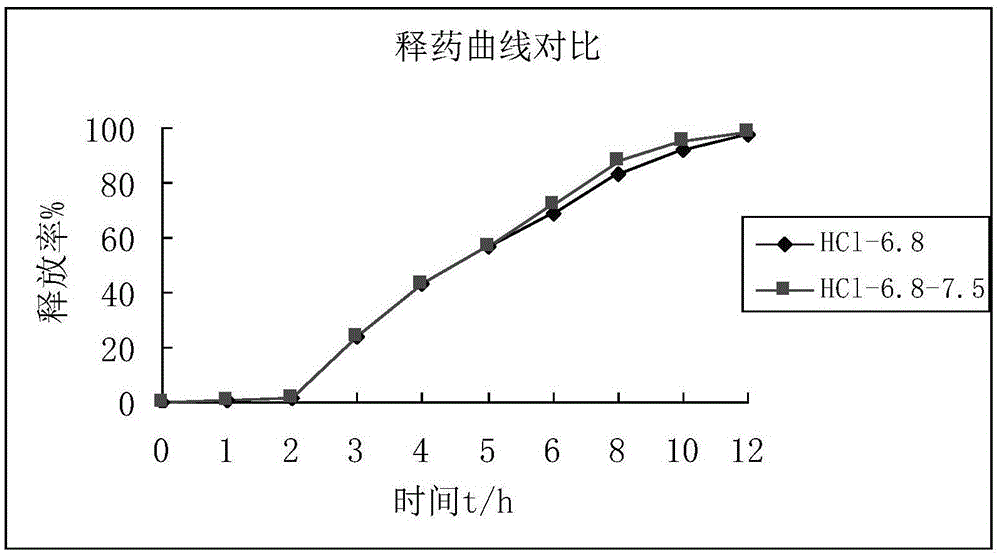
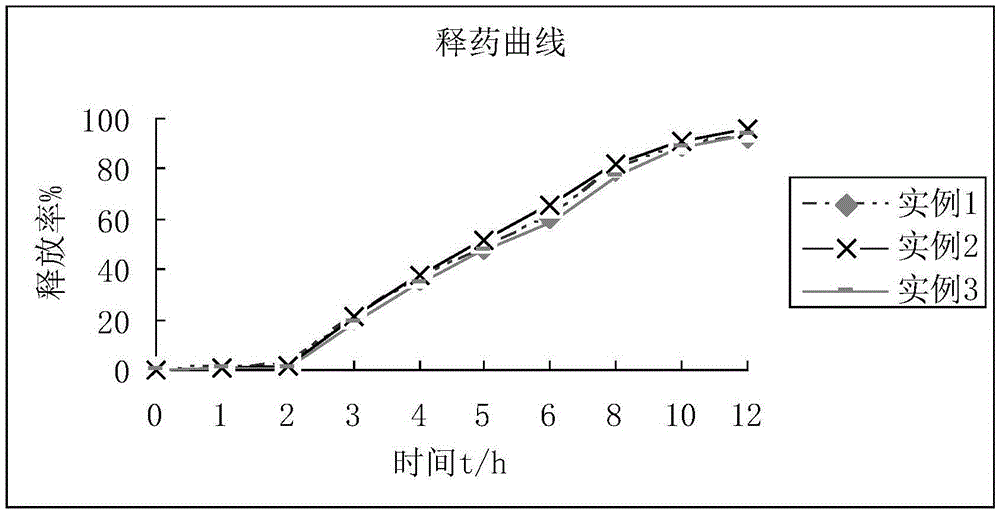
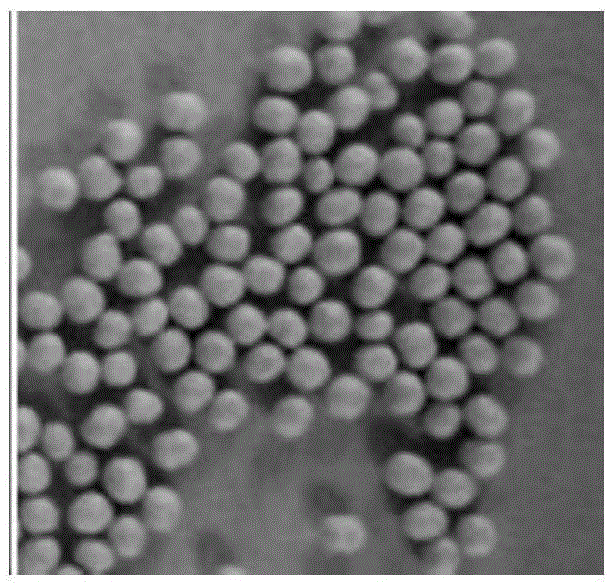
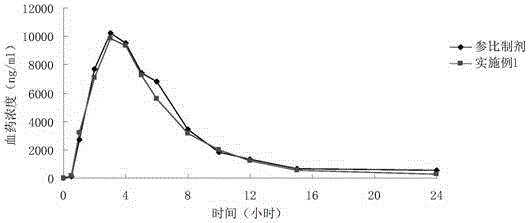
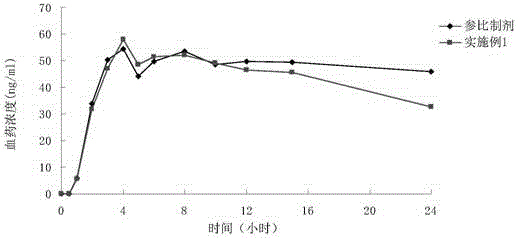
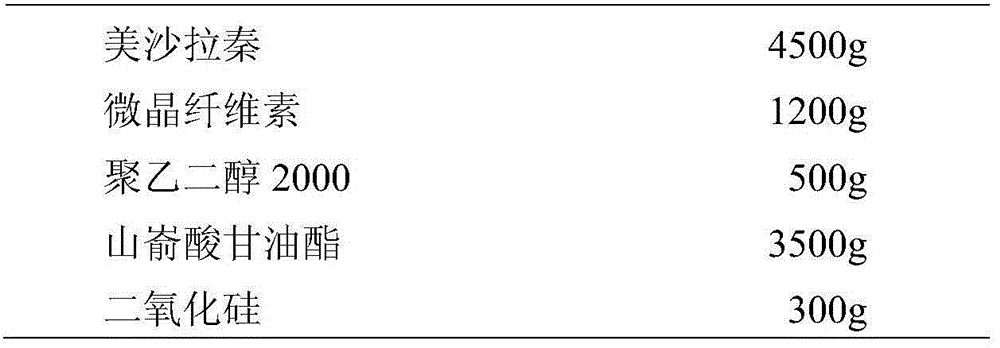
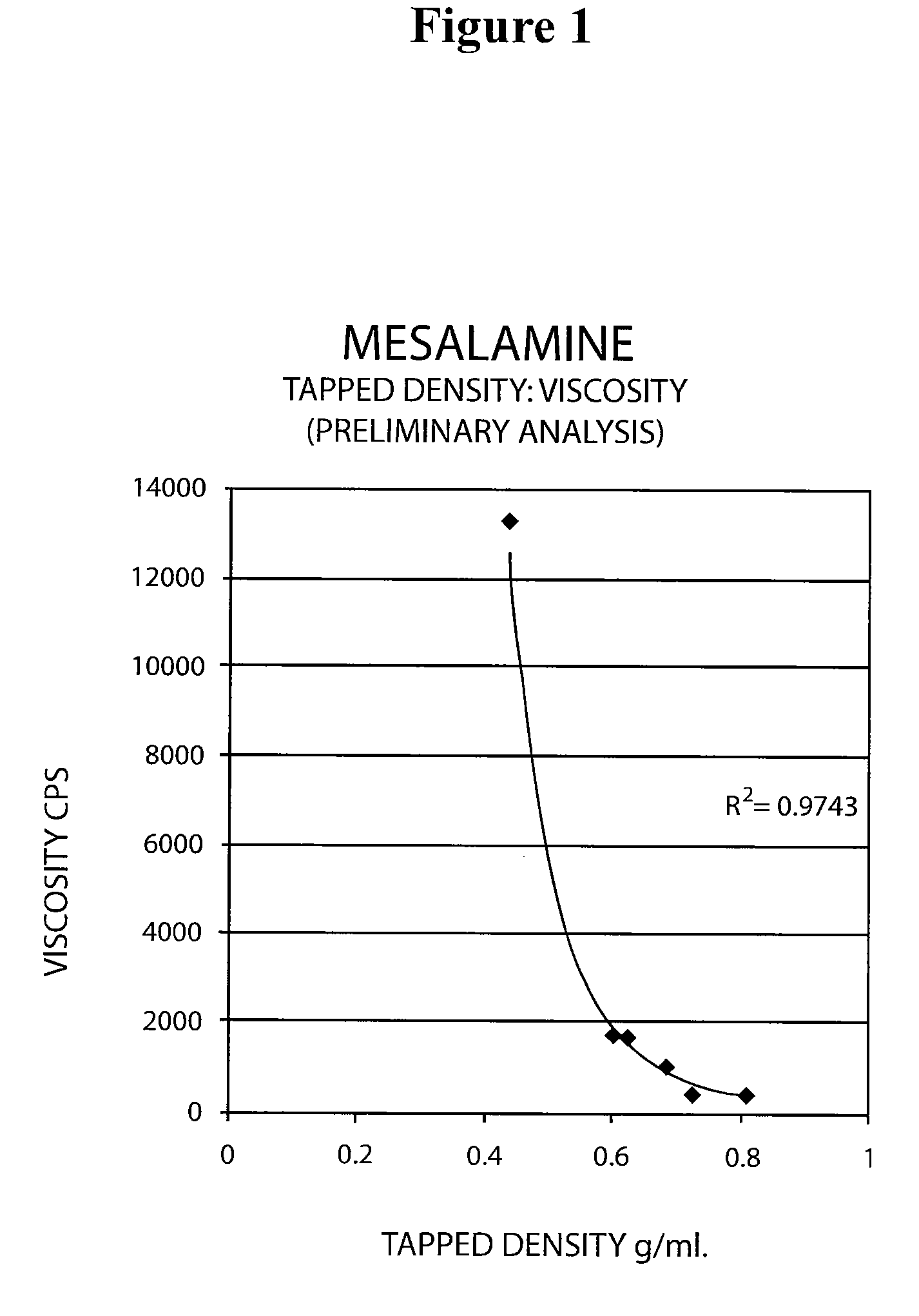
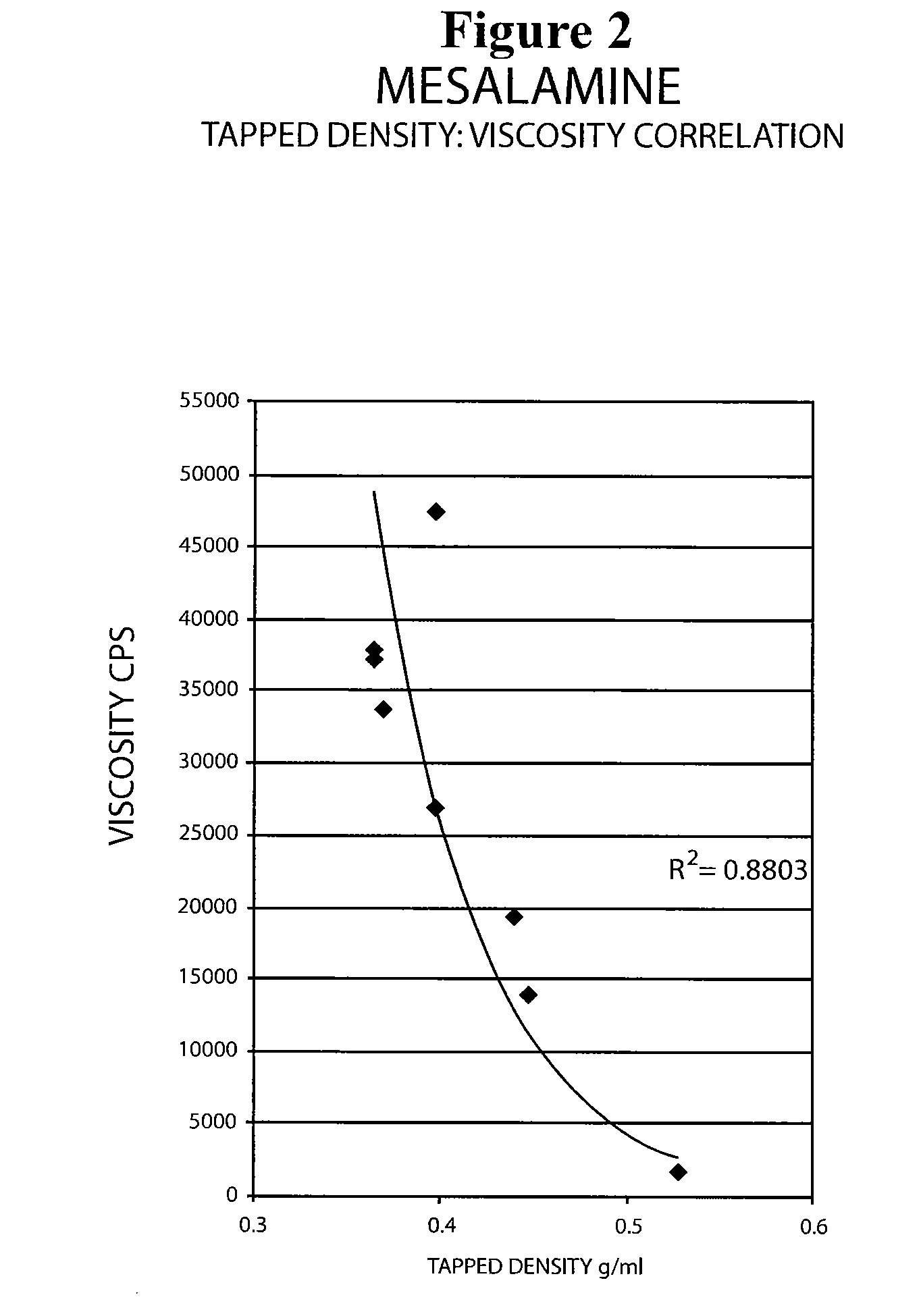
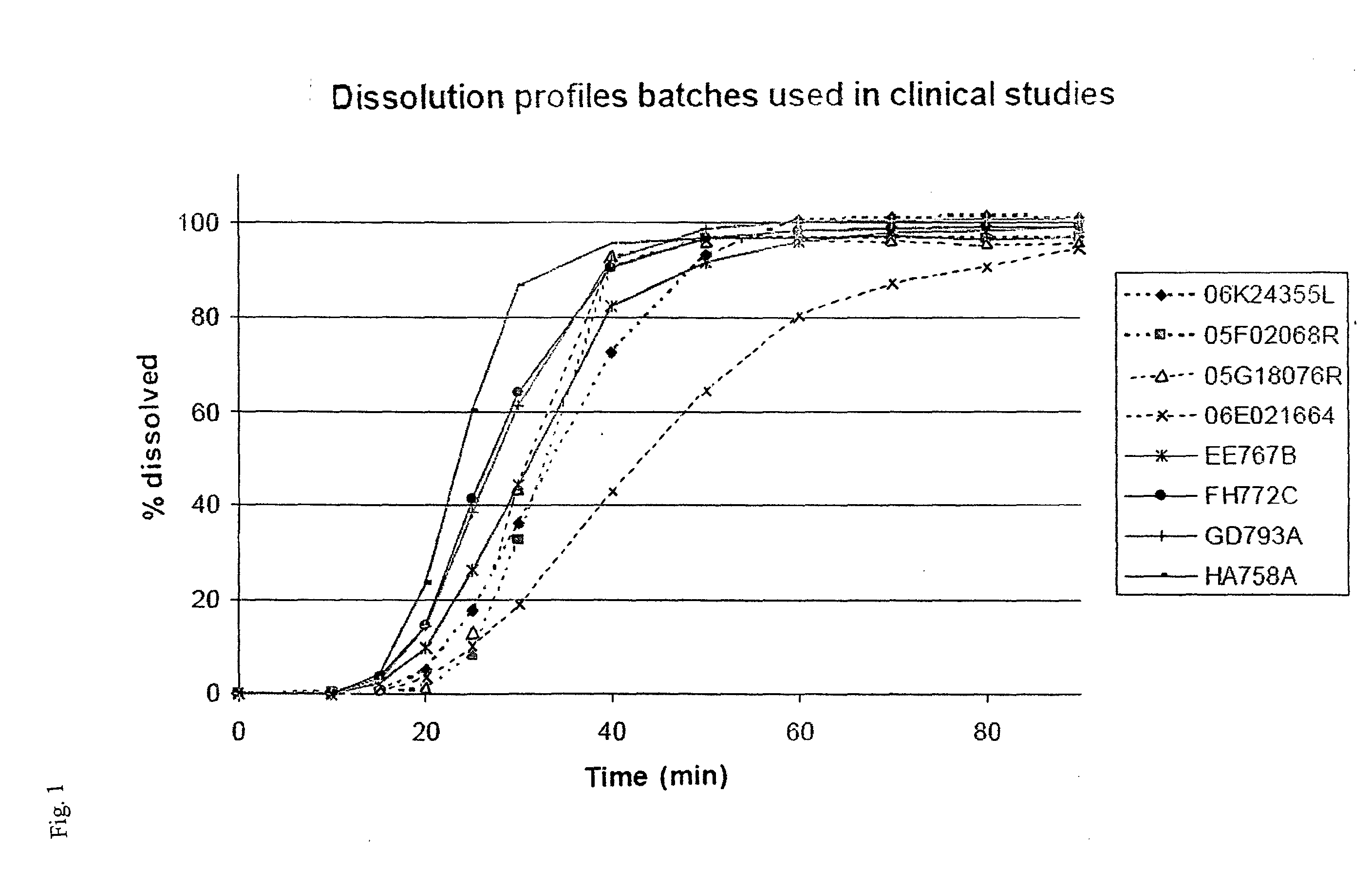
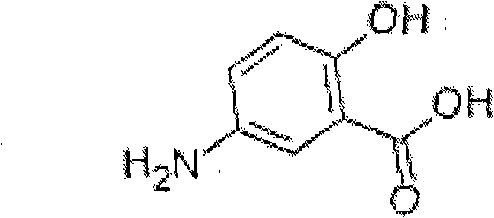
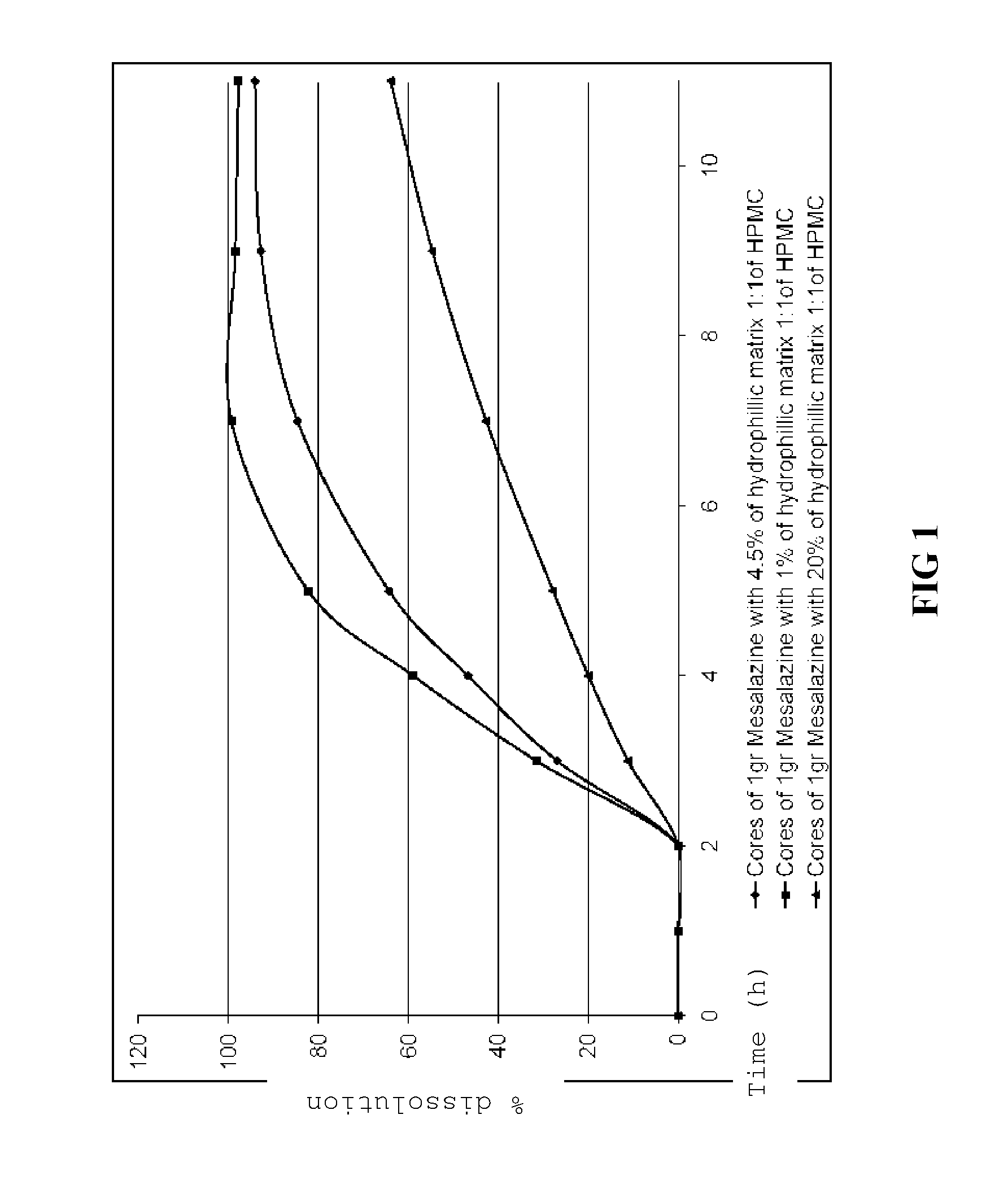
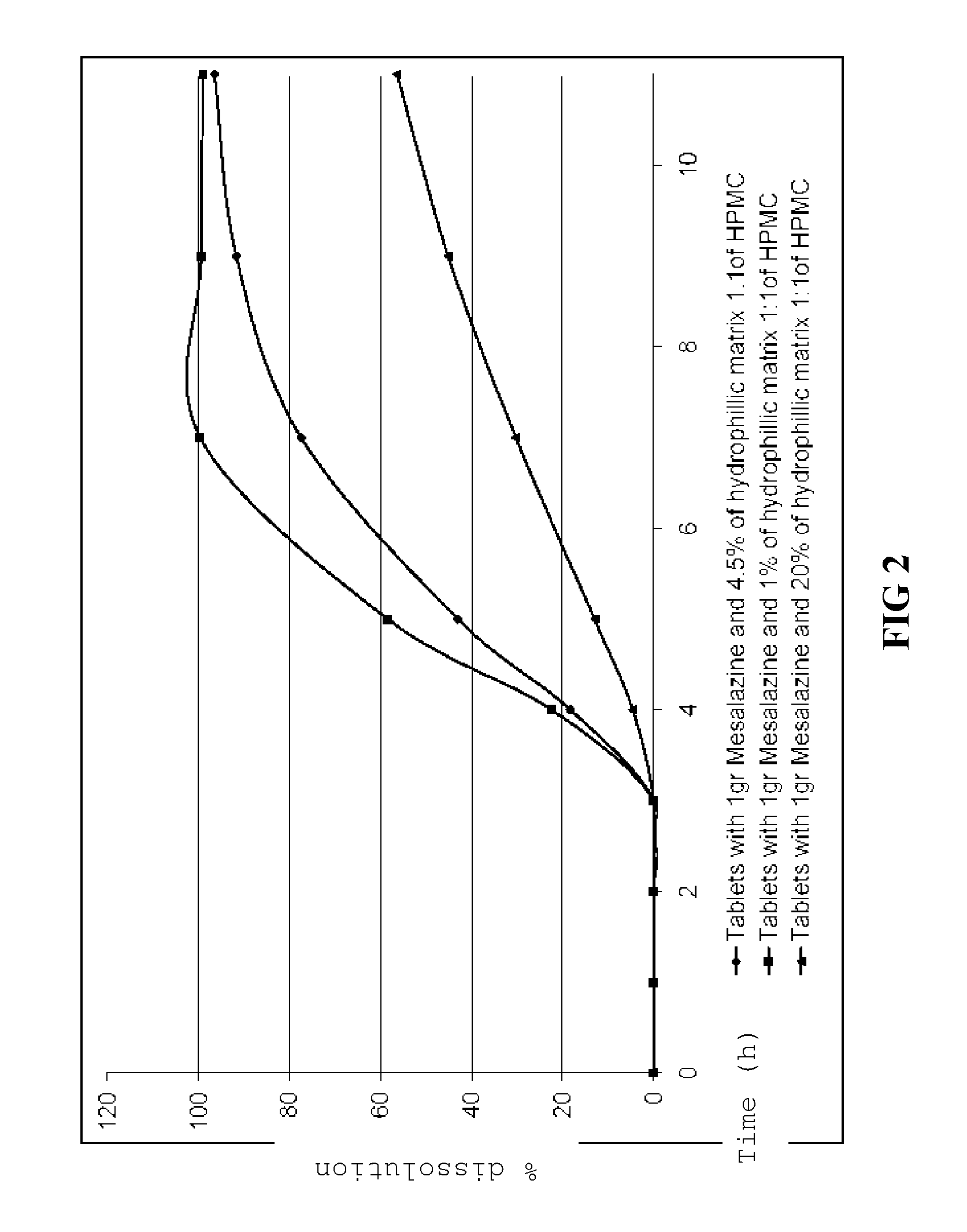
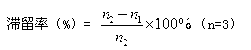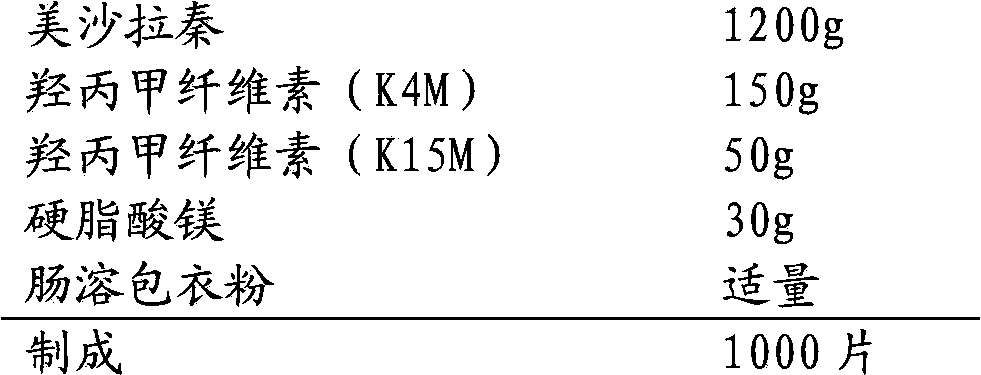Patents
Literature
114 results about "Mesalazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
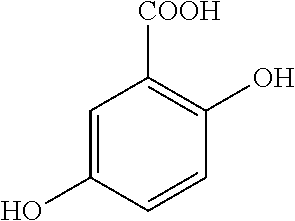
This medication is used to treat a certain bowel disease (ulcerative colitis).
