Patents
Literature
43 results about "Rectal Suppository" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Parenteral delivery systems
Hypertonic sugar compositions administered by other than ingestion and swallowing or intravascular injection, such as by intranasal spray or drops, intraocular drops or ointment, oral spray, intraotic spray or drops, lozenges, chewable tablet, chewing gum, or gargle, pulmonary inhalation, vaginal or rectal suppositories, or transdermal creams, ointments, lotions, or patches, are effective to open the blood-brain barrier to permit entry into the central nervous system of a co-administered chemical compound, such as a nutrient or a therapeutic or diagnostic agent. In this way, the compositions and methods of the invention increase the therapeutic or diagnostic efficacy of such chemical compounds.
Owner:NAITO ALBERT T
Endometriosis treatment protocol
The endometriosis treatment protocol provides for administering to a female patient in need of treatment for endometriosis a pharmaceutical composition in a form suitable for vaginal or rectal delivery having a pharmaceutically effective amount of an aromatase inhibitor, which may be either a steroid or non-steroidal. The pharmaceutical composition may be formed as a vaginal suppository, a rectal suppository, a vaginal gel, a rectal gel, a vaginal cream or a rectal cream. The pharmaceutical composition may optionally have pharmaceutically effective amounts of progesterone and calcitriol, and may be administered in combination with an oral COX-2 inhibitor. Alternatively, the pharmaceutical composition comprises an aromatase inhibitor administered vaginally or rectally and is administered in combination with oral calcitriol and the oral COX-2 inhibitor. The aromatase inhibitor is either steroidal or non-steroidal.
Owner:SHIPPEN EUGENE R
Method, composition, and delivery mode for treatment of prostatitis and other urogenital infections using a probiotic rectal suppository
This invention is a probiotic composition using the delivery mode of a rectal suppository for the treatment and relief of symptoms of urogenital infections including prostatitis. This invention is unique in both mode of delivery and in enabling the treatment of such urogenital infections as prostatitis. The present invention discloses compositions, methodologies, and delivery mode for the utilization of probiotic organisms in therapeutic compositions for treatment and relief from the symptoms of urogenital infections by maintaining and restoring normal flora in humans. The composition includes one or more bacteria selected from the genus Lactobacillus. More specifically, the invention relates to the utilization of one or more species or strains of Lactobacillus producing bacteria for the control of urinary tract bacteria and pathogens, including antibiotic-resistant urinary tract pathogens, and their associated diseases by both a reduction in the rate of colonization of the infection and pathogens. The method for treatment and relief from symptoms includes administration via a rectal suppository with a safe and effective amount of the composition. The delivery mode for administering the probiotic is via a rectal suppository. The delivery through the rectum provides an optimum method of transmission of the treatment.
Owner:NESSA JEFFREY +1
Method of treating HIV infection with suppository containing mammalian liver extract
InactiveUS6156349AImprove natureEffective treatmentBiocidePeptide/protein ingredientsRectal SuppositoryEmulsion
A method for treating human immuno-deficiency virus infection (HIV-1), comprising administering a therapeutically effective amount of a mammalian liver extract characterized by being heat stable, insoluble in acetone, and soluble in water. A rectal suppository colloidal dispersion delivery system for use in the treatment of HIV-1 infection further comprises an emulsion of resolubilized, concentrated mammalian liver extract.
