Combination therapy for the treatment of influenza
A technology for influenza, subjects, applied in the field of compositions for the treatment of viral infections, capable of solving the problems of high case fatality rate, harmful patient survival, impact, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
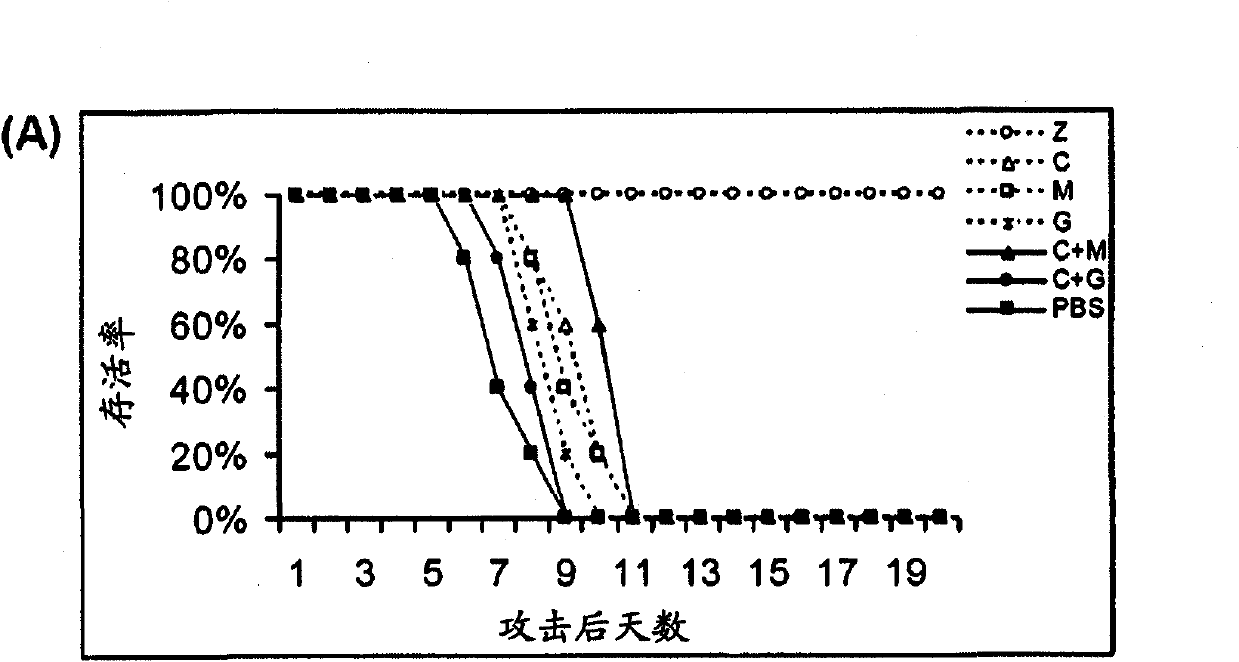
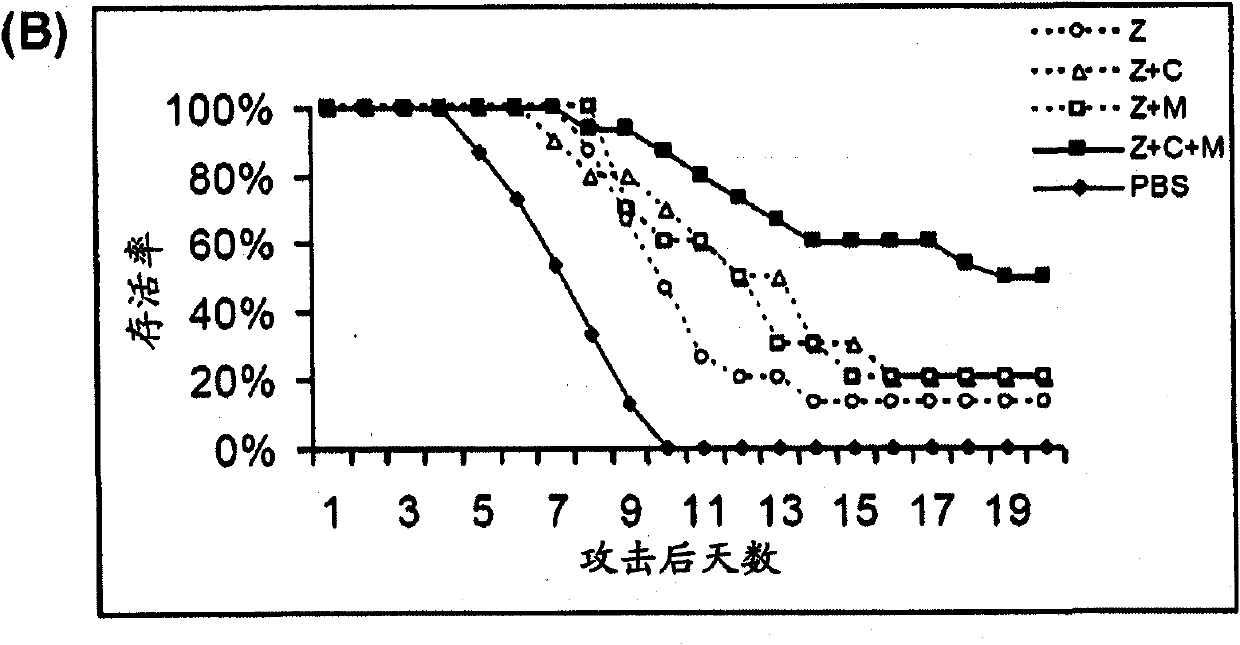
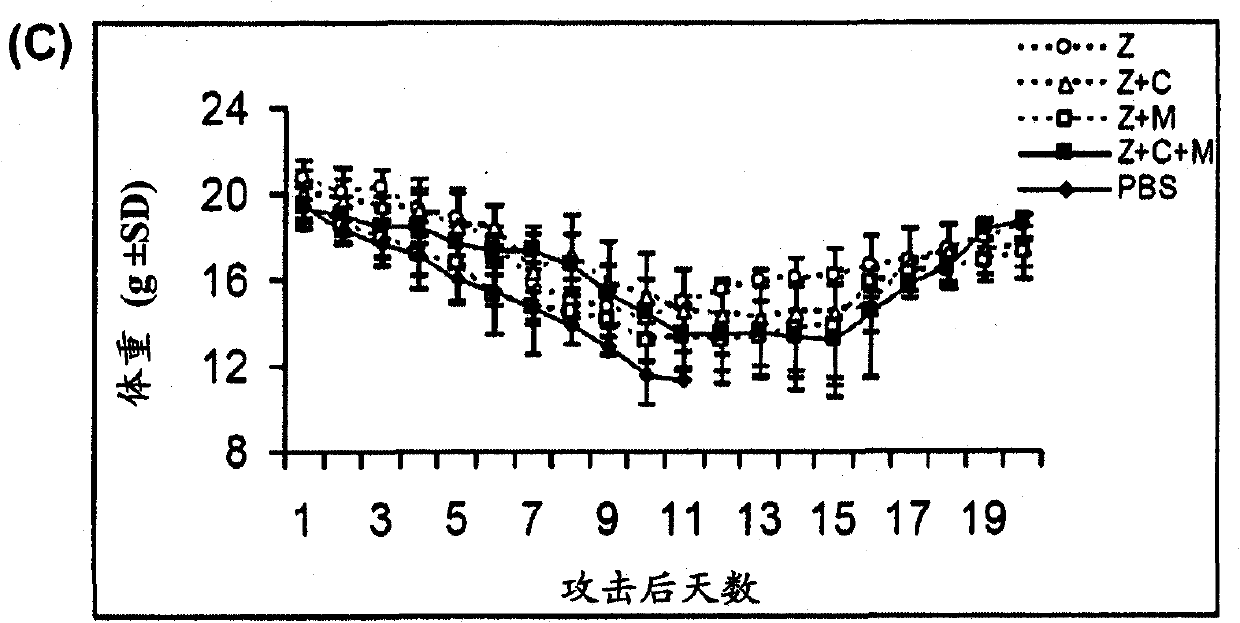
[0073] Example 1: Treatment of Mice with Antiviral Drugs in Combination with Immunomodulators
[0074] Methods and Materials
[0075] Animal models and virus challenge.
[0076] BALB / c female mice aged 5-7 weeks were purchased from the Experimental Animal Unit of the University of Hong Kong. Mice were housed in biosafety level 3 housing with standard pelleted feed and water available ad libitum. Aliquots of influenza A strain A / Vietnam / 1194 / 04 stocks were grown in embryonated eggs. Allantoic fluid containing virus was collected and stored in aliquots at -70°C. The median lethal dose (LD) was determined in mice after serial dilution of virus stock 50 ). All experimental virus attacks used 1000LD 50 . Influenza virus infection was established by intranasal inoculation of isoflurane-anesthetized mice.
[0077] Antiviral and Immunomodulatory Therapy
[0078] Antiviral drugs and immunomodulators were administered by intraperitoneal injection (i.p.) using 0.5ml 29-gauge u...
Embodiment 2
[0093] Example 2: Reduction of virus titer
[0094] Materials and methods
[0095] Virology test.