Mesalazine tablet
InactiveUS20090162434A1Improve efficiencySmall and reduced variationBiocideUrinary disorderColorectal diseasePharmacology
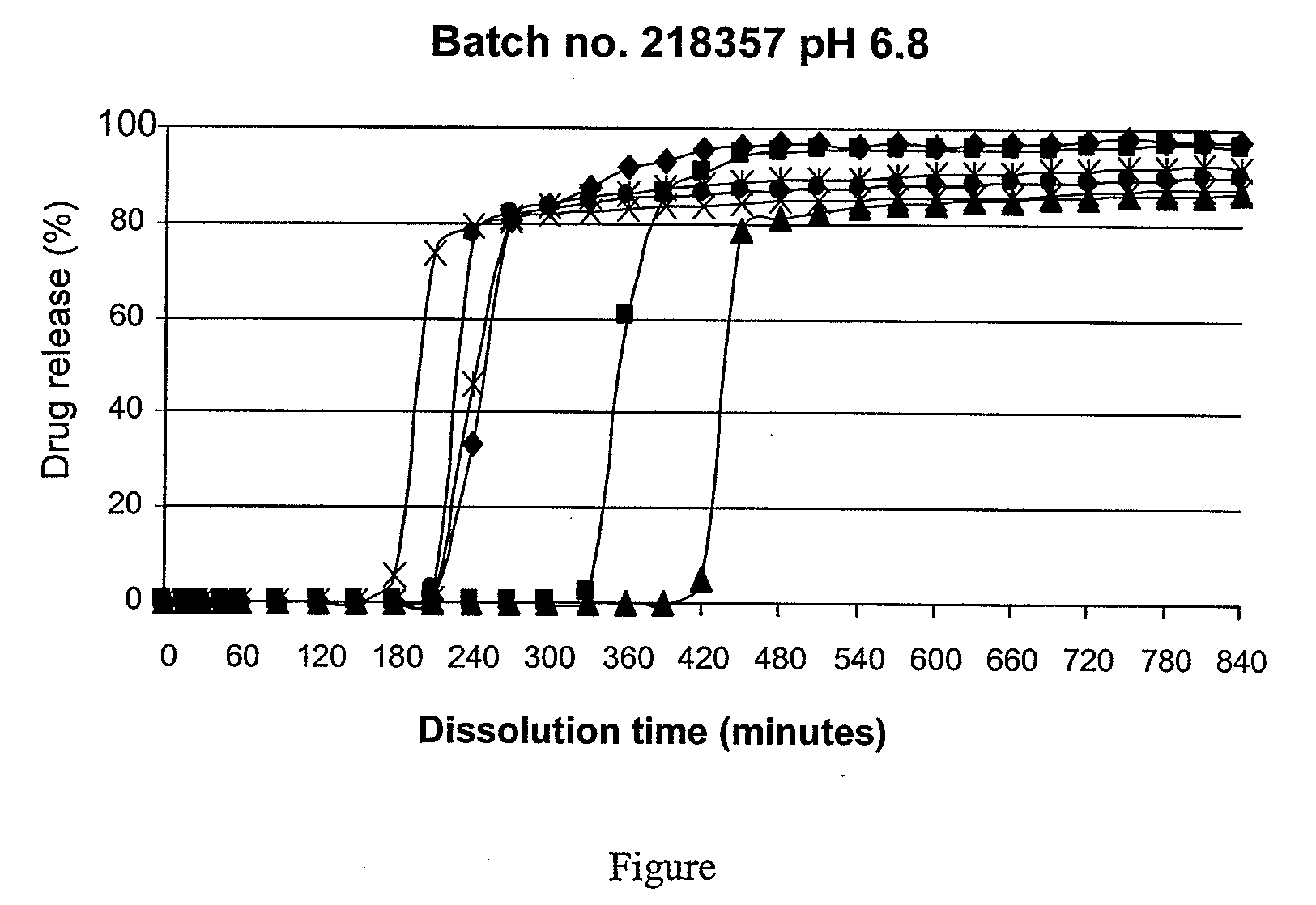
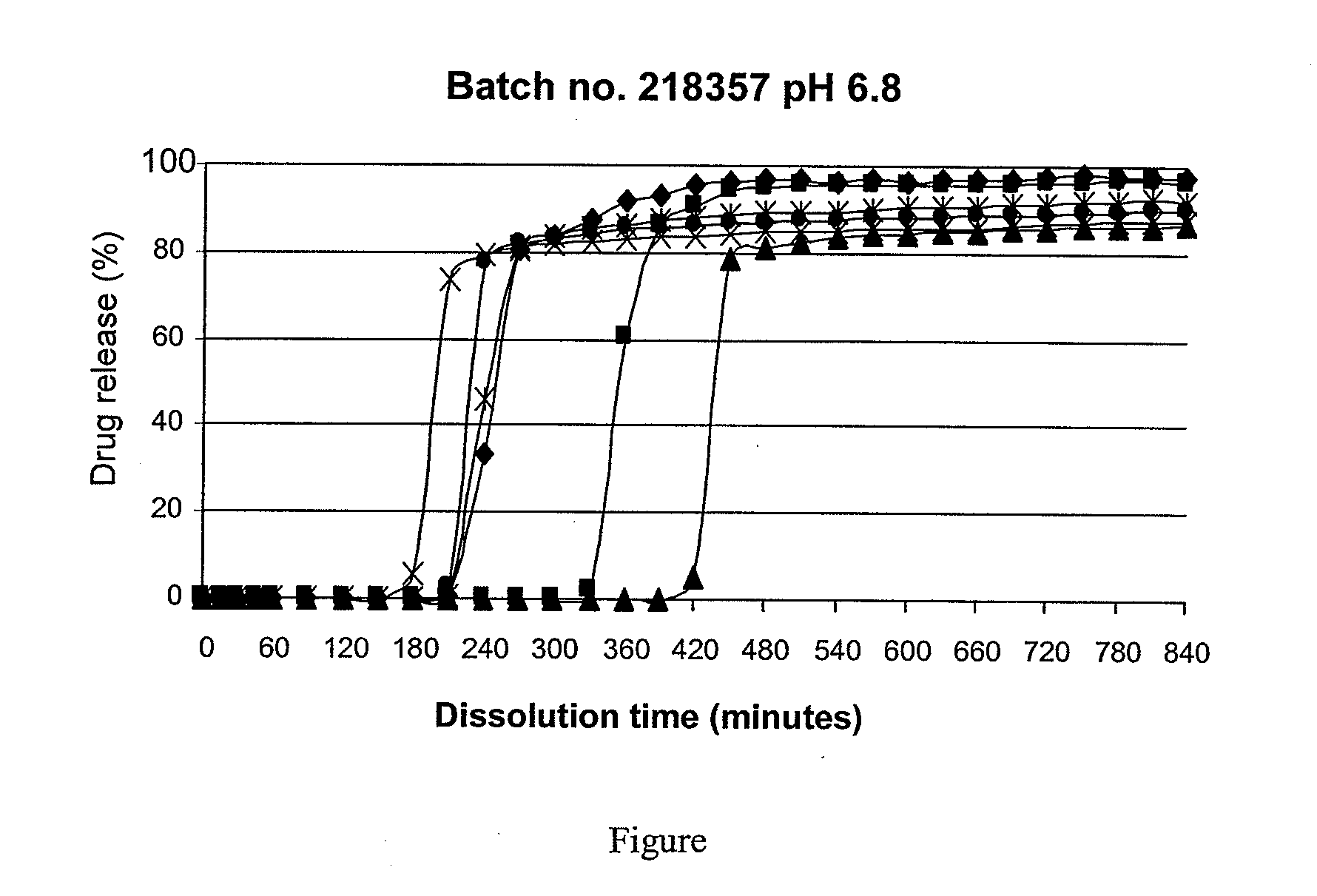
Disclosed are mesalazine tablets and a method for their preparation. The mesalazine tablets comprise a tablet core, a first coating layer, and a second coating layer. The tablet core comprises mesalazine; the first coating layer comprises a cellulose derivative and / or povidone, and the second coating layer comprises methacrylic acid / methyl methacrylate copolymer and an anti-tack agent. The tablets show a high degree of uniformity and reproducibility and release mesalazine starting at pH 6.8 and are used in the treatment of colorectal diseases.
