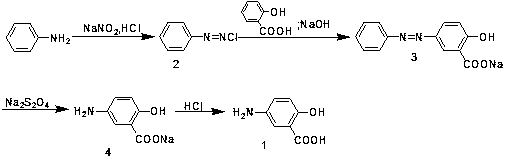
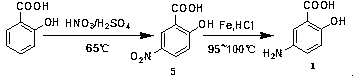
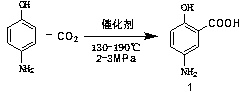
Industrialized preparation method of mesalazine
A technology of mesalazine and potassium hydroxide, applied in the field of industrialized preparation of mesalazine, can solve the problems of backward production technology, increase of synthesis cost, unfavorable large-scale production, etc., achieve optimized post-treatment mode, reduce environmental hazards, high The effect of industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of (II)
[0037]
[0038] Put 14 kg (250 mol) of potassium hydroxide into a 100 L reactor, add 50 L of water to fully dissolve it, keep the temperature at 20-40°C, and slowly add 10 kg (49.6 mol) of 2-chloro-5-nitrate Benzoic acid (I), warming up to 120-140 ° C, reacted at this temperature for 6 hours, TLC followed the reaction process, until the raw materials were completely reacted, cooled to room temperature, and the pH of the reaction system was adjusted between 1-3 with 17L hydrochloric acid , continue to stir for 1.5 hours, filter, wash the filter cake with 50L water, beat the filter cake with 30L water at 30°C, filter, continue to wash the filter cake with 50L water, and dry at 65°C for 12 hours to obtain 2-hydroxy-5-nitrate 8.81 kg of benzoic acid (intermediate II), yield: 97%, purity: 99.88%.
[0039] (2) Preparation of crude product of mesalamine (1)
[0040]
[0041] Put 3.26kg (30.6mol) of sodium carbonate into a 100 L reactor, add 64...
Embodiment 2
[0045] (1) Preparation of (II)
[0046]
[0047] Put 50g (0.893mol) of sodium hydroxide into a 500 mL reactor, add 179 mL of water to fully dissolve it, keep the temperature at 20-40°C, and slowly add 35.7g (0.177mol) of 2-chloro-5-nitro Benzoic acid (I), heated up to 120-140°C, reacted at this temperature for 6 hours, followed the reaction process by TLC, after the raw materials were completely reacted, cooled to room temperature, and adjusted the pH of the reaction system between 1-3 with 60.7mL hydrochloric acid , continue to stir for 1.5 hours, filter, wash the filter cake with 179 mL of water, beat the filter cake with 107 mL of water at 30 ° C, filter, continue to wash the filter cake with 179 mL of water, and dry at 65 ° C for 12 hours to obtain 2-hydroxy-5-nitrate 31.11 g of benzoic acid (intermediate II), yield: 96%, purity: 99.78%.
[0048] (2) Preparation of crude product of mesalamine (1)
[0049]
[0050] Put 11.6g (0.109mol) of sodium carbonate into a 500...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com