Owner:STEINBACH PYLANT & HERMAN L L C
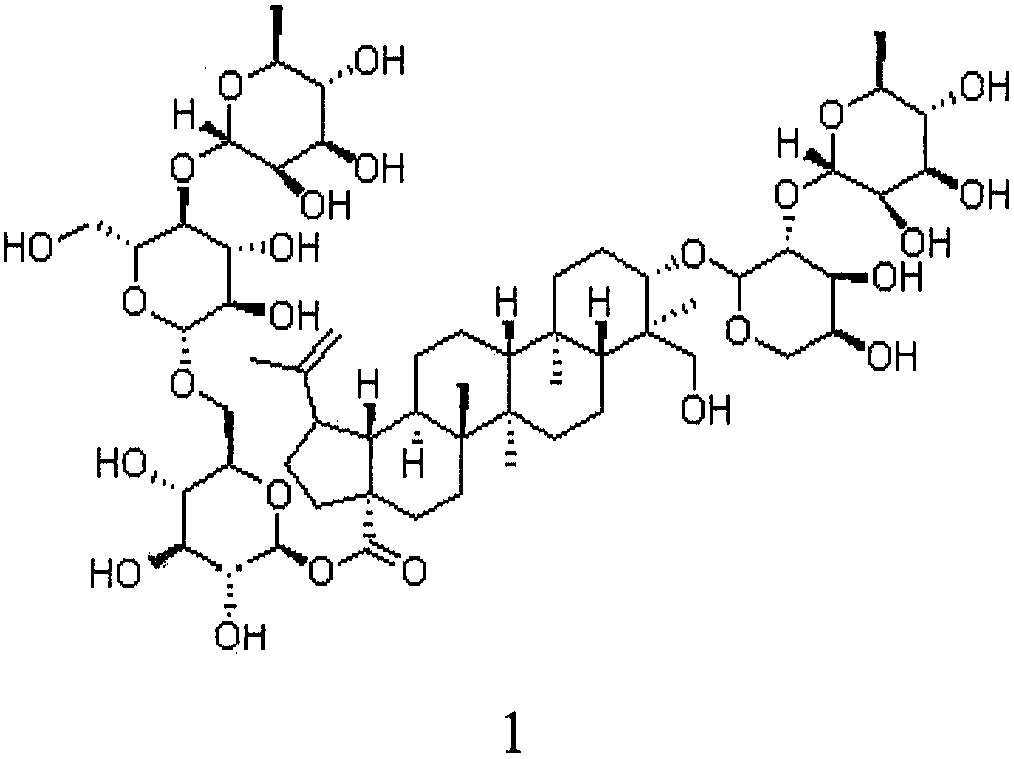
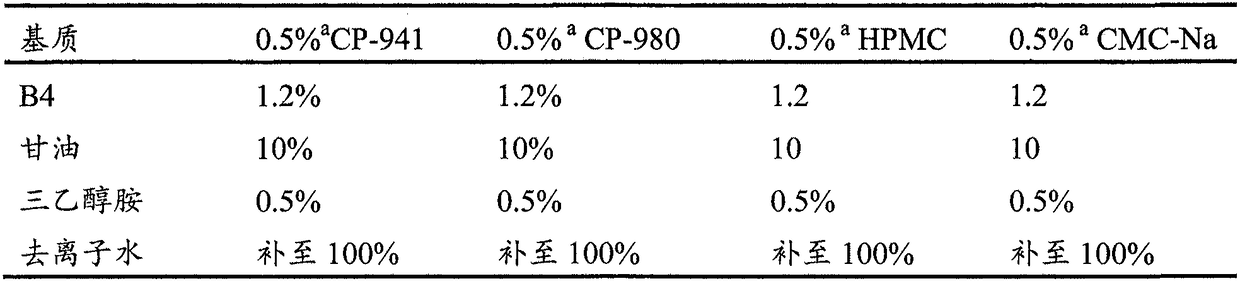
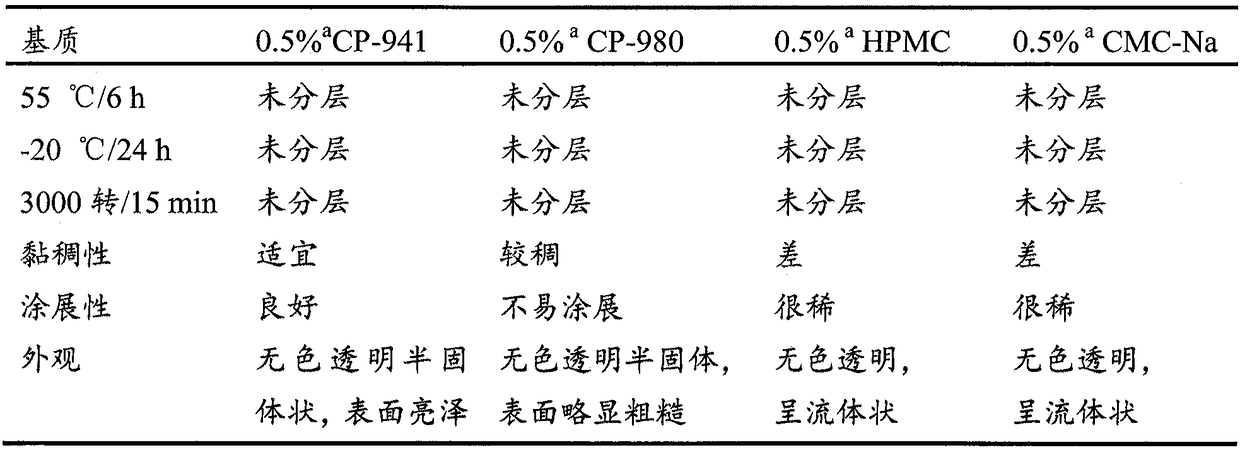
Preparation of anemoside B4 for rectal mucosal administration and preparation method thereof
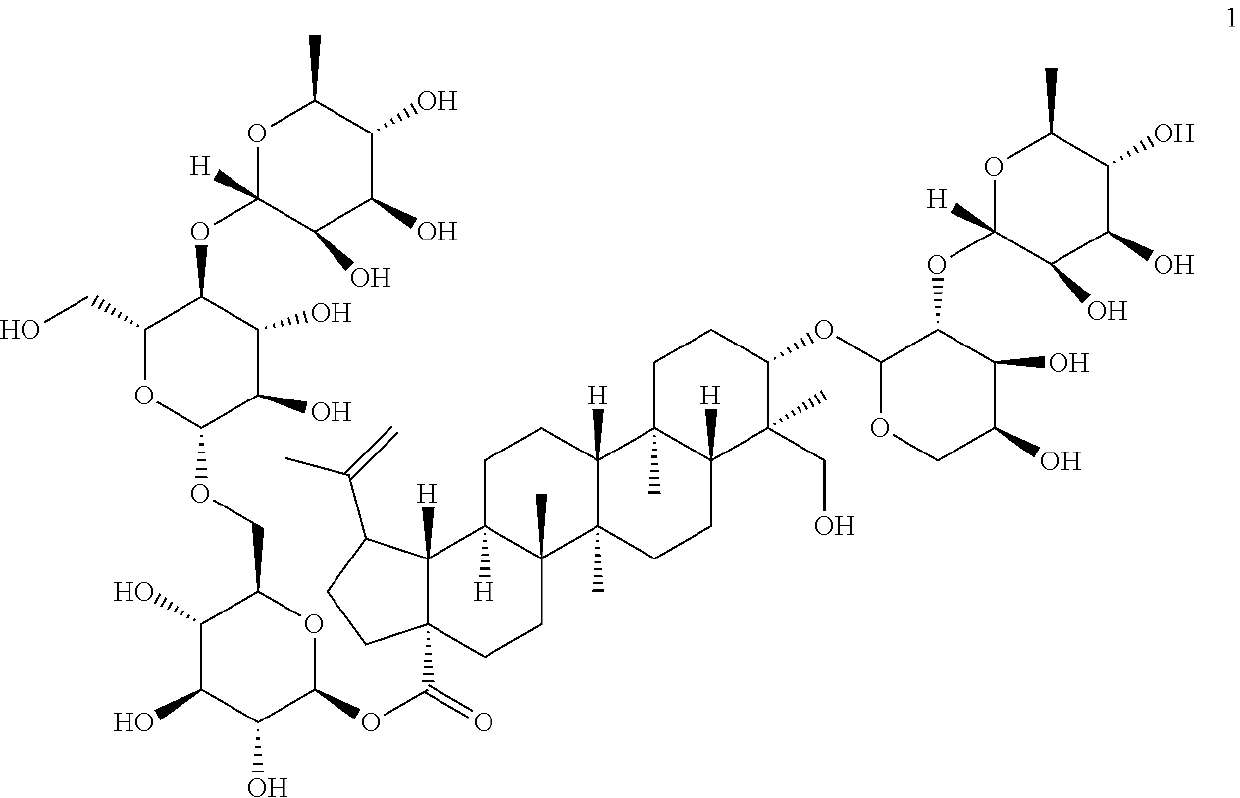
The invention relates to a preparation of anemoside B4 for rectal mucosal administration, comprising anemoside B4 and a pharmaceutically acceptable matrix; the preparation herein is selected from oneor two of rectal gel and rectal suppository. The invention also specifically provides rectal gel of anemoside B4, rectal suppository of anemoside B4, and their preparation methods. The preparation ofanemoside B4 for rectal mucosal administration provided herein requires less dosage than oral administration and has better effect under the same the dosage.