Owner:DISPHAR INTERNATIONAL BV
Modified release formulations of Anti-irritability drugs
InactiveUS20090017110A1Provide flexibilityBiocideOrganic active ingredientsFda approvalAnti allergic drug
Modified or extended release formulations containing mesalamine compounds and associated methods are disclosed and described. In some aspects, such formulations may be substantially bioequivalent to known FDA approved mesalamine formulations such as PENTASA®.
Owner:CAPRICORN PHARMA INC
Mesalazine oral controlled release medicine composition
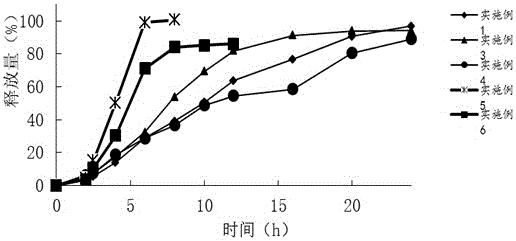
The invention relates to a mesalazine oral colon-targeted sustained release medicine composition which is characterized by containing: (a) a sustained release table core containing mesalazine or medicinal salts or solvates thereof and hydrophilic stroma, wherein the mesalazine or the medicine salts thereof are dispersed in the hydrophilic stroma; and (b) a coating wrapped outside the table core and containing acid-resistant materials; wherein the one-hour release of the composition in simulated intestinal fluid with the pH of 7.2 is less than 20 percent, the four-hour release thereof is 30-60 percent, and the eight-hour release thereof is greater than 70 percent. The composition has simple manufacturing process and low cost, slowly releases the mesalazine in small intestines and colons, and achieves the colon-targeted medicine delivery once a day and the local curative effect.
Owner:CHONGQING PHARMA RES INST
High drug load mesalazine sachet
ActiveUS20070043004A1Easy to managePatient compliance is goodBiocideAntipyreticCosmetic appearancePharmaceutical formulation
The present invention is directed to a high drug formulation having desirable properties in terms of ease of manufacture and visual appearance as well as a sachet for the formulation.
Owner:FERRING BV
Modified release formulations of anti-irritability drugs
Modified or extended release formulations containing mesalamine compounds and associated methods are disclosed and described. In some aspects, such formulations may be substantially bioequivalent to known FDA approved mesalamine formulations such as PENTASA®.
Owner:CAPRICORN PHARMA INC
Mesalazine enteric sustained-release tablet preparation method
InactiveCN108159012ASolve the preparation processLow densityOrganic active ingredientsDigestive systemSustained Release TabletTableting
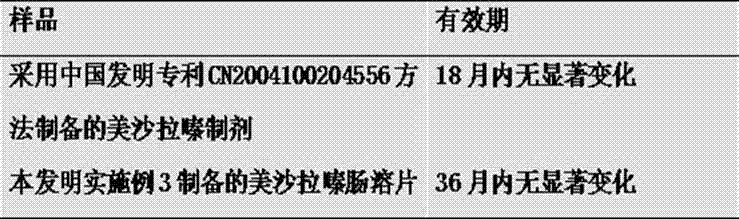
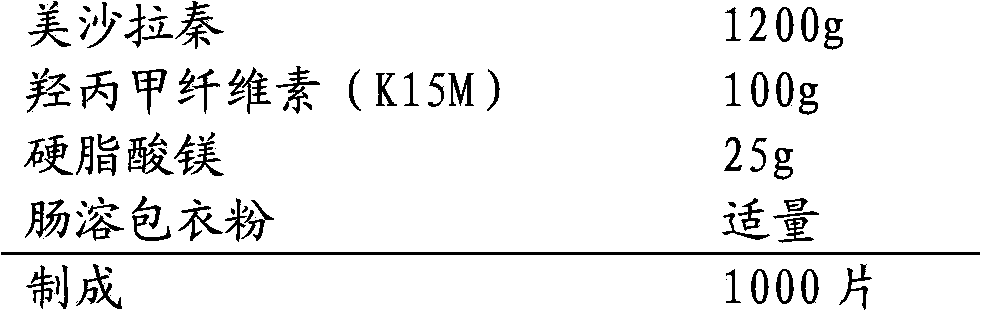
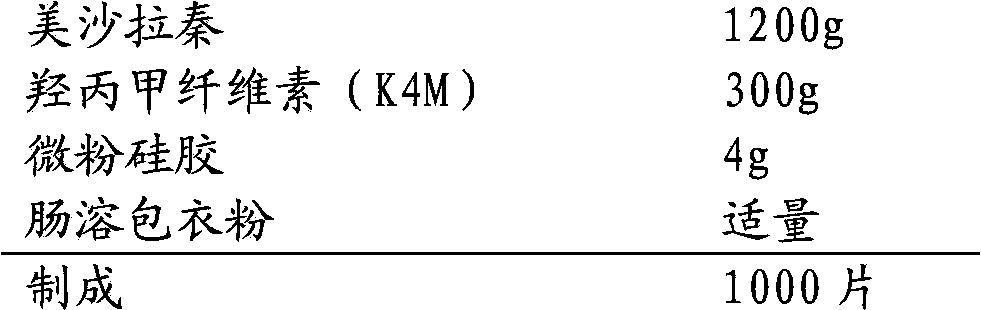
The invention relates to a mesalazine enteric sustained-release tablet preparation method, and belongs to the technical field of medicine. According to the present invention, a mesalazine enteric sustained-release tablet core is prepared by using wet granulation and tableting, and the mesalazine enteric sustained-release tablet is prepared through enteric coating, wherein each tablet contains 1.2g of mesalazine.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Oral mesalazine colon-specific adhesive pellet
InactiveCN103211780AHas absorptionBioadhesiveOrganic active ingredientsDigestive systemTolerabilityPatient compliance
The invention discloses an oral mesalazine colon-specific adhesive preparation with a high drug-loading rate, which can be used for effectively treating ulcerative colitis and segmental ileitis, as well as a preparation method of the oral mesalazine colon-specific adhesive preparation. The preparation is composed of a pill-containing core and an enteric coating layer, wherein the pill-containing core consists of mesalazine, excipient, adhesive and boning agent. Compared with the other oral preparations, the oral mesalazine colon-specific adhesive pellet has the effects of improving the curative effect and lowering adverse reactions when the dosage is the same. Compared with enema and suppository, the pellet has better tolerance. The preparation method can be used for effectively controlling the temperature in the production process and avoiding an overheating phenomenon in the preparation process, and is simple in preparation process and high in drug-loading rate. Besides, the oral mesalazine colon-specific adhesive pellet is applicable to the large-dose dosing characteristic of mesalazine, thereby reducing the medicine taking inconvenience of a patient and improving the dependence of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
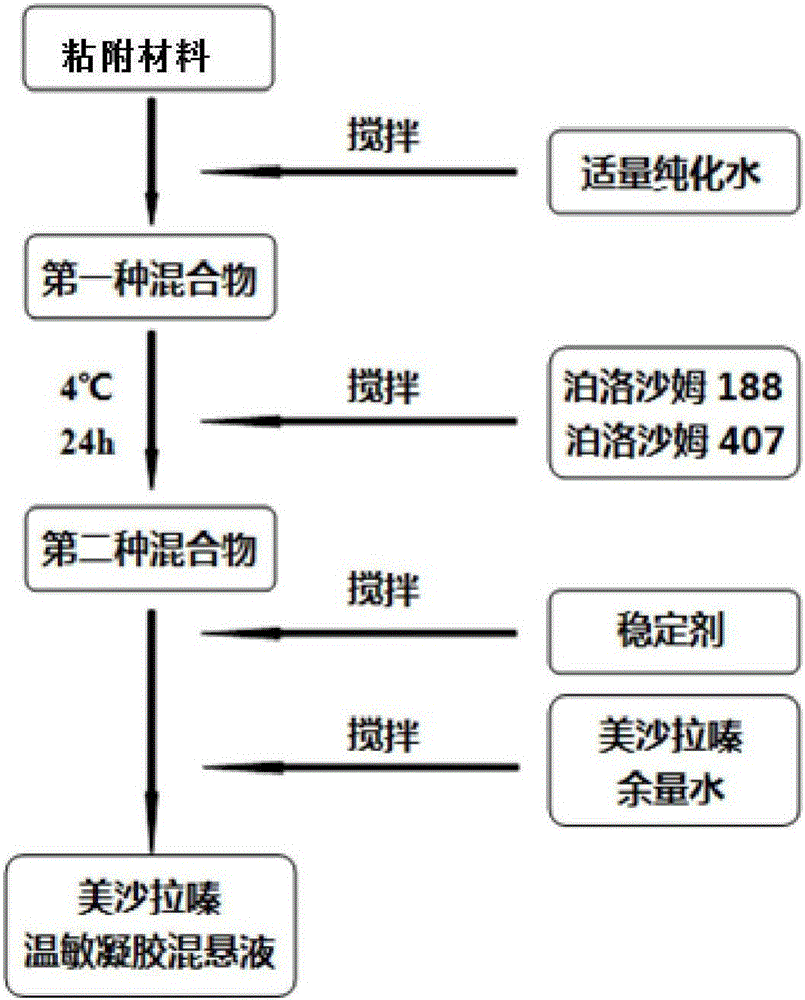
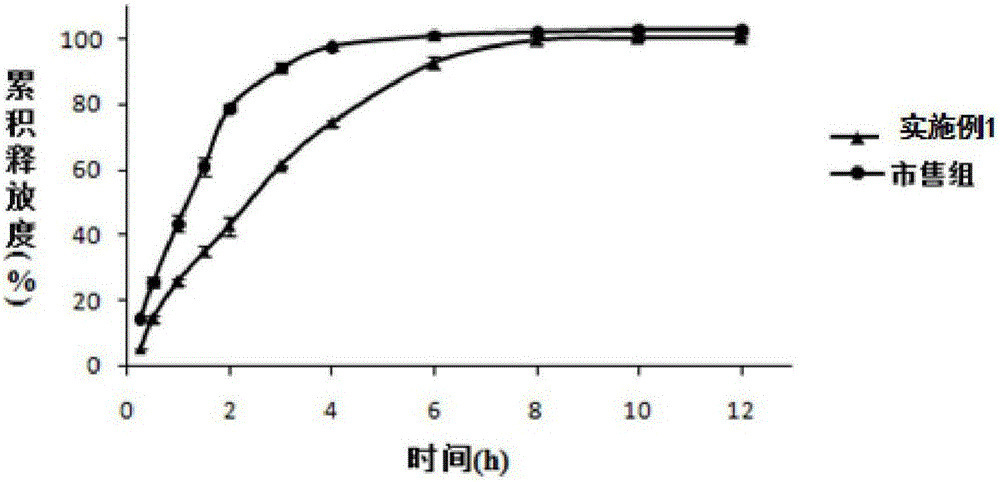
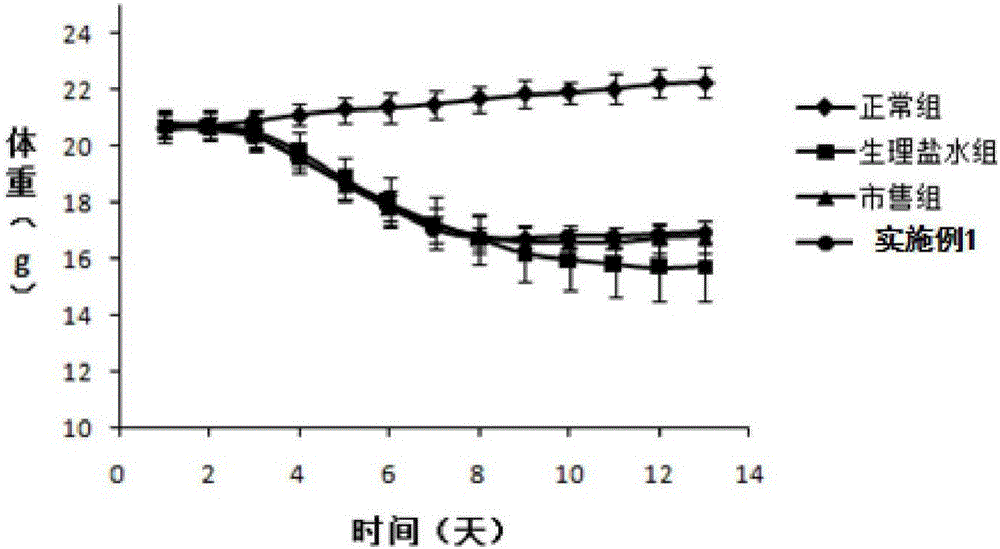
Mesalazine temperature-sensitive gel enema and preparation method thereof
InactiveCN105878177AGels quicklyExtended stayOrganic active ingredientsAerosol deliveryIrritationTemperature sensitive
The invention discloses a temperature-sensitive gel enema capable of prolonging the detention time of mesalazine in colons and a preparation method of the temperature-sensitive gel enema. The mesalazine temperature-sensitive gel enema is prepared from mesalazine as a main drug, temperature-sensitive gel substrate poloxamer, a bio-adhesive material, a stabilizer and an appropriate amount of water. The mesalazine temperature-sensitive gel enema for colons disclosed by the invention is a liquid freely flowing at a room temperature, is injected to the colons in a liquid form, and has the advantages of uniform drug distribution and good spreadability. Then, the mesalazine temperature-sensitive gel enema is subjected to phase transformation fast under a body temperature condition to form non-chemically-crosslinked semisolid gel, thereby prolonging the detention time of the drug in the colons in addition to the mucous membrane adhesion effect of the bio-adhesive material. Compared with a conventional mesalazine enema, the mesalazine temperature-sensitive gel enema disclosed by the invention can be used for effectively reducing drug liquid leakage and improving the curative effect, and has the advantages of simple preparation and low irritation.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Mesalazine sustained-release pellets, preparation method thereof and mesalazine sustained-release capsule
ActiveCN105456223ASimple prescriptionHigh drug loadingOrganic active ingredientsDigestive systemSustained release pelletsProcedure Agents
The invention provides mesalazine sustained-release pellets, a preparation method thereof and a mesalazine sustained-release capsule. The preparation method comprises the steps that 5-aminosalicylic acid, microcrystalline cellulose and a binding agent are mixed, and cores with pills are obtained through an extrusion rolling technology; materials comprising ethyecellulose are adopted, and cores with the pills are coated with sustained-release coating layers; the sustained-release coating layers are coated with enteric coating layers by the adoption of enteric materials and a processing aid, and mesalazine sustained-release pellets are obtained; the enteric materials are methacrylic acid and ethyl acrylate copolymer. PH dependent form and time dependent form drug release mechanisms are combined to achieve an ideal drug release curve, and the drug release position accuracy is improved. The mesalazine sustained-release capsule comprises the mesalazine sustained-release pellets, can be better released in the gastrointestinal tract and is beneficial to treatment on ulcerative colitis and Crohn's disease.
