Mesalazine oral controlled release medicine composition
A technology for oral salazine tablets and compositions, which is applied in directions such as drug combinations, sugar-coated pills, and pharmaceutical formulations, can solve the problems of complex preparation process, high production cost, no sustained-release effect, etc., and achieves improved compliance, low cost, The effect of reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
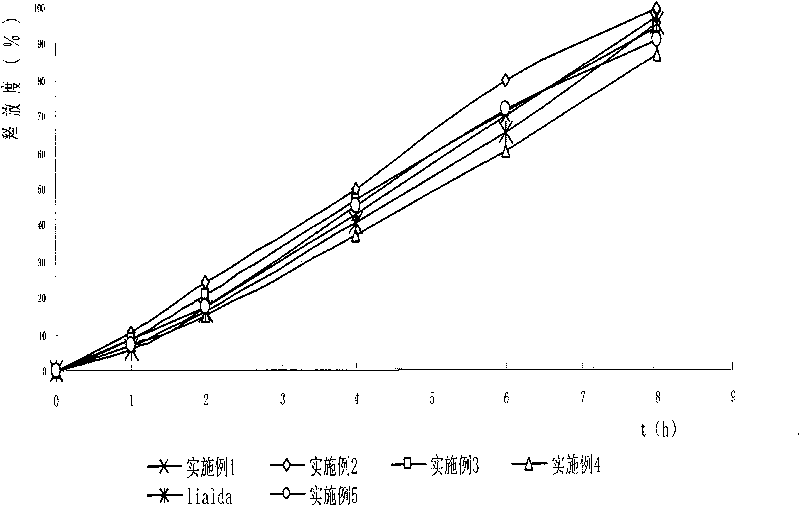
Image
Examples
Embodiment 1
[0032] Tablet prescription:
[0033] Mesalamine 1200g
[0034] Sodium Carboxymethyl Cellulose 96g
[0035] Sodium carboxymethyl starch 48g
[0036] Magnesium Stearate 13.4g
[0037]
[0038] 1000 pieces
[0039] The preparation process of the tablet core: mix mesalazine, carboxymethylcellulose sodium and carboxymethyl starch sodium evenly, add appropriate amount of distilled water, granulate and dry. Add magnesium stearate, mix uniformly, measure the granule content, compress into tablets, and the main ingredient content of each tablet is 1.2g.
[0040] Coating Solution Prescription:
[0041] Coating Material Weight
[0042] Eudragit S100 200g
[0043] Eudragit L100 100g
[0044] Triethyl citrate 60g
[0046] Titanium dioxide 30g
[0047] Iron Oxide Red 30g
[0048] Ethanol 4750ml
[0049] water 250ml
[0050] Coating process: Prepare the above coating solution a...
Embodiment 2
[0052] Tablet core prescription
[0053] Mesalamine 1200g
[0054] Hypromellose K 100 80g
[0055] Sodium carboxymethyl starch 30g
[0056]
[0057] Magnesium Stearate 13.0g
[0058]
[0059] 1000 pieces
[0060] Tablet core preparation process: uniform mesalazine and hypromellose K100, add appropriate amount of distilled water, granulate, and dry. Add magnesium stearate, mix uniformly, measure the granule content, compress into tablets, and the main ingredient content of each tablet is 1.2g.
[0061] Coating Solution Prescription:
[0062] Coating Material Weight
[0063] Eudragit S100 300g
[0064] Triethyl citrate 60g
[0066] Titanium dioxide 30g
[0067] Iron Oxide Red 30g
[0068] Ethanol 4750ml
[0069] water 250ml
[0070] Coating process: Prepare the above coating solution according to the con...
Embodiment 3
[0072] Tablet prescription:
[0073] Mesalamine 1200g
[0074] Carbomer 971 25g
[0075] Dextran-40 75g
[0076] Magnesium Stearate 13.0g
[0077]
[0078] 1000 pieces
[0079] The preparation process of the tablet core: mesalazine, carbomer 971, dextran-40, adding an appropriate amount of distilled water, granulating, and drying. Add magnesium stearate, mix uniformly, measure the granule content, compress into tablets, and the main ingredient content of each tablet is 1.2g.
[0080] Coating Solution Prescription:
[0081] Coating Material Weight
[0082] Eudragit S100 200g
[0083] Eudragit L100 150g
[0084] Triethyl citrate 60g
[0086] Titanium dioxide 30g
[0087] Iron Oxide Red 30g
[0088] Ethanol 4750ml
[0089] water 250ml
[0090] Coating process: Prepare the above coating solution according to the conventional method, and coat with a high-efficienc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com