Owner:刘琦
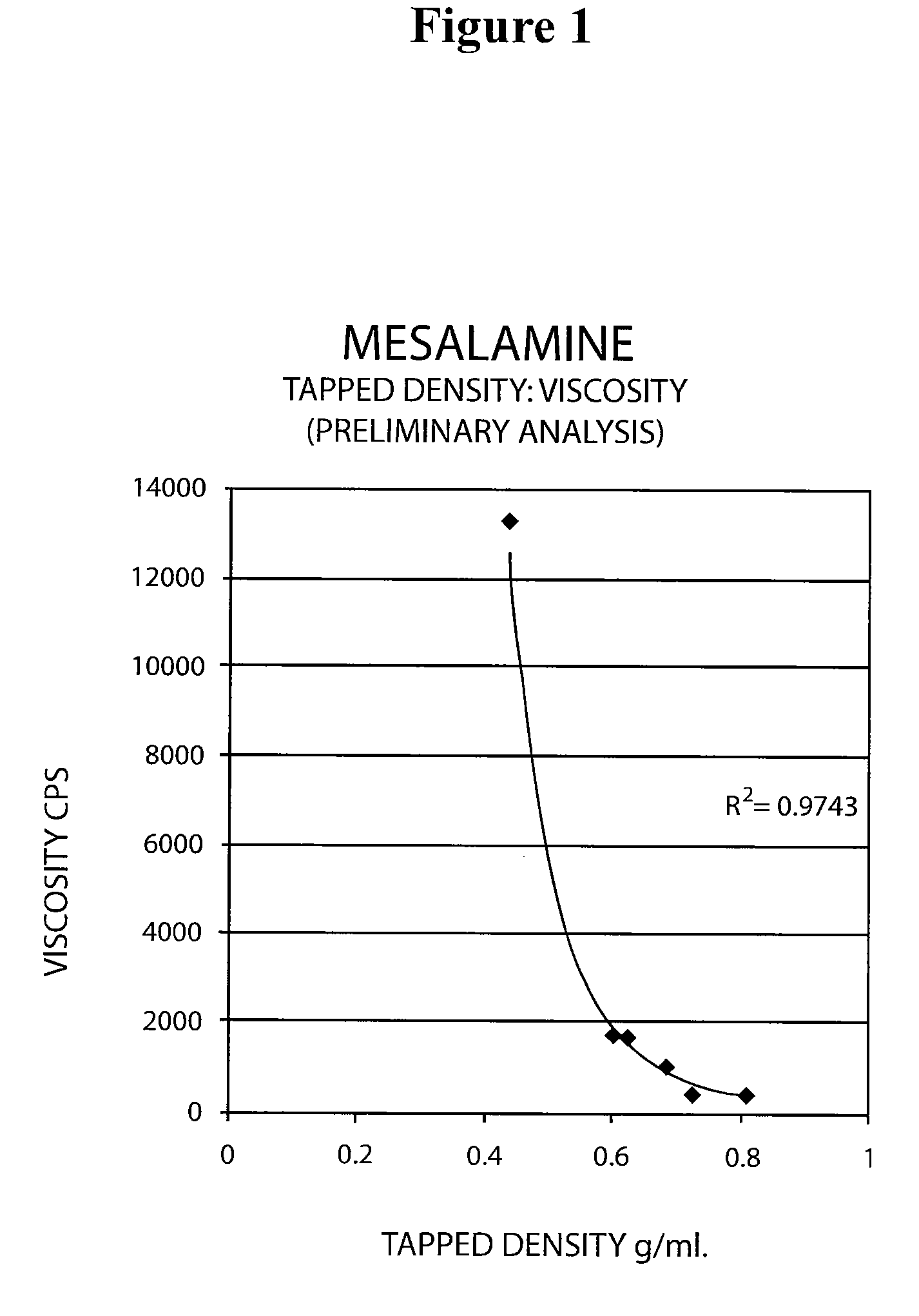
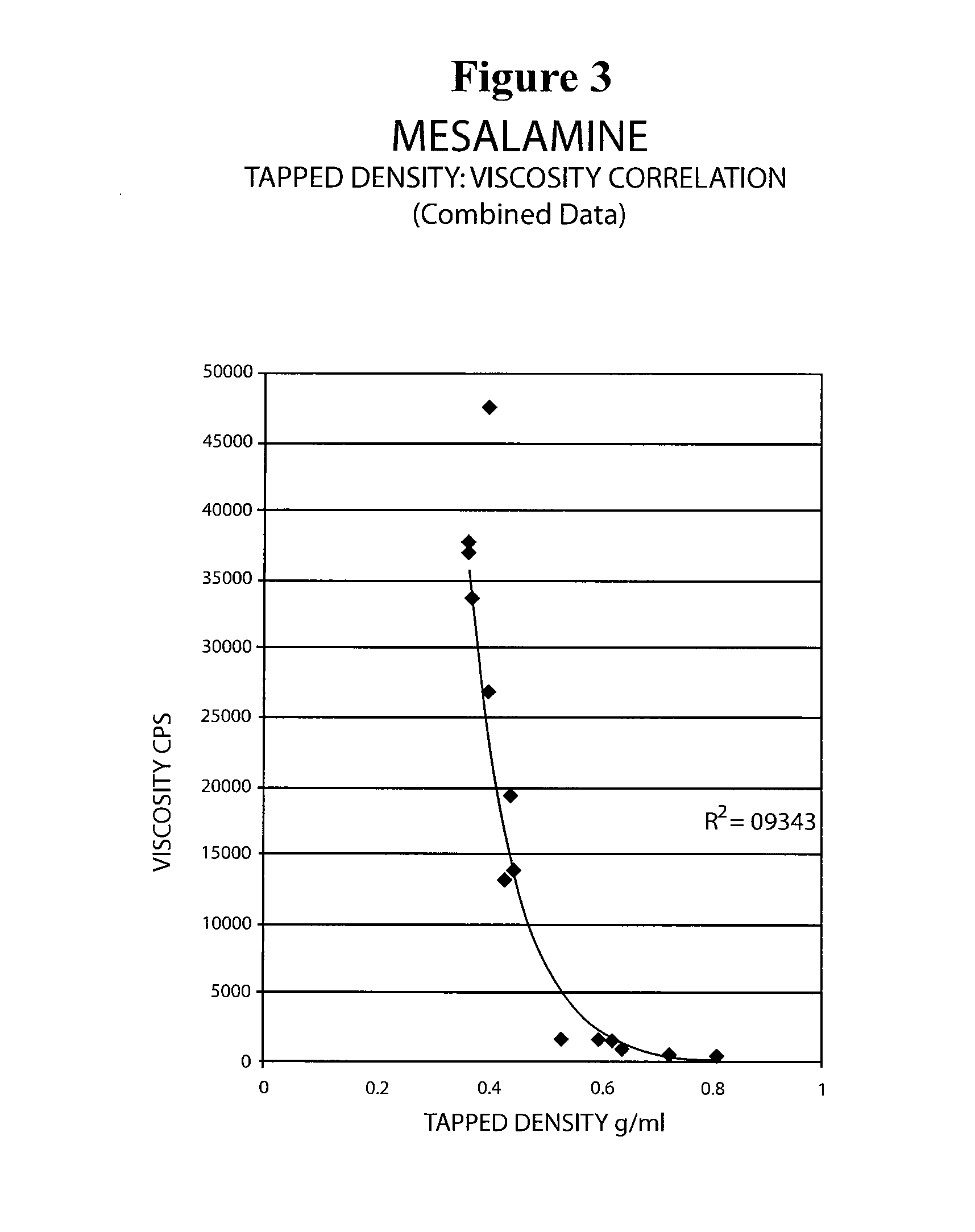