Owner:XINAN PHARMA
Mesalazine enteric-coated sustained-release pellet and preparation method thereof
The invention provides a mesalazine enteric-coated sustained-release pellet, which consists of a slow-release pellet core and an enteric-coated dressing. The slow-release pellet core contains 40-45 wt.% of mesalazine, 45-50 wt.% of a matrix sustained-release composite, 5-10% of a chaotropic agent and 0-5 wt.% of other additives; the matrix sustained-release composite consists of glyceryl behenate and microcrystalline cellulose in the weight ratio of 2-4:1. The invention also provides a preparation method of the mesalazine enteric-coated sustained-release pellet. The method provided by the invention employs a skeleton controlled-release technology to control drug release, reaches ideal drug release rate and good inter-batch reproducibility in preparation, does not have high requirement on equipment, and is in favor of ndustrial production.
Owner:广东暨大基因药物工程研究中心有限公司
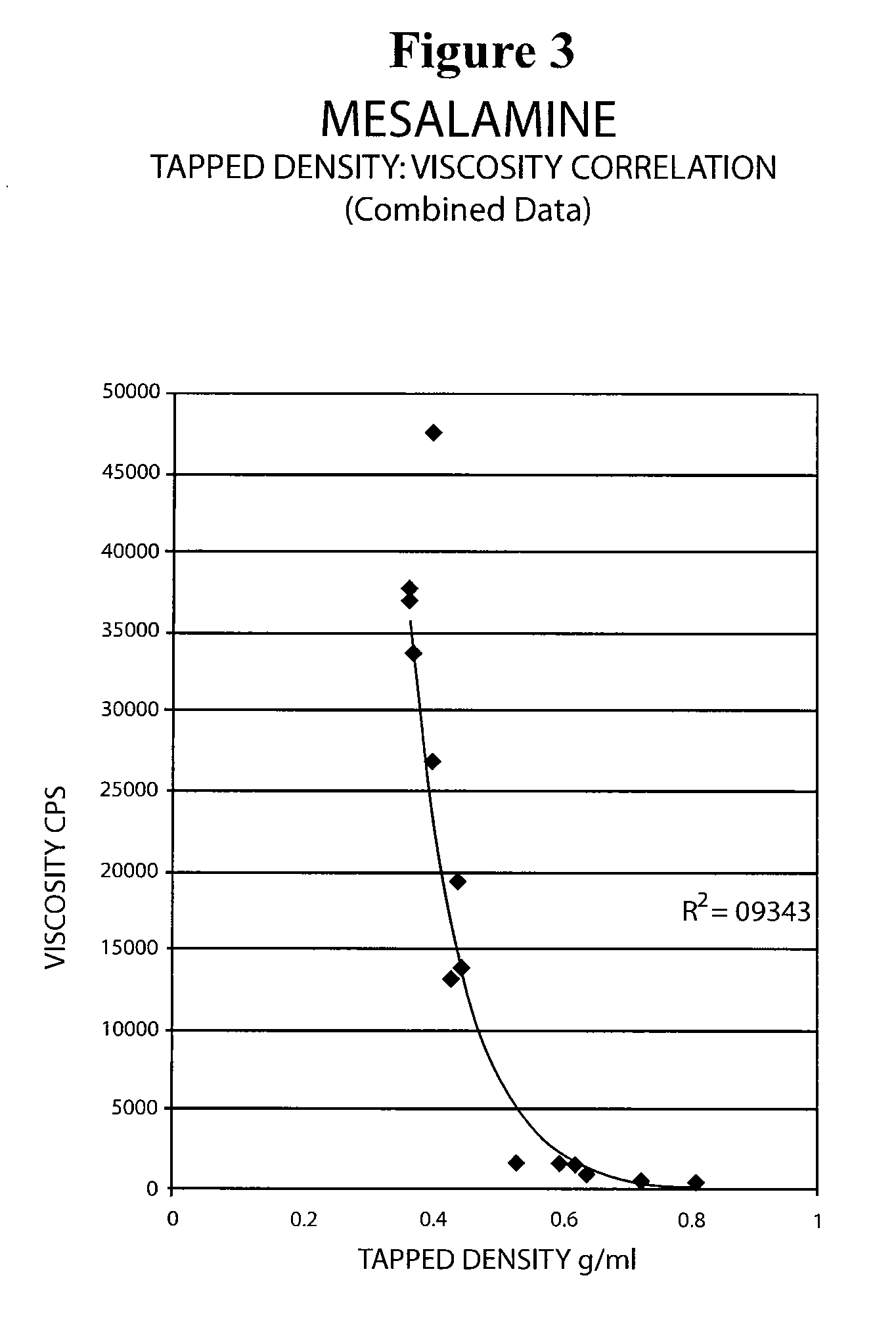
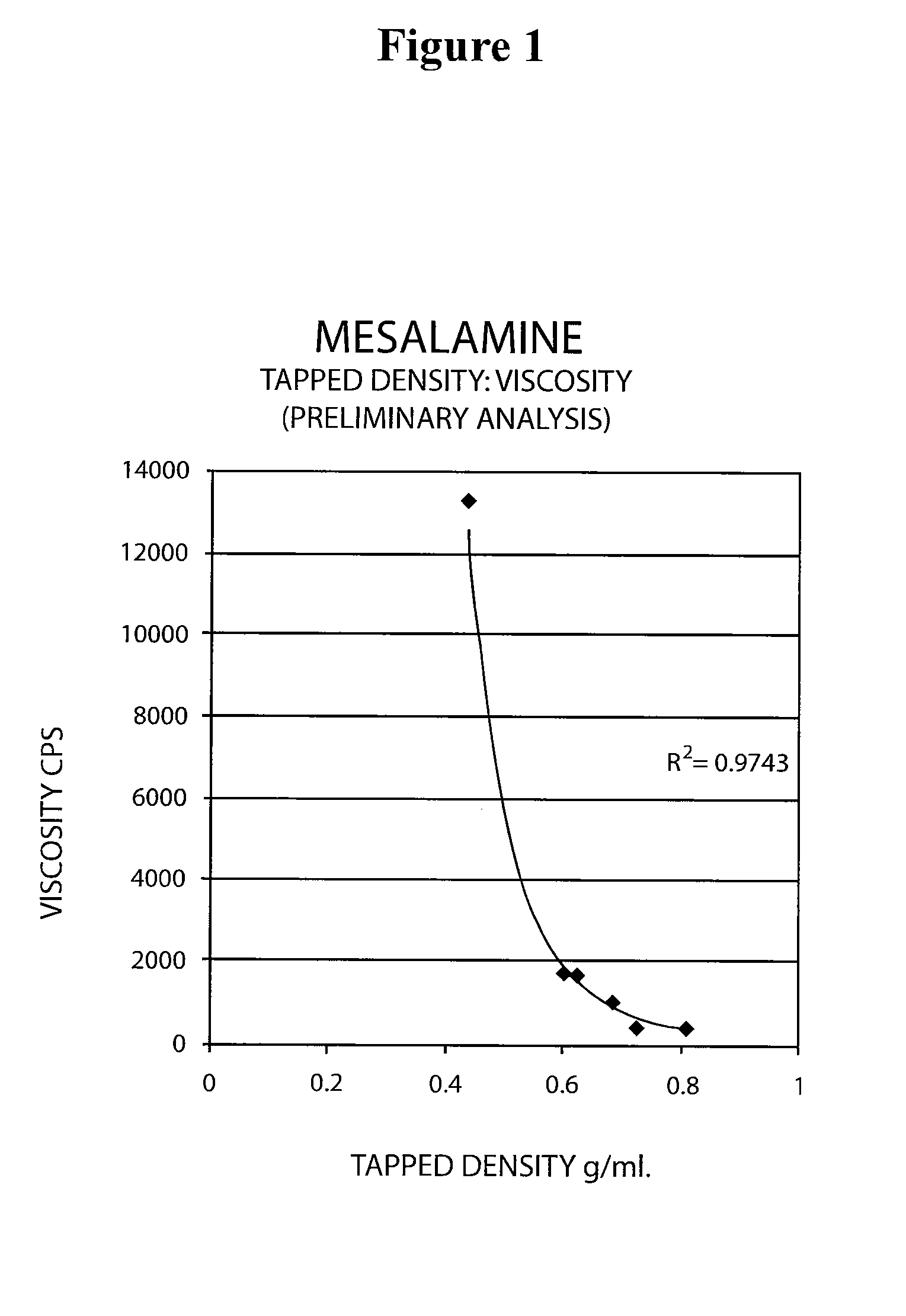
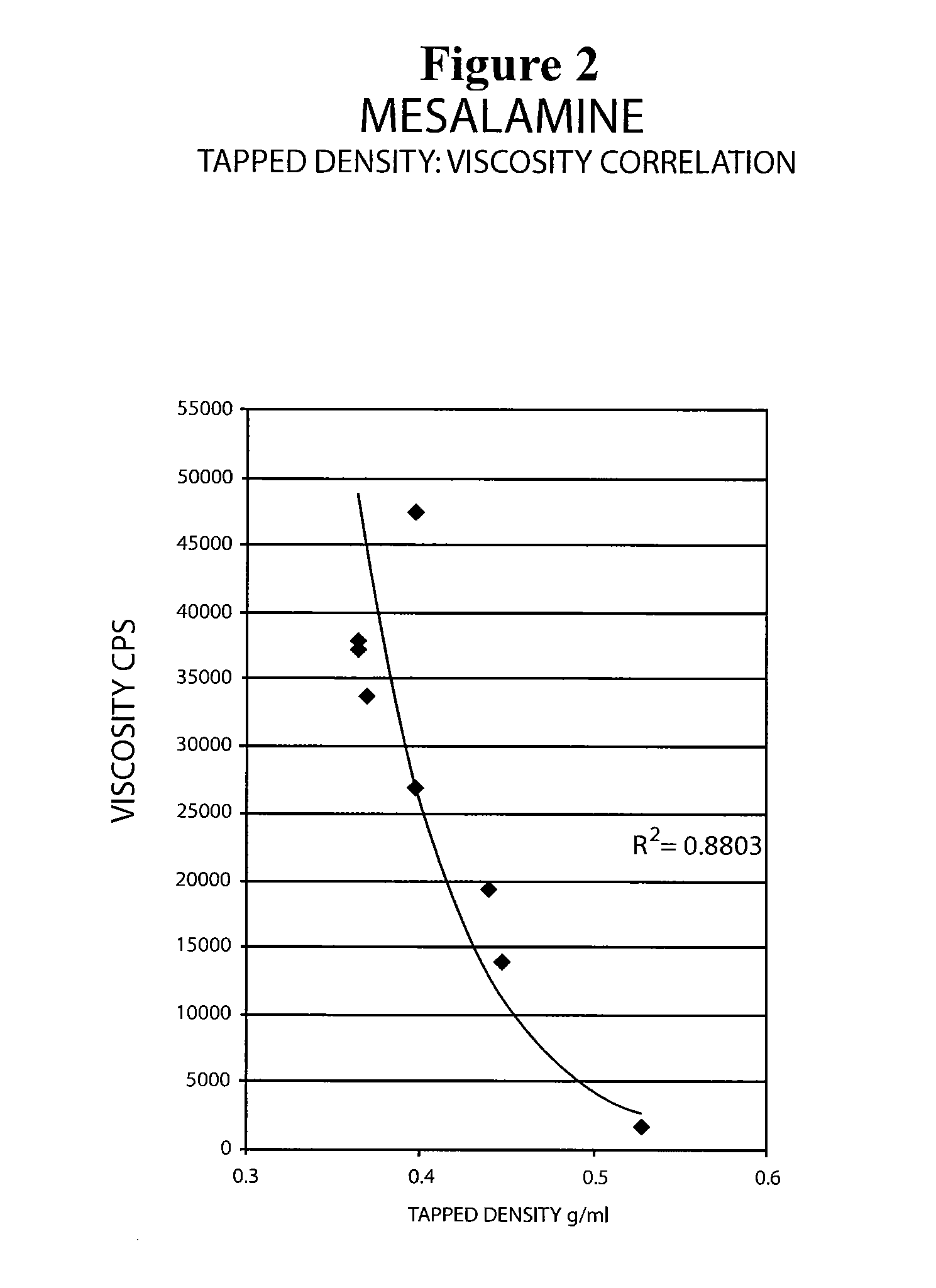
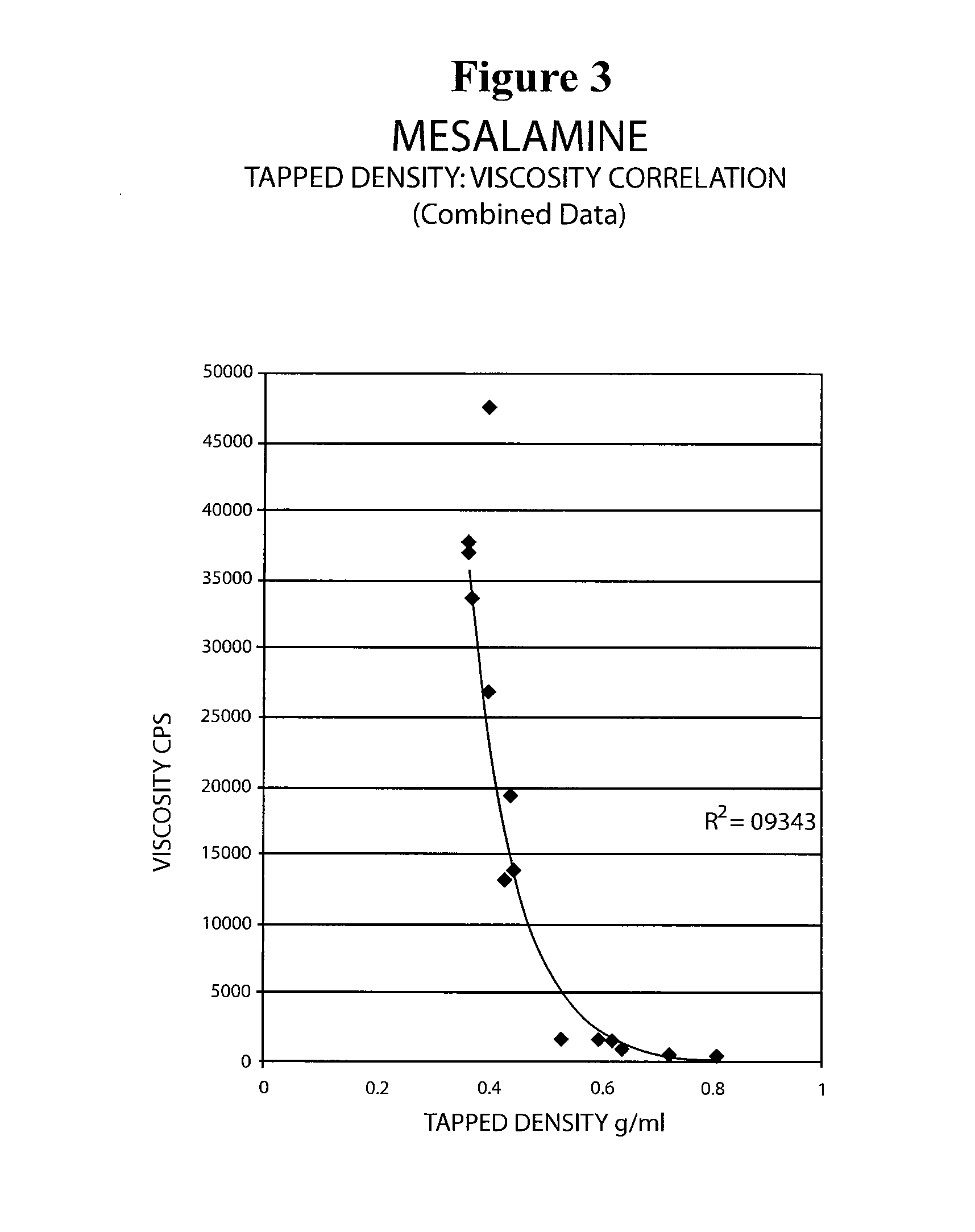
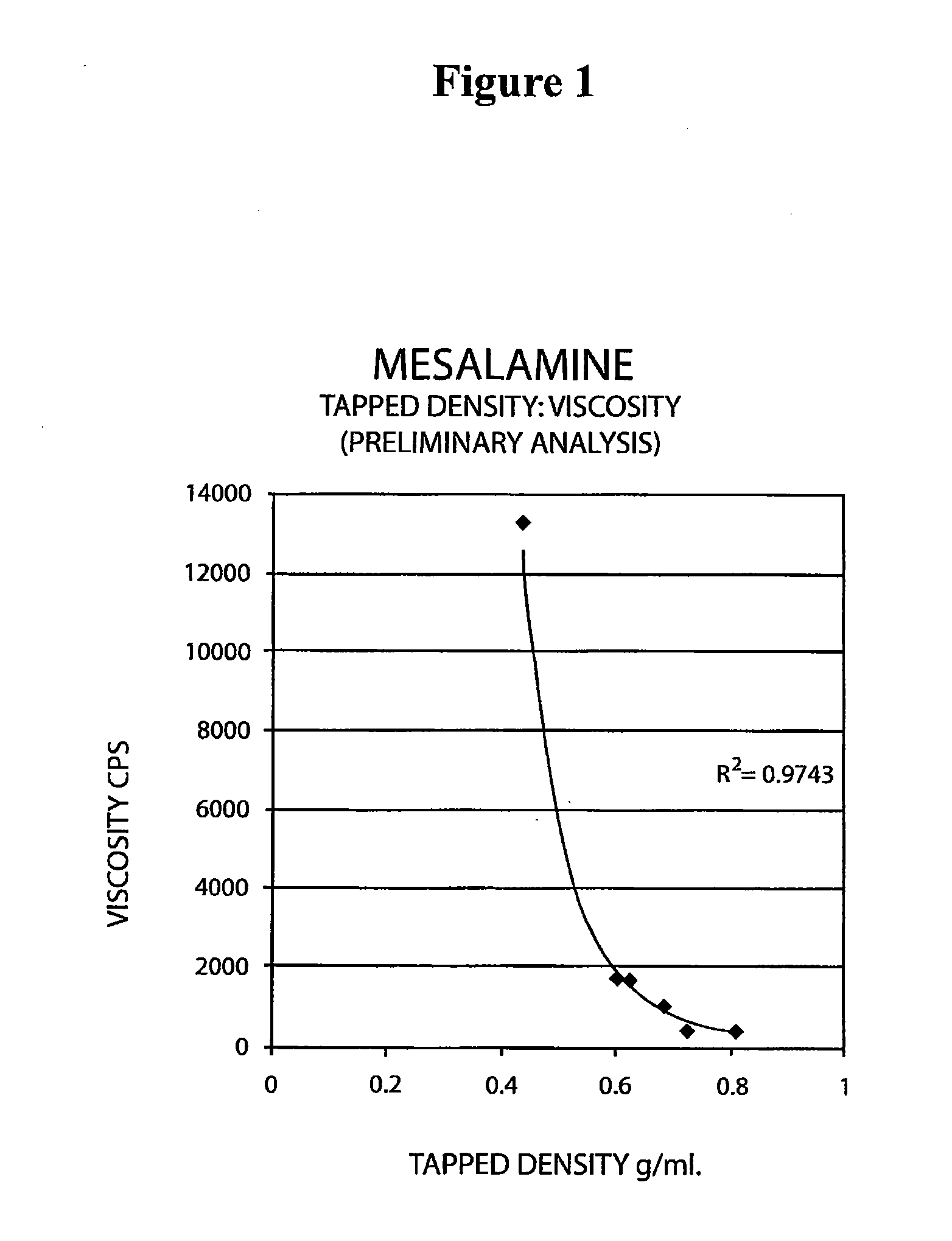
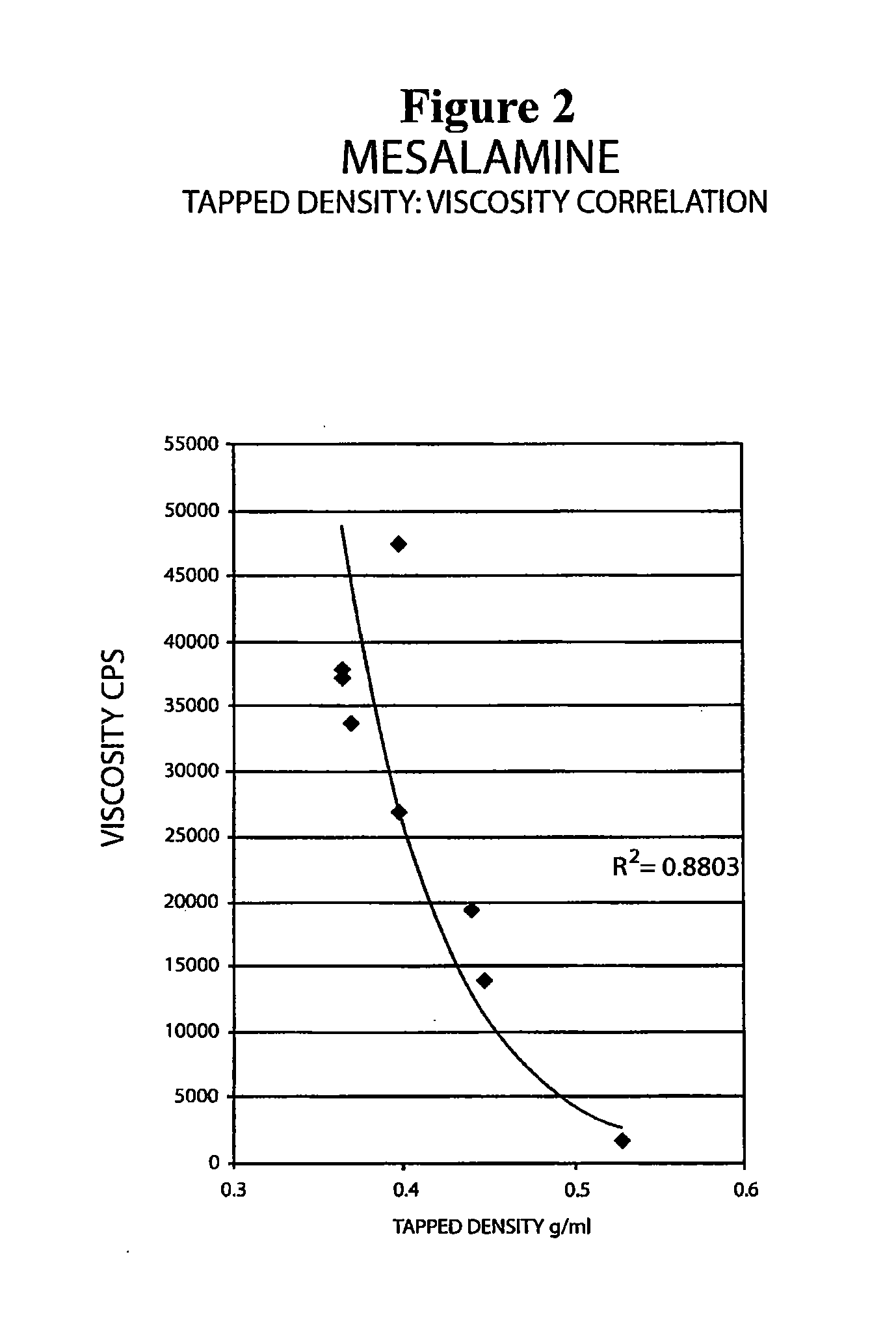
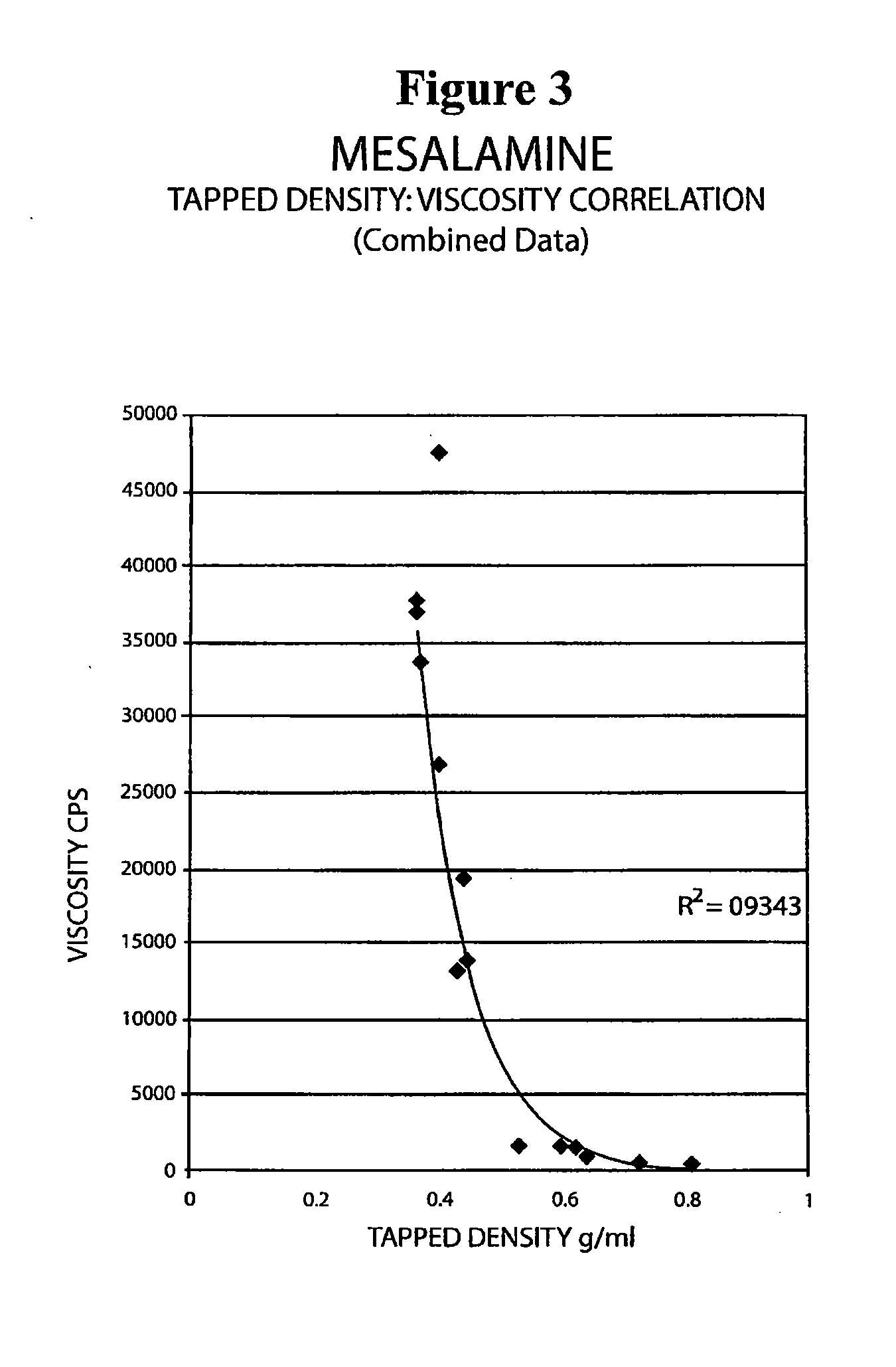
Mesalamine suppository
ActiveUS20090022793A1Improve the comfort of useHigh tap densityBiocideSalicyclic acid active ingredientsRectal SuppositoryPhosphate
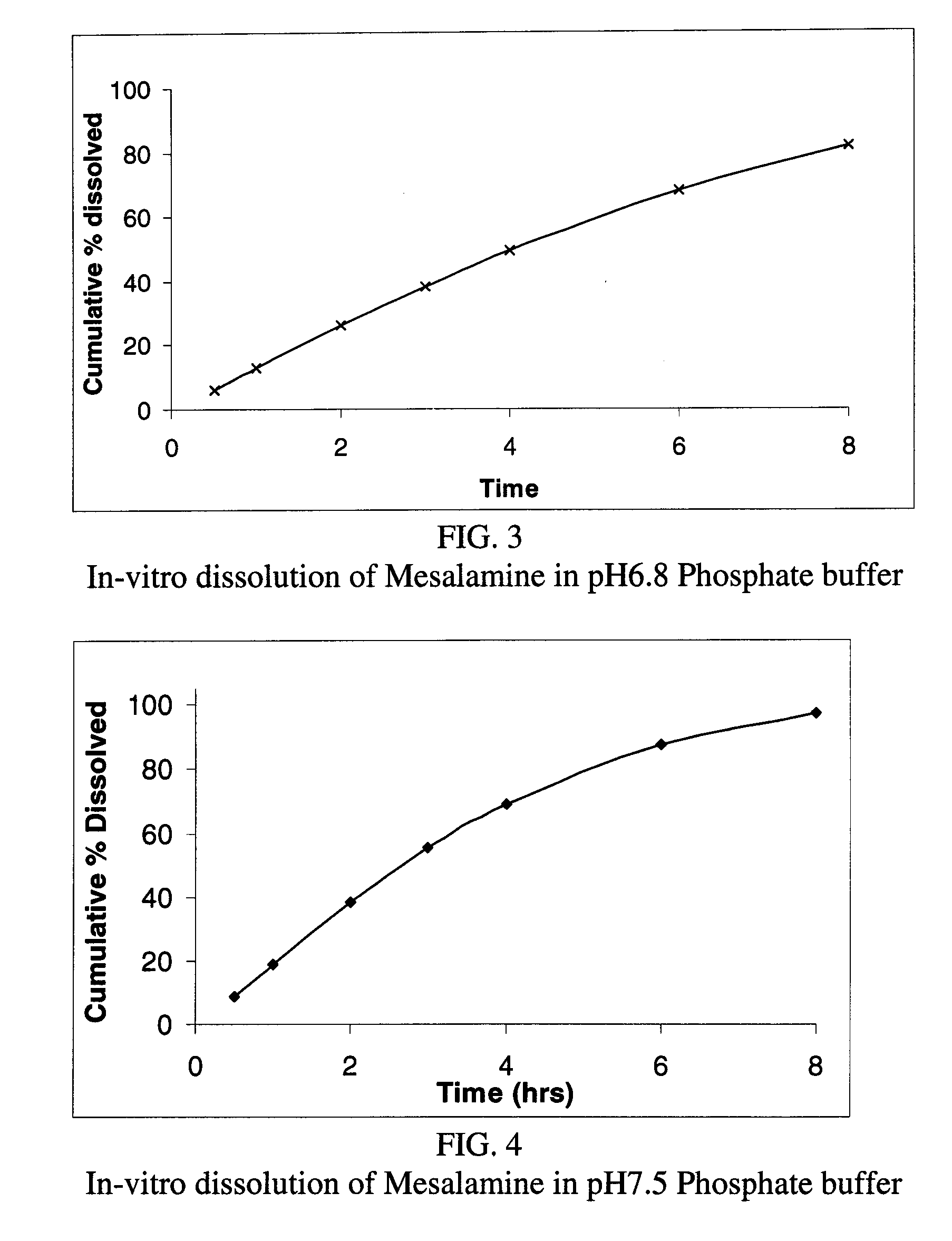
