Mesalazine enteric positioned controlled-release preparation and preparation method thereof
A technology of mesalazine and controlled-release preparations, which is applied in the field of medicine, can solve the problems of aggravating the condition of patients with colonic ulcers, complex control of industrial preparation parameters, and low drug loading of mesalazine, achieving remarkable technological progress, Symptom improvement, effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
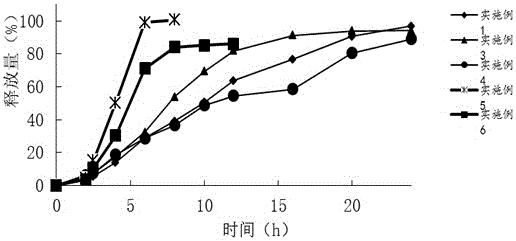
Embodiment 1
1.1%
[0035] The preparation method of the above mesalazine enteric-coated positioning and controlled-release preparation is as follows:
[0036] (1) Preparation of mesalazine-containing pellets
[0037] Take the mesalazine with a composition of 66.7% by weight and pass through an 80-mesh screen to make the volume average particle size of the drug particles ≤200μm; then take the composition of hydroxypropyl cellulose (Klucel MF) with a composition of 9.2% by weight and the composition by weight as After passing through a 60-mesh sieve, 5.8% lactose is mixed with mesalazine; take a small amount of the above mixture and place it in a fluidized bed, and use ethanol-water (70:30) solvent for fluidized granulation, and control the fluidized bed temperature 45 ℃, after the granules are dried, 40 meshes are sized, and 40-60 mesh granules are selected as the mother core. The pellets are prepared in the WL-300 pill making machine, and the rotation speed of the turntable is controlled to 200...
Embodiment 2
1.1%
[0046] The preparation method of the above mesalazine enteric controlled-release preparation is as follows:
[0047] (1) Preparation of mesalazine-containing pellets
[0048] Take the 66.7% by mass of mesalazine and pass it through an 80-mesh sieve to make the volume average particle size of the drug particles ≤200μm; then take the 9.2% by mass of hydroxypropyl cellulose and 10.8% of lactose and pass through a 60-mesh sieve After meshing, mix with mesalazine; take a small amount of the above mixture and place it in a fluidized bed, use ethanol-water (70:30) solvent for fluidized granulation, control the temperature of the fluidized bed at 45°C, and dry the granules to 40 mesh Select the 40-60 mesh particles as the mother core, prepare the pellets in the WL-300 pill making machine, control the rotating speed of the turntable to 200-300rpm, and the inlet temperature of 40℃ to prepare the drug-containing pellets. The diameter of the pellets is controlled to be 0.8- 1.5mm; Mix t...
Embodiment 3
1.1%
[0055] The preparation method of the above mesalazine enteric-coated positioning and controlled-release preparation is as follows:
[0056] (1) Preparation of mesalazine-containing pellets
[0057] Take mesalazine with a mass percentage composition of 79.5% and pass it through an 80-mesh sieve to make the volume average particle size of the drug particles ≤200μm; then take the hydroxypropyl cellulose with a mass percentage composition of 8.5% and pass it through a 60-mesh sieve. Mix the salazine; take the above-mentioned small amount of mixture and place it in a fluidized bed, use ethanol-water (70:30) solvent for fluidized granulation, control the temperature of the fluidized bed at 45°C, and dry the granules to 40 meshes and sieving to 40~ The 60-mesh particles are used as the core, and the pellets are prepared in the WL-300 pelletizing machine, the rotating speed of the turntable is controlled to be 200-300rpm, the inlet temperature is 40°C, and the drug-containing pellets...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com