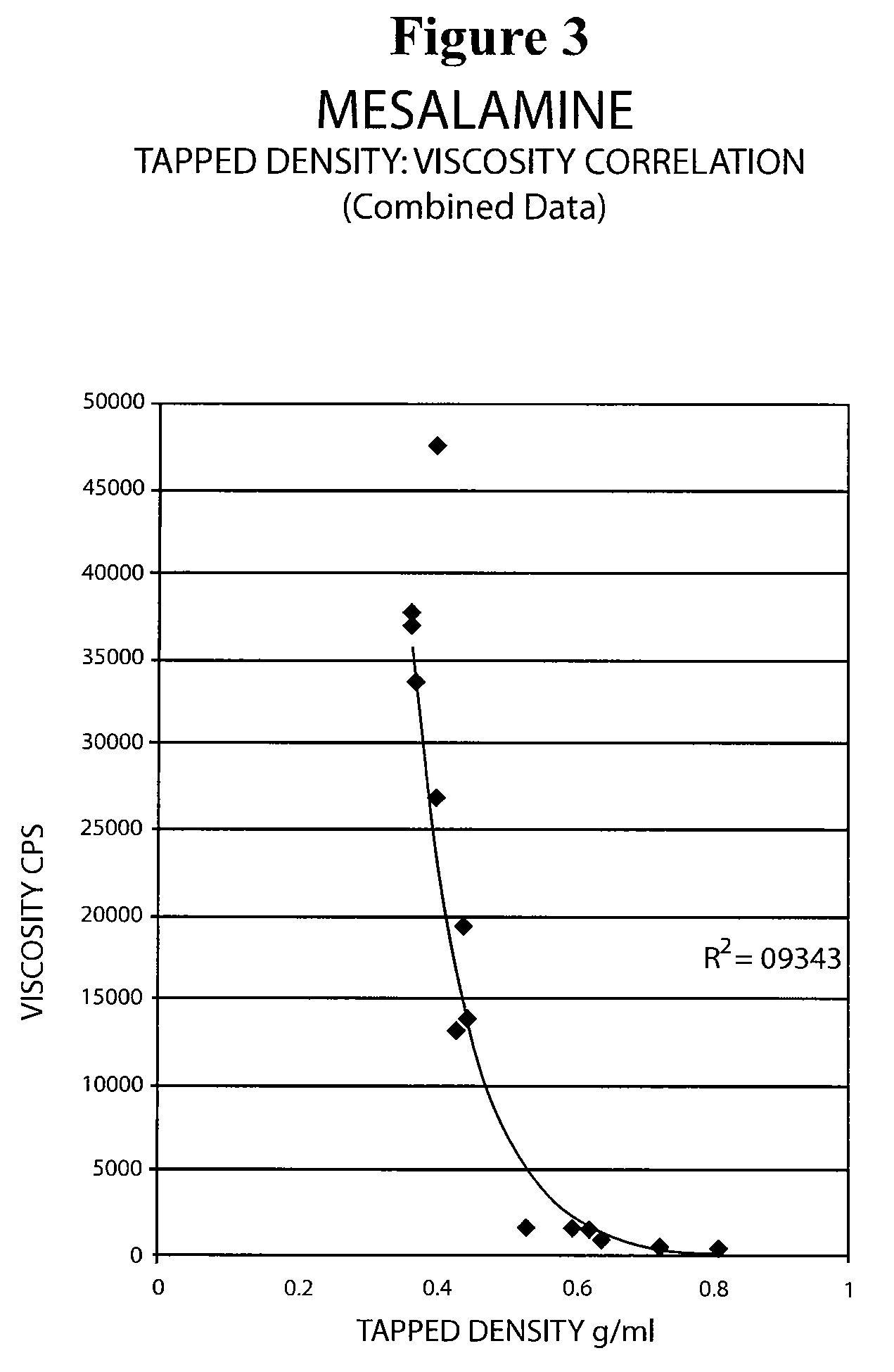
Mesalamine suppository