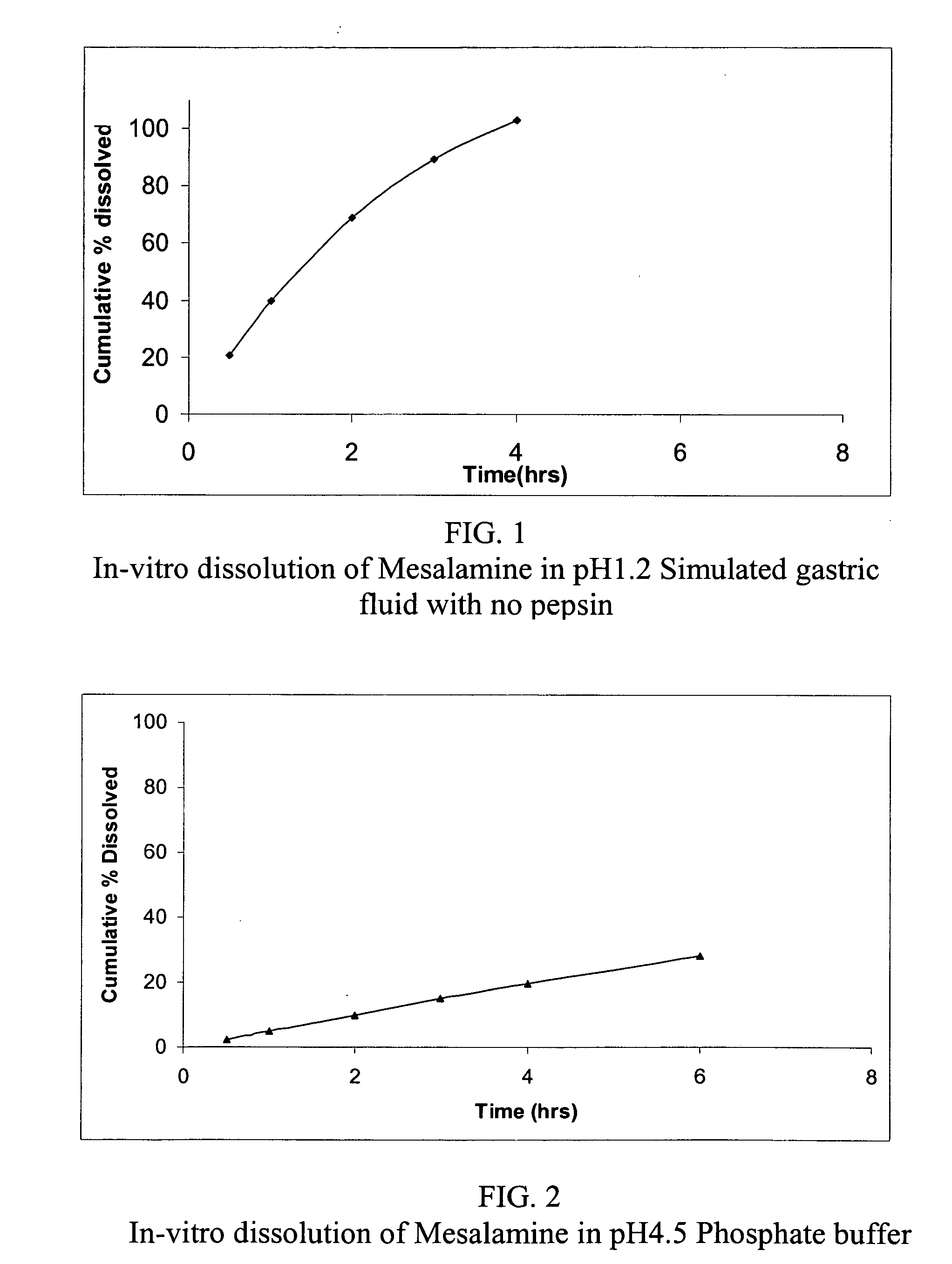
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 850 to about 1150 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 2250 to about 2700 mg. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:APTALIS PHARMA CANADA
Mesalamine suppository
ActiveUS20090264386A1Improve the comfort of useHigh tap densitySalicyclic acid active ingredientsBiocideRectal SuppositoryPhosphate
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 850 to about 1150 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 2250 to about 2700 mg. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 400 to about 600 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 870 to about 1715 mg. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:AXCAN PHARM CANADA INC
Mesalazine enteric coatel tablet and preparation method thereof
ActiveCN102784154AReduce lossesImprove stabilityOrganic active ingredientsAntipyreticCurative effectBuffer solution
The invention discloses a mesalazine enteric coatel tablet and a preparation method of the mesalazine enteric coatel tablet. The mesalazine enteric coatel tablet is characterized in that a nanotechnology, an enteric coating technology and a pellet tabletting technology are adopted to reduce the amount of release in the mesalazine enteric coatel tablet, increase the amount of release in a buffer solution, improve the curative effect, prolong the expiration date, guarantee the product quality, and obtain the promising result.