[0096] Virus titers released in tracheo-lung lavage fluid by TCID 50 Assay, viral RNA in lung tissue cells was quantified by real-time RT-PCR (Li, B.J. et al. Nat Med 11: 944-951 (2005); Zheng, B.J. et al. Antivir Ther 10: 393-403 (2005); Wang, M . et al. Emerg Infect Dis 12: 1773-1775 (2006)). Briefly, the total RNA of the lysed lung tissue was extracted using the RNeasy Mini kit (Qiagen, Germany), and the applied SuperScript II Reverse Transcriptase TM (Invitrogen, USA) to reverse transcribe it into cDNA. The NP gene of the virus and the internal control actin gene were measured by SYBR green Mx3000 Real-Time PCR System (real-time PCR system) (Stratagene, USA), wherein the primer NP-forward: 5'-GAC CAG GAG TGG AGG AAA CA- 3' (SEQ ID NO: 1), NP-reverse: 5'-CGG CCA TAA TGG TCA CTC TT-3' (SEQ ID NO: 2); - Actin-forward: 5'-CGT ACC ACT GGC ATC GTGAT-5' (SEQ ID NO: 3), -act...
Embodiment 3
[0103] Example 3: Histology
[0104] Materials and methods
[0105] Histopathological analysis
[0106] Lung, brain, spleen, kidney and liver tissues from challenged mice were immediately fixed in 10% buffered formalin and embedded in paraffin. Sections 4-6 μm thick were mounted on glass slides. According to the method described by Zheng, B.J. et al. Eur J Immun 32:3294-3304 (2002); Zheng, B.J. et al. Int J Cancer 92:421-425 (2001), hematoxylin Determination by sperm and eosin (H&E) staining.
[0107] Immunohistochemical assay
[0108] Lung sections were stained with the following reagents as previously described (28, 30): 1:5000 dilution of anti-influenza nucleoprotein monoclonal antibody (HB65, ATCC, USA), 1:2000 dilution of goat anti-mouse IgG H and L Strept-specific biotin conjugate (Calbiochem, USA) and streptavidin / peroxidase complex reagent (Vector Laboratories, USA).
[0109] Flow Cytometry
[0110] Blood cells from mice were stained with fluorescein-labeled m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com