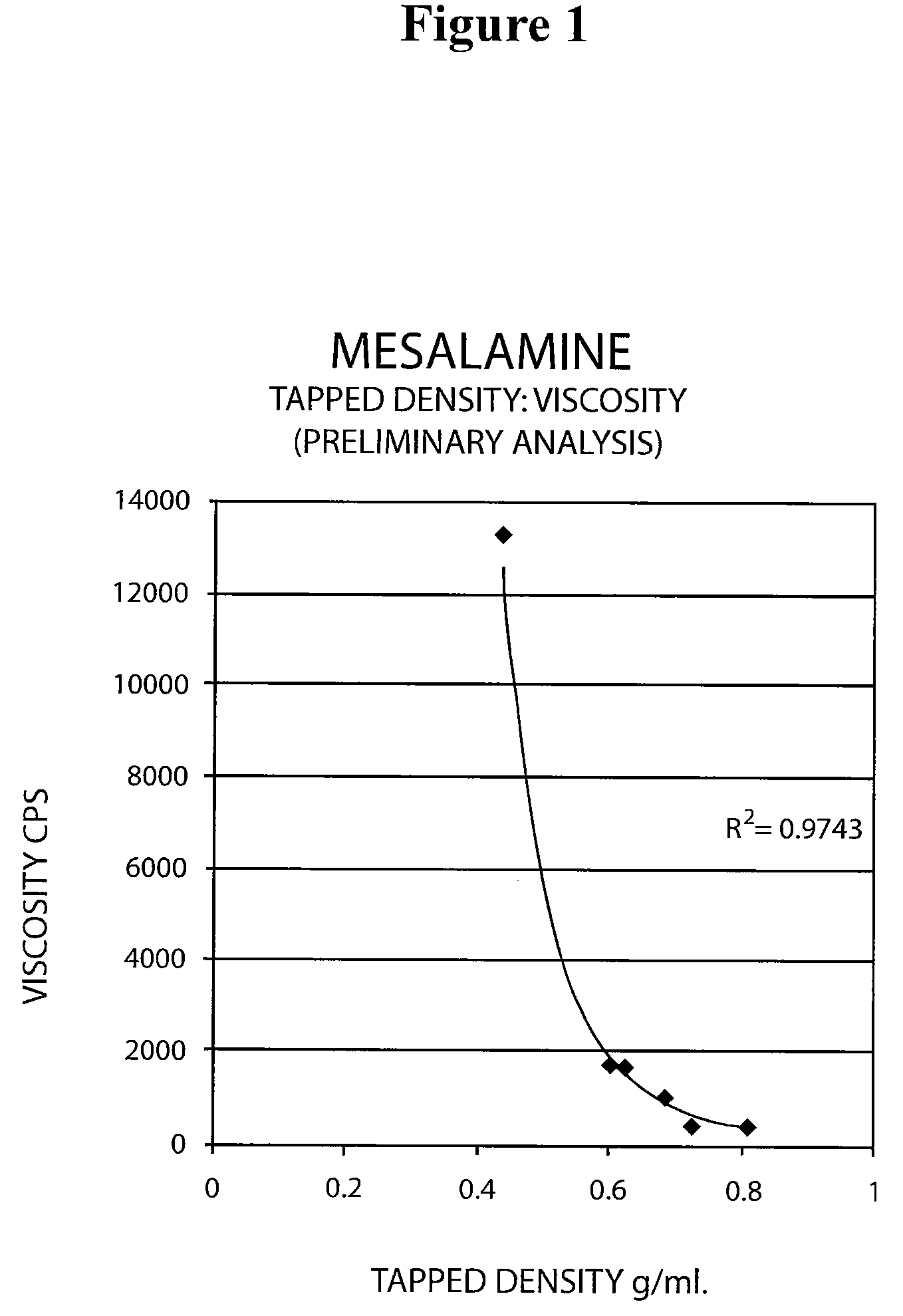
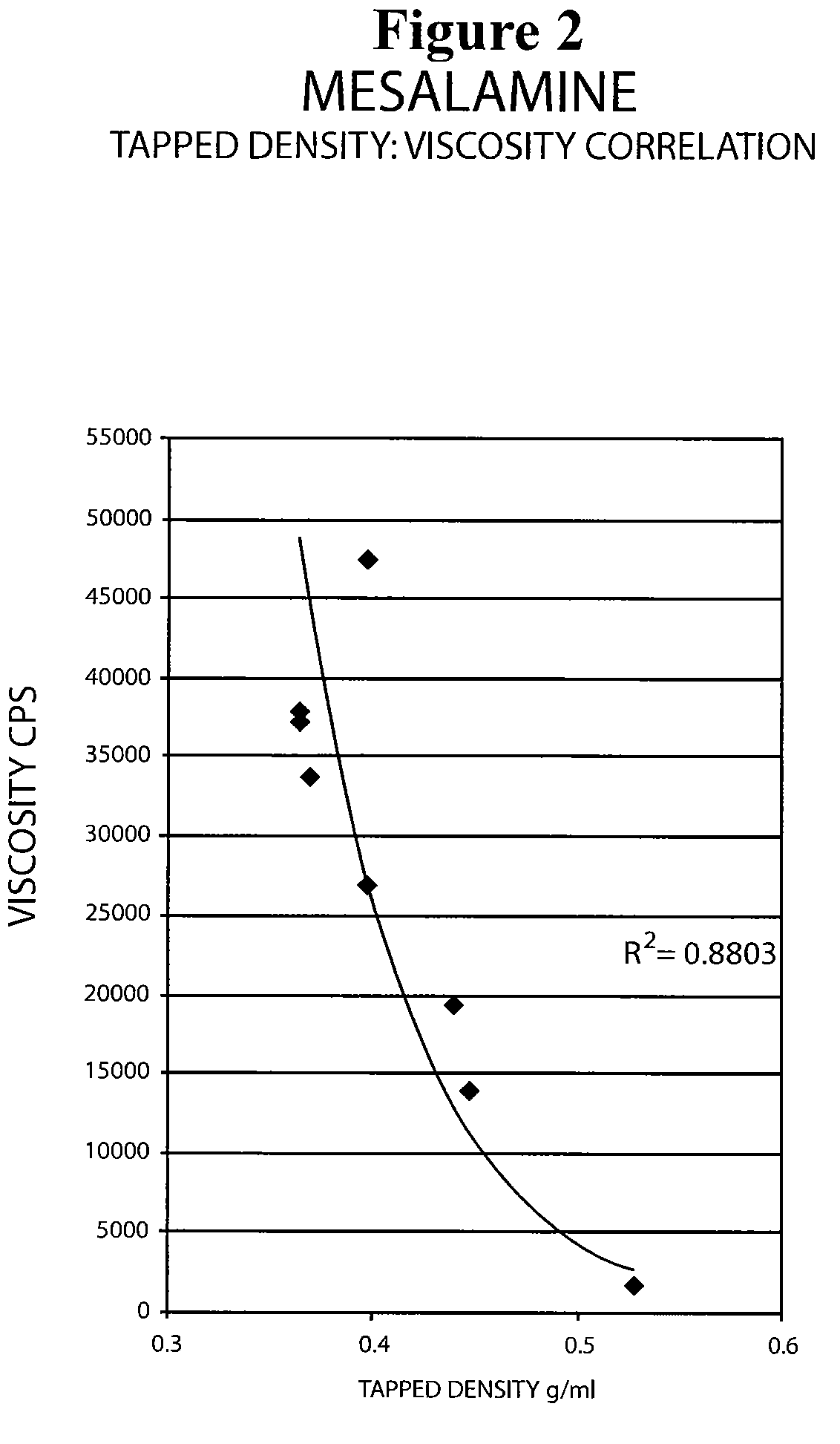