Owner:JIAMUSI LULING PHARMA CO LTD SUNFLOWER PHARMA GRP
Mesalazine enteric-coated sustained release tablet
InactiveCN103565766AImprove uniformityEasy to prepareOrganic active ingredientsDigestive systemSustained Release TabletIntestinal fluid
The invention discloses a mesalazine enteric-coated sustained release tablet. The mesalazine enteric-coated sustained release tablet is characterized by consisting of the following components in a unit dose: 1.2g of mesalazine, 0.1-0.3g of a sustained release material, 0.004.0.03g of a lubricant and 0.02-0.1g of enteric-coated powder. The release of the mesalazine enteric-coated sustained release tablet in one hour in simulated intestinal fluid of which the pH value is 7.2 is less than 30%, the release in four hours is not more than 70%, and the release in eight hours exceeds 80%.
Owner:四川健能制药有限公司
Production of mesalazine colon-specific drug administration sustained-release tablet
The invention discloses a production of a mesalazine colon-specific drug administration sustained-release tablet. The mesalazine (5-ASA) is widely applied clinically, and becomes a priority drug for treating inflammatory bowel diseases. The mesalazine is ineffective when being orally taken, because the mesalazine is quickly absorbed in the small intestine, then acetylated and discharged along with urine, thus being not able to reach the colon part. 5-ASA can only play the treatment function when reaches the colon lesion part in a prototype, and is produced into a pH-dependent colon-specific drug administration sustained-release tablet, so that the 5-ASA drug which reaches the colon has enough concentration to have an anti-inflammatory effect. Eudragit L100 and Eudragit S100 can be used to produce a film coating which meets the colon-specific release requirement according to a certain proportion. The mesalazine is produced into a coating sustained-release tablet, so that the 5-ASA drug which reaches the colon has enough concentration to achieve the anti-inflammatory effect.
Owner:JILIN INST OF CHEM TECH
Industrialized preparation method of mesalazine
InactiveCN107778189AHarm reductionAvoid Refining Difficult ProblemsOrganic compound preparationAmino-carboxyl compound preparationSolventSubstitution reaction
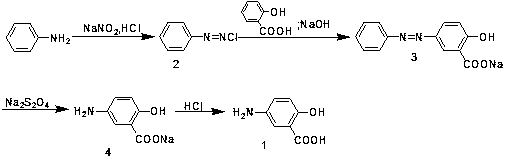
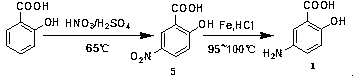
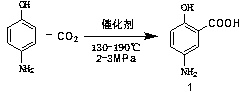
The invention discloses an industrialized preparation method of mesalazine. The preparation method is characterized by comprising the following steps: 1) taking 2-chlorine-5-nitrobenzoic acid (I) as astarting raw material, adding alkali, and performing substitution reaction at certain temperature to generate 2-hydroxy-5-nitrobenzoic acid (intermediate II); and 2) reducing nitryl by using raney nickel under certain pressure, at certain temperature and under the catalyst and alkaline conditions to prepare the finished product mesalazine. The process synthesis cost is low, the reaction conditionis mild, the whole process takes water as a solvent, a poisonous reagent is not used, the catalyst is recycled, and the whole process adopts green production; and the product prepared by the processhas high yield and high purity.
Owner:KAMP PHARMA
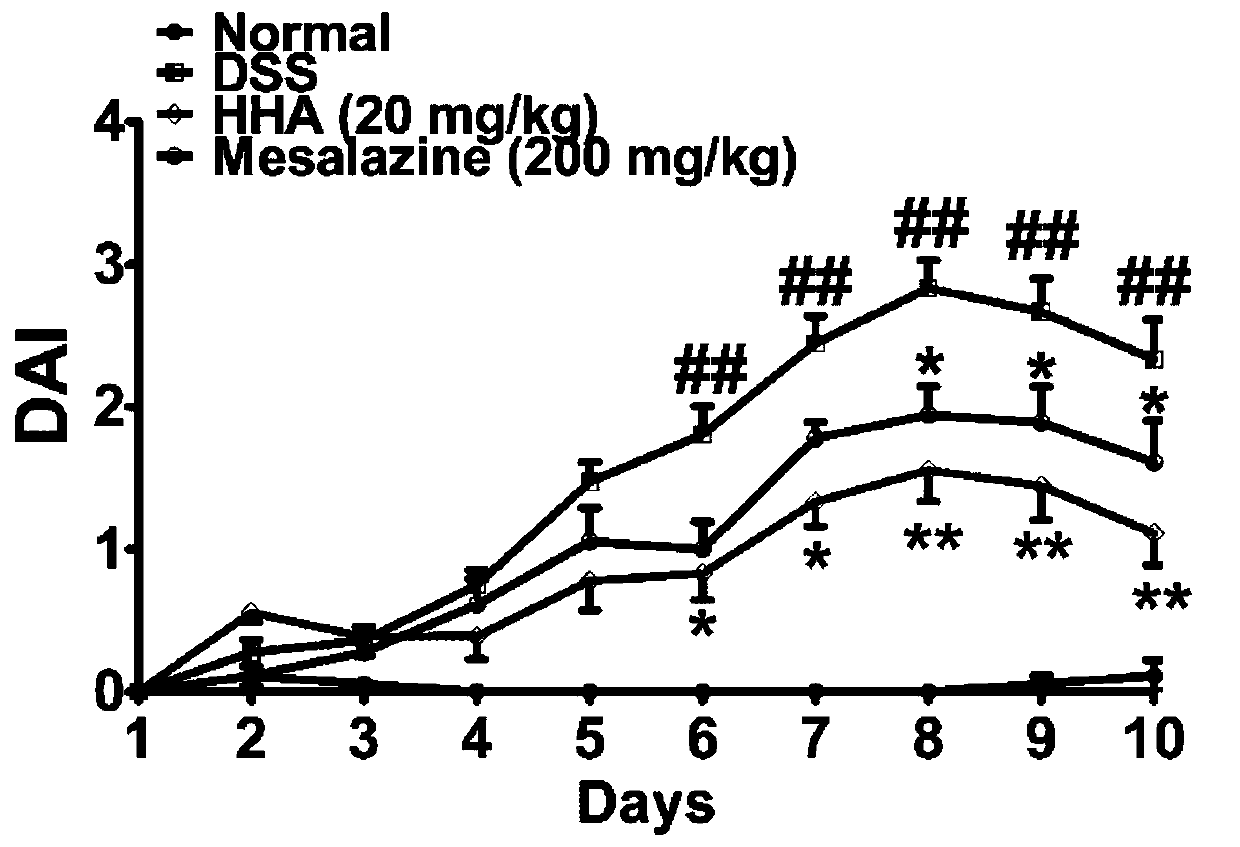
Application of p-hydroxybenzoic acid in preparing drug for treating inflammatory bowel disease
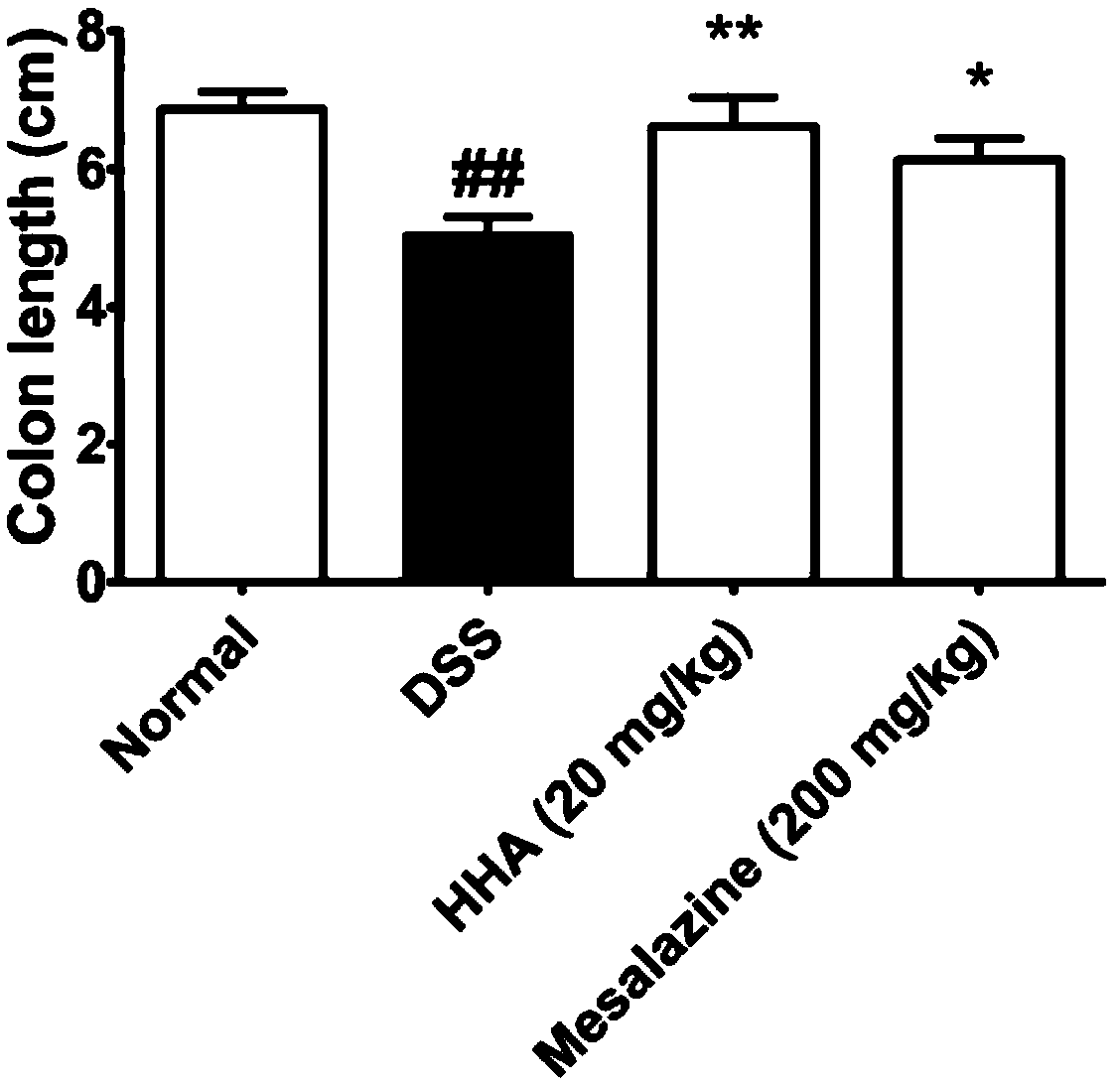
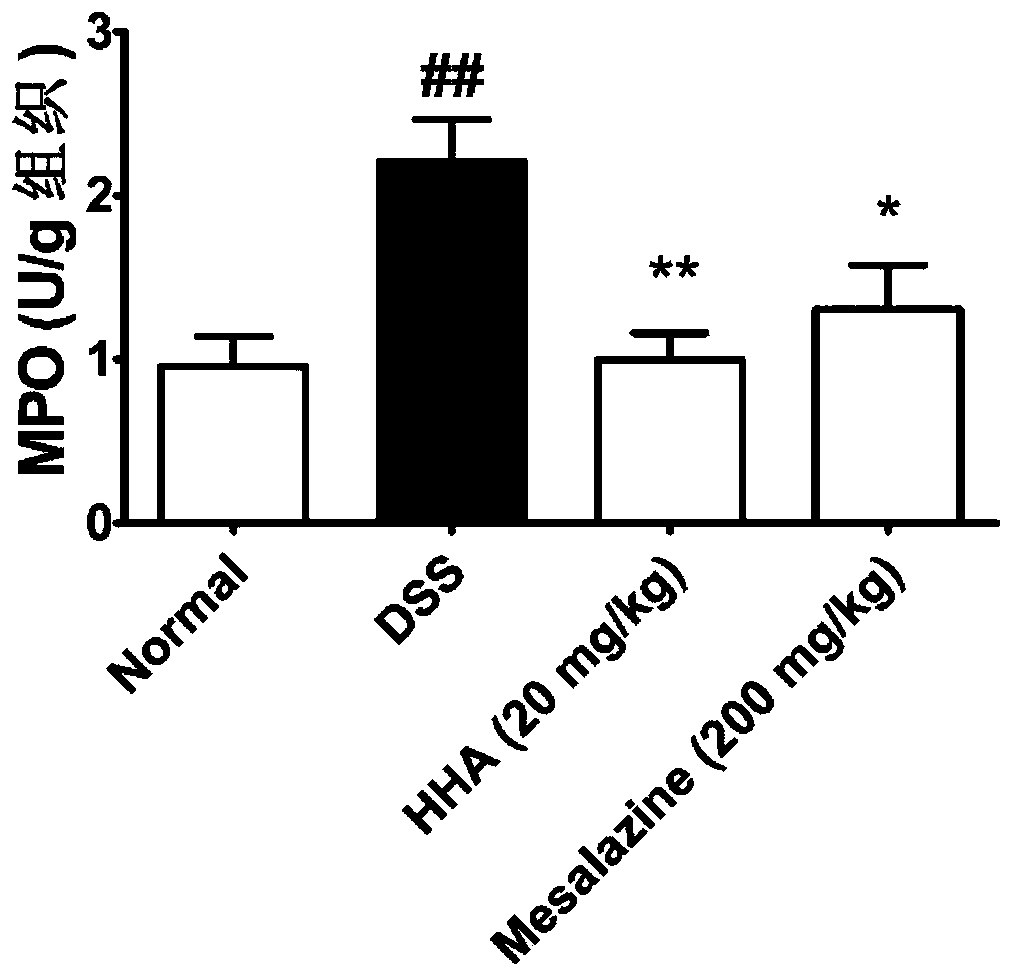
The invention discloses application of p-hydroxybenzoic acid in preparing a drug for treating an inflammatory bowel disease. At a dose significantly lower than mesalazine, the p-hydroxybenzoic acid can significantly inhibit the increase in disease activity index scores of enteritis model mice induced by dextran sodium sulfate, improve colon length shortening of the colitis model mice, reduce the colonic MPO activity of the mice, and reduce the inflammatory cell infiltration of colon tissues in the colitis model mice. According to the application, the p-hydroxybenzoic acid can alleviate the inflammatory reaction of the DSS-induced inflammatory bowel disease model mice and improve the severity of the inflammatory bowel disease, and can be used for preparing the drug or a health care productfor treating the inflammatory bowel disease.
