Mesalazine enteric coatel tablet and preparation method thereof
A kind of mesalamine, the technology of extracting mesalamine, applied in the field of chemical medicine preparation, can solve the problems of low buffer release, high release in acid, insufficient curative effect, etc., to ensure hardness and friability, and increase stability , the effect of reducing losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Take 250g of mesalamine, 15g of calcium carbonate, 5g of talcum powder, and 2g of sodium carboxymethyl starch, mix them evenly, put them in a high-energy nano ball mill to make a nano-mixed powder of 100-300nm, put them in a coating granulator, add paste Essence 5g, mannitol 5g, mix well, use 10% povidone K30 ethanol solution as binder, make pellets, increase weight by 3%, get drug-containing pellets, put them in a fluidized coating machine, take LE enteric-coated Coating powder 18g, made into 10% ethanol solution, sprayed and coated to obtain enteric-coated pellets, added 15g of pregelatinized starch, 5g of pectin, 5g of microcrystalline cellulose, 5g of hydroxypropyl cellulose, and micronized silica gel 1g, mixed evenly, tableted to obtain tablet cores, placed in a film coating machine, 15g of LE enteric coating powder was taken, mixed with 8% ethanol liquid, sprayed and coated to obtain mesalazine enteric-coated tablets.
specific Embodiment 2
[0015] Take 250g of mesalamine, 25g of calcium carbonate, 15g of talcum powder, and 4g of sodium carboxymethyl starch, mix them evenly, put them in a high-energy nano ball mill to make a nano-mixed powder of 100-300nm, put them in a coating granulator, add paste Essence 15g, mannitol 15g, mix well, use 15% povidone K30 ethanol solution as binder, make pellets, increase weight by 5%, get drug-containing pellets, put them in a fluidized coating machine, take LE enteric-coated Coating powder 22g, made into 10% ethanol solution, sprayed and coated to obtain enteric-coated pellets, added 25g of pregelatinized starch, 15g of pectin, 15g of microcrystalline cellulose, 15g of hydroxypropyl cellulose, and micronized silica gel 3g, mixed evenly, tableted to obtain tablet cores, placed in a film coating machine, 25g of LE enteric coating powder was taken, mixed with 8% ethanol liquid, sprayed and coated to obtain mesalazine enteric-coated tablets.
specific Embodiment 3
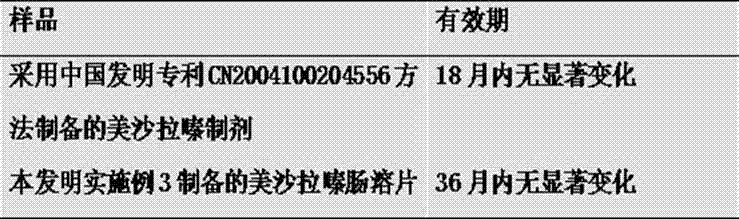
[0017] Take 250g of mesalamine, 20g of calcium carbonate, 10g of talcum powder, and 3g of sodium carboxymethyl starch, mix them evenly, put them in a high-energy nano ball mill to make a nano-mixed powder of 100-300nm, put them in a coating granulator, add paste Essence 10g, mannitol 10g, mix well, use 12% povidone K30 ethanol solution as binder, make pellets, increase weight by 4%, get drug-containing pellets, put them in a fluidized coating machine, take LE enteric-coated Coating powder 20g, made into 10% ethanol solution, sprayed and coated to obtain enteric-coated pellets, added 20g of pregelatinized starch, 10g of pectin, 10g of microcrystalline cellulose, 10g of hydroxypropyl cellulose, and micronized silica gel 2g, mixed evenly, compressed to obtain tablet cores, placed in a film coating machine, 20g of LE enteric coating powder was taken, mixed with 8% ethanol liquid, sprayed and coated to obtain mesalazine enteric-coated tablets.
[0018] The raw material standards us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com