Owner:GUILIN MEDICAL UNIVERSITY +1
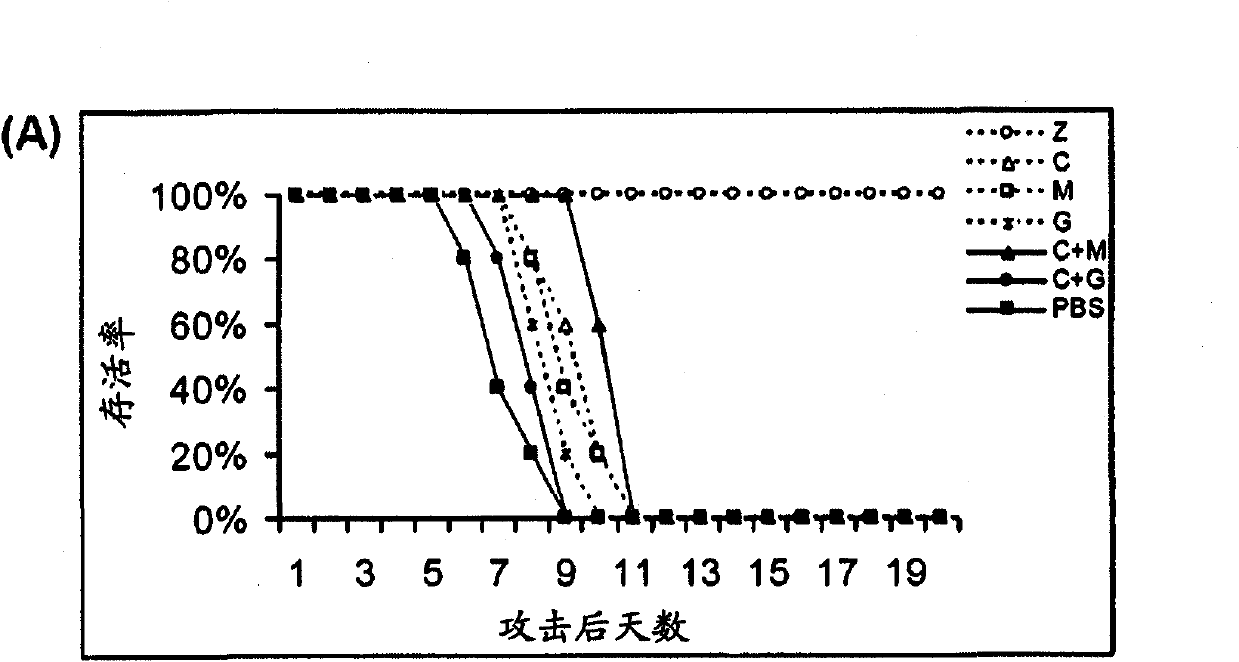
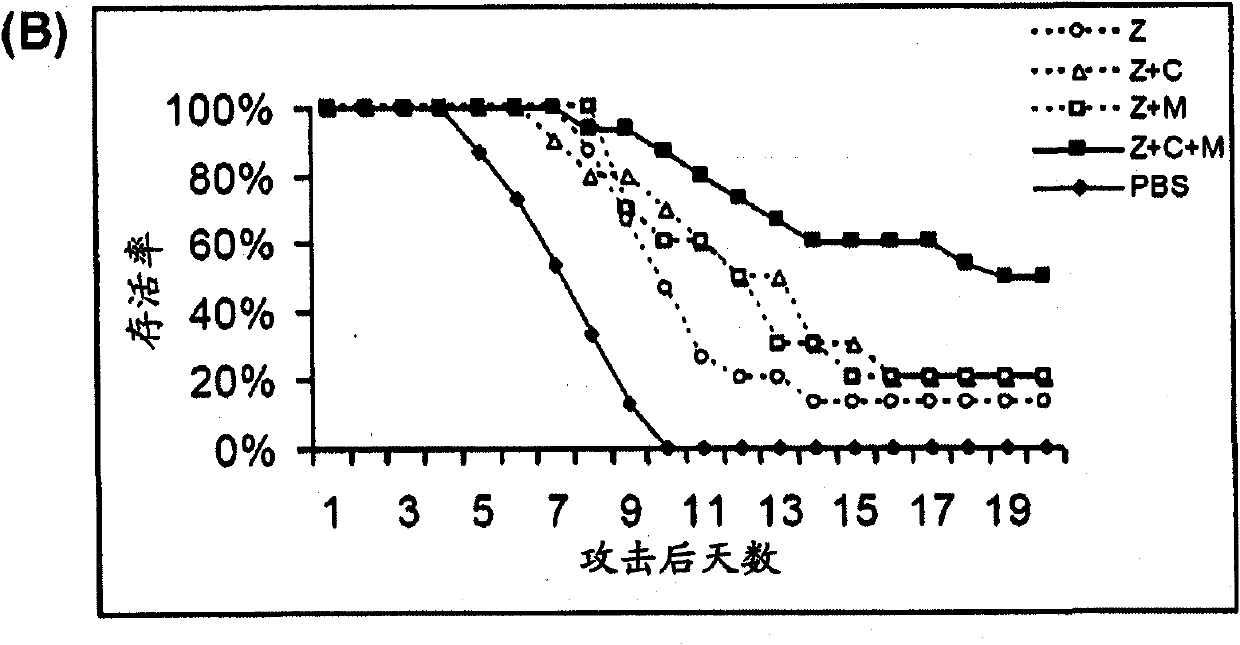
Combination therapy for the treatment of influenza
InactiveCN102036658AInhibit or reduce the effective amountOrganic active ingredientsAntiviralsInfected cellImmunomodulating Agent
Compositions and methods for treating one or more symptoms of influenza, preferably influenza due to infection with influenza A (H5N1) are provided. It has been discovered that administration of a combination of a neuraminidase inhibitor with two immunomodulators increases survivability in subjects 24, 48, or even 72 hours post infection compared to administration of the neuraminidase inhibitor alone. A preferred neuraminidase inhibitor includes, but is not limited to zanamivir. Preferred immunomodulators include, but are not limited to celecoxib and mesalazine. Another embodiment provides a method for treating influenza, preferably, influenza due to infection with avian influenza A (H5N1) by administering to subject infected with the influenza virus, an effective amount of a neuraminidase inhibitor to inhibit or reduce budding of the influenza virus from infected cells of the subject, and an effective amount of at least two immunomodulators effective to reduce or inhibit one or more symptoms of inflammation in the subject.
Owner:THE UNIVERSITY OF HONG KONG
Method of treatment for inflammatory bowel disease
The present invention provides methods of treating inflammatory bowel disease comprising administering to a subject in which remission has not been achieved after a first treatment of about 4-8 weeks of 5-ASA (mesalamine or 5-ASA), a further daily dose greater than about 4 g of 5-ASA in controlled release form to achieve a favorable response to treatment.
Owner:SHIRE DEV
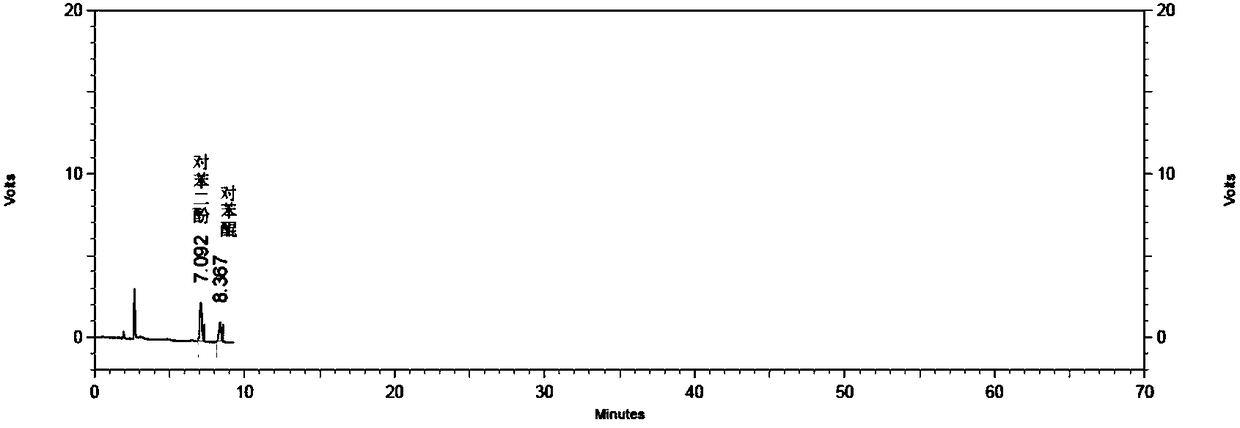
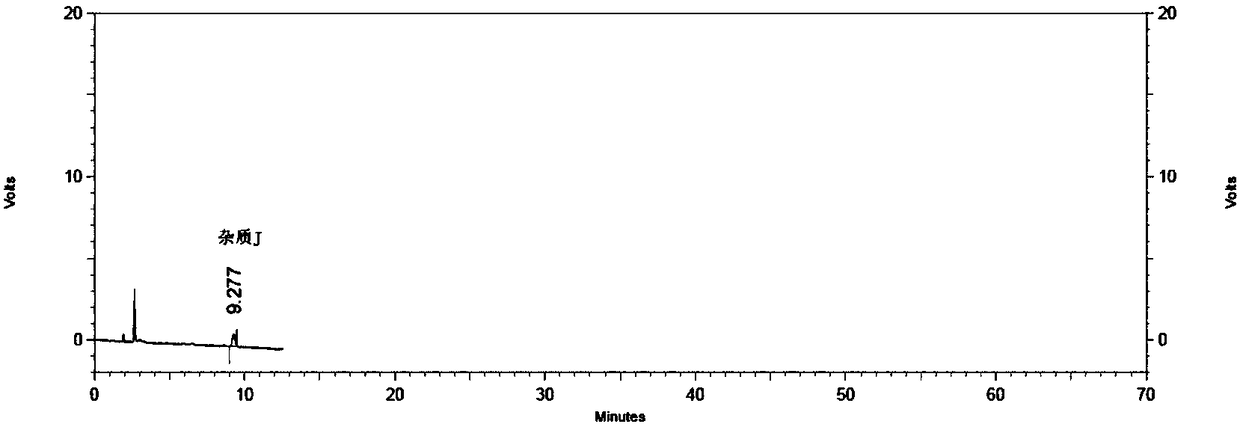
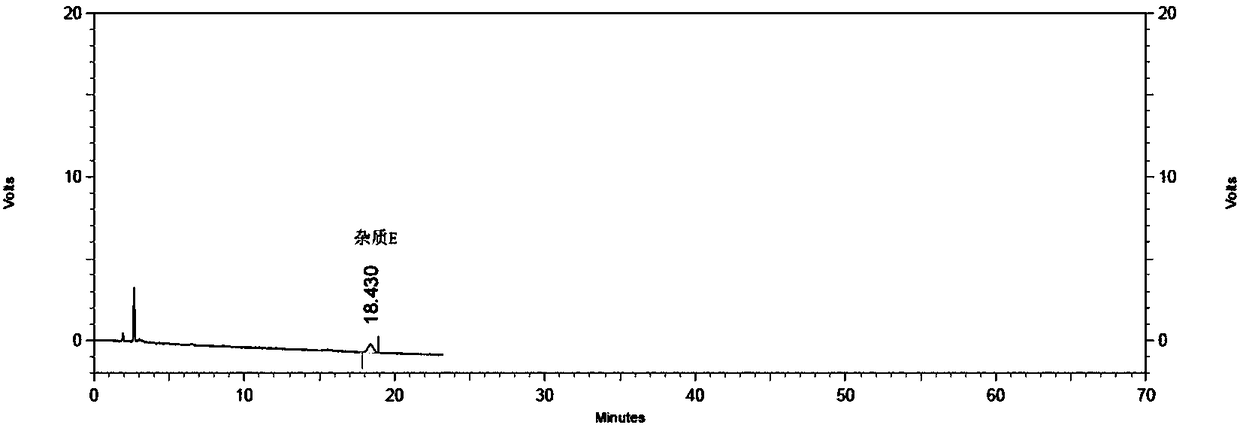
Method for detecting specific impurities of mesalazine
InactiveCN108169344AStrong specificityHigh detection sensitivityComponent separationElutionSilica gel
The invention relates to a method for detecting specific impurities of mesalazine, belonging to the technical field of medicines. According to the invention, a phenyl bonded silica gel is used as a monomer for a chromatographic column, a mobile phase is prepared by mixing an organic phase with a buffer salt solution containing an ion-pairing agent according to a certain ratio, and elution is carried out. The method of the invention can simultaneously detect a plurality of specific impurities of mesalazine, has good specificity, high detection sensitivity and good precision, and is applicable to detection of specific impurities of mesalazine bulk drugs and to specificity of detection of specific impurities of delayed-release mesalazine tablets.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Mesalazine enteric positioned controlled-release preparation and preparation method thereof
ActiveCN105902500ASignificant technological progressEfficient preparation methodOrganic active ingredientsDigestive systemCellulosePosition control
The invention discloses mesalazine enteric positioned controlled-release preparation, comprising a core, a controlled-release coating layer, an adhesive coating layer, and an enteric coating layer. The core includes mesalazine, hydroxyl propyl cellulose and talc powder, the controlled-release coating layer is composed of ethyl cellulose and triethyl citrate, the adhesive coating layer is made from sodium alginate, the enteric coating layer is composed of methacrylic acid and methyl methacrylate copolymer, triethyl citrate and talc powder. The invention also provides a preparation method of the enteric positioned controlled-release preparation. The preparation method is simple with controllable parameters, prepared pellets do not release in gastric acid and may release slowly for 24 hours in a dissolution medium at pH 7.5. This preparation is irritating to gastrointestinal adhesion and may be used in acute stage treatment for ulcerative colitis (inflammation accompanied ulcers) and maintenance treatment for preventing recurrence.
Owner:UNIV OF SHANGHAI FOR SCI & TECH +1
Mesalazine tablet having improved dissolution
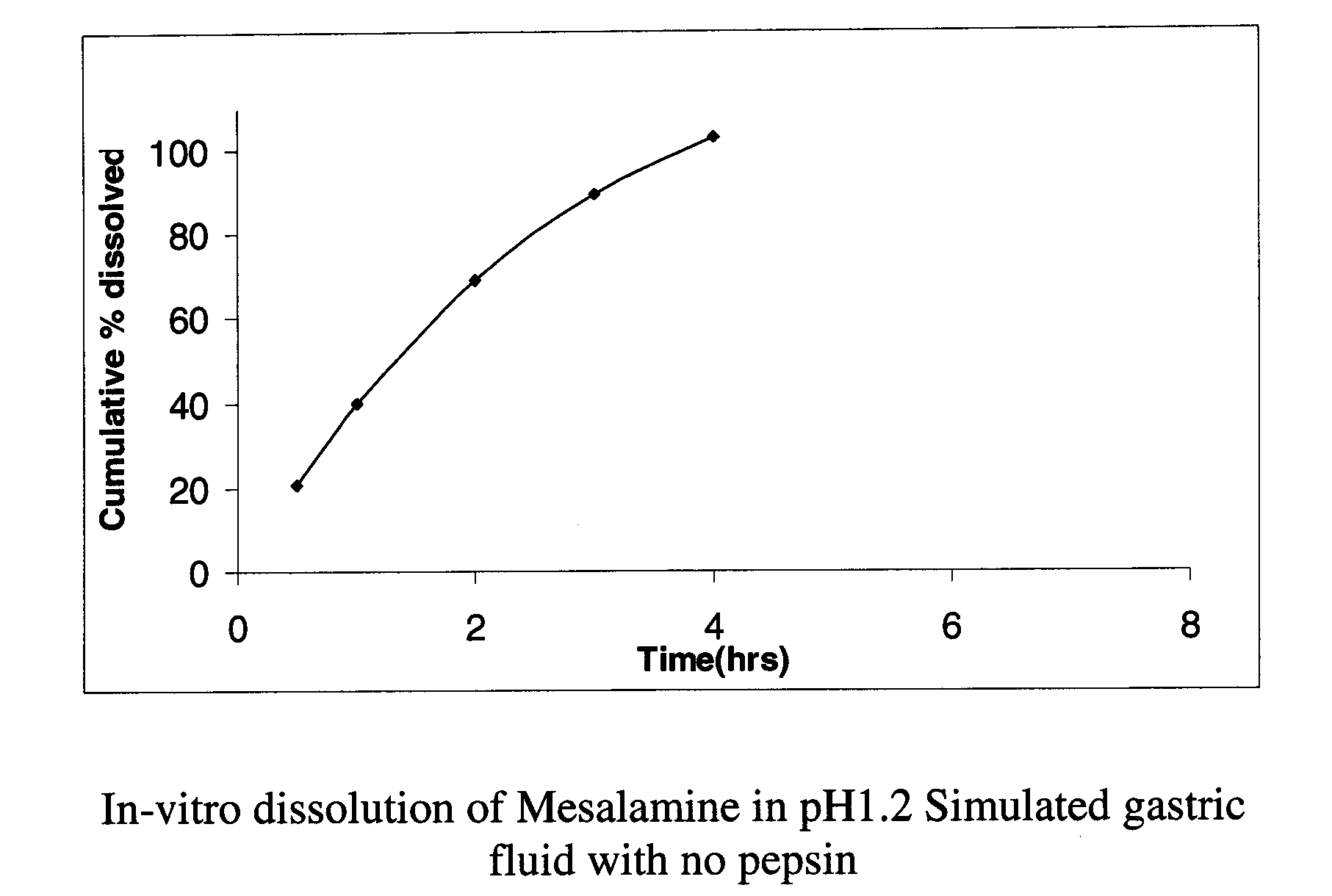
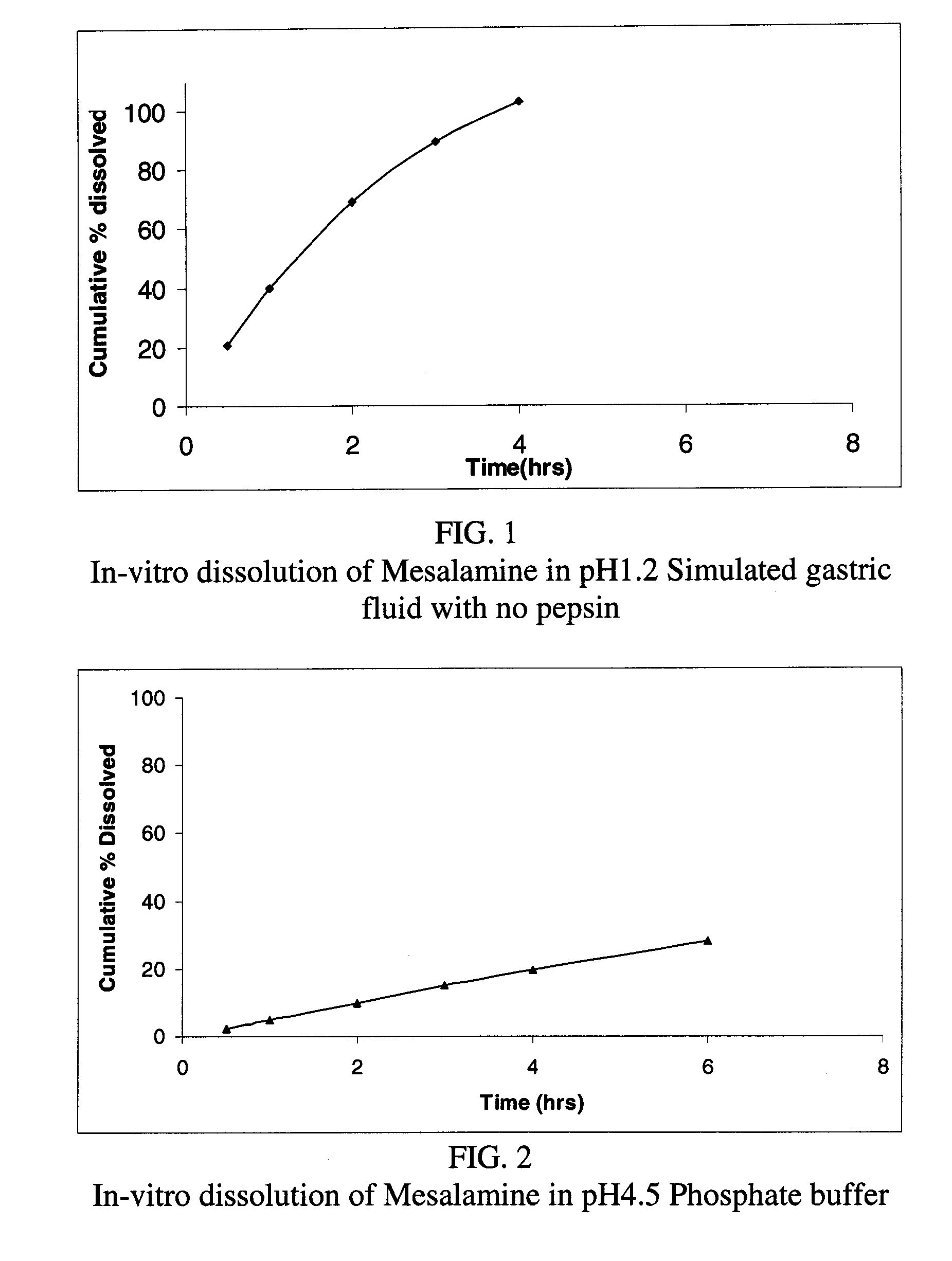
InactiveUS20130183434A1Sustained-release formulationLow dissolution rateOrganic active ingredientsDigestive systemHardnessDissolution
The invention provides a method for preparing a mesalazine enteric coated tablet comprising: (i) granulating a composition comprising mesalazine, a pharmaceutically acceptable salt, or ester thereof, into mesalazine granulates; (ii) tabletting a core composition comprising the mesalazine granulates obtained in (i) to obtain a tablet core; (iii) coating the tablet core obtained in (ii) with at least an intermediate layer and an enteric coating; where the tablet core hardness is controlled to be comprised between 80 N and 105 N and the intermediate layer represents less than 2% by weight of the tablet.
Owner:DISPHAR INTERNATIONAL BV
Mesalazine dripping pills and preparation method thereof
InactiveCN103230375AIncrease surface areaHas a wetting effectOrganic active ingredientsDigestive systemColloidBioavailability
The invention provides mesalazine dripping pills and a preparation method thereof. According to the invention, mesalazine and a substrate are combined according to a weight ratio of 1:1-100, such that the dripping pills are obtained. According to the invention, a mesalazine preparation form is changed from tablets unto dripping pills, such that the medicine is dispersed into the substrate in a state of molecules, colloid or microcrystalline. Medicine total surface area is increased. The substrate is hydrophilic, such that a wetting effect is provided for the medicine, and the medicine can be rapidly dispersed into micro-particles or solution. Therefore, medicine dissolving and absorption are accelerated, and bioavailability and medicine stability are improved. The dripping pills are highly efficient and convenient. Therefore, the mesalazine dripping pills provided by the invention has the advantages of accelerated dissolving and absorption, convenient application, high bioavailability and medicine stability, reduced adverse reaction, fast effect, convenient carrying, simple production process, and suitability for large-scale productions.
Owner:TIANJIN KANGRUI PHARMA
Oral pharmaceutical tablet for controlled release of mesalazine and process for obtaining it
InactiveUS20130273156A1Control releaseInhibition of burst releaseOrganic active ingredientsBiocideBULK ACTIVE INGREDIENTExcipient
The invention provides an oral pharmaceutical tablet for controlled release of mesalazine or a pharmaceutically acceptable salt thereof as active ingredient with a core and a gastro-resistant outer coating, wherein the core comprises mesalazine and a hydrophilic matrix consisting of a mixture of hydroxypropylmethyl cellulose (HPMC) having a different viscosity and the gastro-resistant outer coating comprises a pH-dependent release polymer, with the pharmaceutically acceptable excipients. The invention also refers to the process for obtaining said oral pharmaceutical tablet and to said oral pharmaceutical tablet of controlled release of mesalazine for treating ulcerative colitis.
Owner:LAB LICONSA
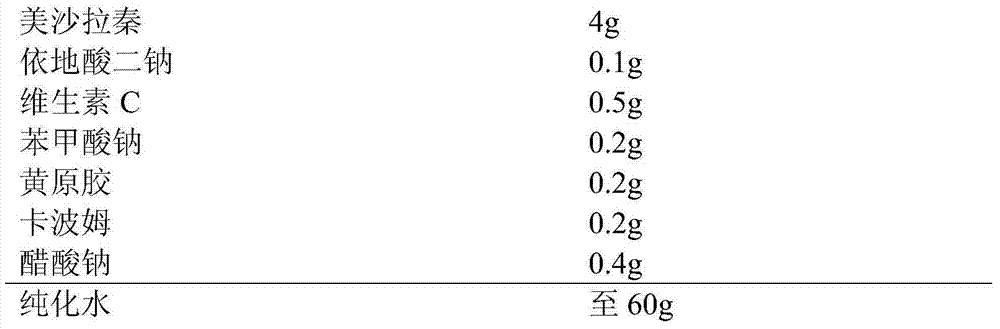
Mesalazine-containing drug composition
InactiveCN104706582AGood redispersibilityEvenly dispersedOrganic active ingredientsDigestive systemPreservativeSuspending Agents
The present invention provides a mesalazine-containing drug composition, wherein per 60 g of the composition comprises 4 g of mesalazine, 0.04-0.1 g of a metal chelating agent, 0.1-0.5 g of an antioxidant, 0.01-0.2 g of a preservative, 0.1-0.4 g of an auxiliary suspending agent, 0.1-0.4 g of a pH value adjusting agent, and the balance of water. The enema liquid has good stability.
Owner:四川健能制药有限公司
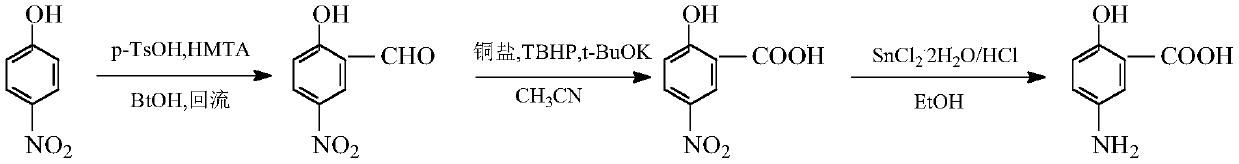
Method for synthesizing mesalazine
ActiveCN111533663ALow costAvoid generatingOrganic compound preparationAmino-carboxyl compound preparationIce waterSalicylaldehyde
A method for synthesizing mesalazine is disclosed. The method comprises the following steps: 1) adding p-nitrophenol, p-toluenesulfonic acid, absolute ethyl alcohol and hexamethylenetetramine, stopping heating after the reaction is finished, heating to room temperature while stirring with ice water, separating out solids, filtering, washing and drying to obtain 5-nitrosalicylaldehyde; 2) adding the 5-nitrosalicylaldehyde, potassium tert-butoxide, copper salt and acetonitrile, adding tert-butyl hydroperoxide while stirring, after the reaction is finished, performing vacuum concentration to remove the solvent, pouring cold water into residues, stirring, performing suction filtration, adjusting the pH value of the filtrate with hydrochloric acid, performing suction filtration, and drying to obtain 5-nitrosalicylic acid; and 3) adding stannous chloride dihydrate, concentrated hydrochloric acid, the 5-nitrosalicylic acid and ethanol, carrying out vacuum concentration after the reaction is finished, dissolving residues in water, adjusting the pH value with a concentrated hydrochloric acid solution, standing for crystallization, carrying out suction filtration, washing filter cake with water, and drying to obtain mesalazine. No isomer is generated, and the yield is high; the method does not need high-temperature and high-pressure conditions; the reaction cost is low; and raw materialsand auxiliary materials with high toxicity and heavy environmental pollution are not used.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
Mesalamine suppository
ActiveUS20100105639A1Improve the comfort of useHigh tap densityPowder deliverySalicyclic acid active ingredientsRectal SuppositoryPhosphate
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Yet another embodiment is a mesalamine rectal suppository comprising mesalamine particles and one or more pharmaceutically acceptable excipients, where the mesalamine particles have a surface area of from about 0.1 m2 / g to about 2.8 m2 / g (e.g., from about 0.1 m2 / g to about 1.3 m2 / g). Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:AXCAN PHARM CANADA INC
Mesalazine oral sustained-release preparation
InactiveCN109464409AReduce releaseSmooth releaseOrganic active ingredientsDigestive systemCarboxymethyl starchStearic acid
The invention relates to a Mesalazine oral sustained-release pharmaceutical composition which is characterized by comprising the following ingredients by weight per one unit of preparation: 0.9g-1.3gof Mesalazine, 0.01g-0.05g of carnauba wax, 0.01g-0.05g of stearic acid, 0.05g-0.2g of hydrogenated castor oil, 0.05g-0.2g of sodium carboxymethylcellulose, 0.05g-0.2g of sodium carboxymethyl starch,0.005g-0.02g of silicon dioxide, 0.005g-0.02g of magnesium stearate, 0.005g-0.02g of talcum powder and 0.02g-0.1g of enteric coating powder. The invention also provides a preparation method of the Mesalazine oral sustained-release pharmaceutical composition. All the ingredients have a synergistic effect, so that the finally prepared composition is good in sustained-release effect and stable in property.
Owner:重庆健能医药开发有限公司
Mesalamine suppository
InactiveUS20160175329A1Small sizeEasy to keepOrganic active ingredientsBiocideRectal SuppositoryGynecology
A mesalamine rectal suppository having improved comfort of use and better retention in lower rectum for a long period of time is provided. The suppository contains a suppository base, at least one surfactant and at least one mucoadhesive agent. A method for manufacturing the suppository and methods for treating ulcerative colitis, such as active ulcerative proctitis, using such suppository is also provided.
Owner:LUPIN ATLANTIS HLDG
Method for determination of related substances in mesalazine enemas
ActiveCN109799292AEfficient separationStrong specificityComponent separationUltraviolet detectorsSilica gel
The invention provides a method for determination of related substances in mesalazine enemas. In the method, high performance liquid chromatography is adopted, octadecyl silane bonded silica gel is used as column filler, a ultraviolet detector is adopted, appropriate organic phase, buffer solution and water are selected as mobile phase, and gradient elution is adopted to effectively solve the problem of separation between mesalazine and impurities. The method has high specificity, good repeatability and can ensure controllability of the process and degradation of the impurities in mesalazine.
Owner:重庆健能医药开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com