ActiveUS20090022793A1Improve the comfort of useHigh tap densityBiocideSalicyclic acid active ingredientsRectal SuppositoryPhosphate
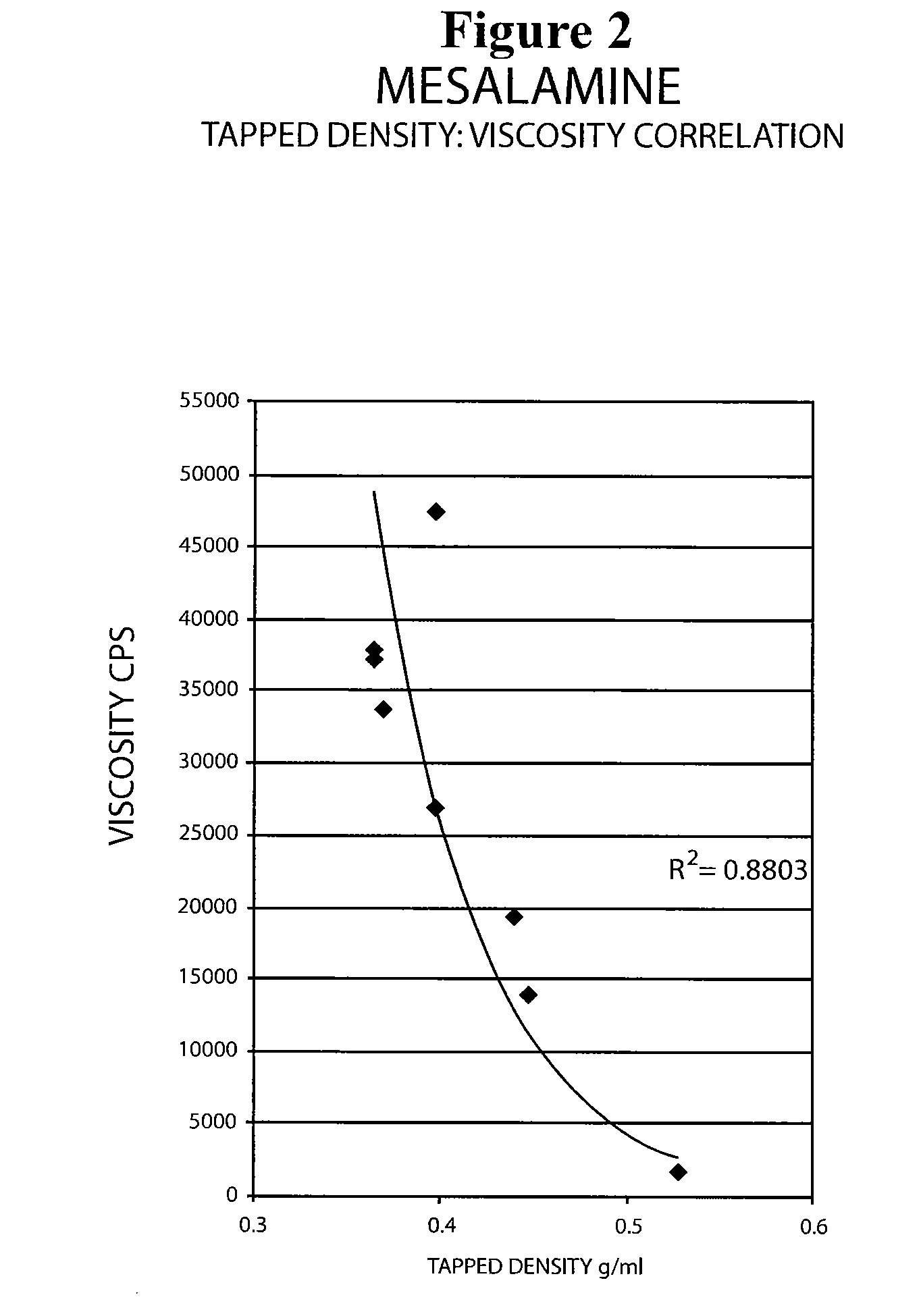
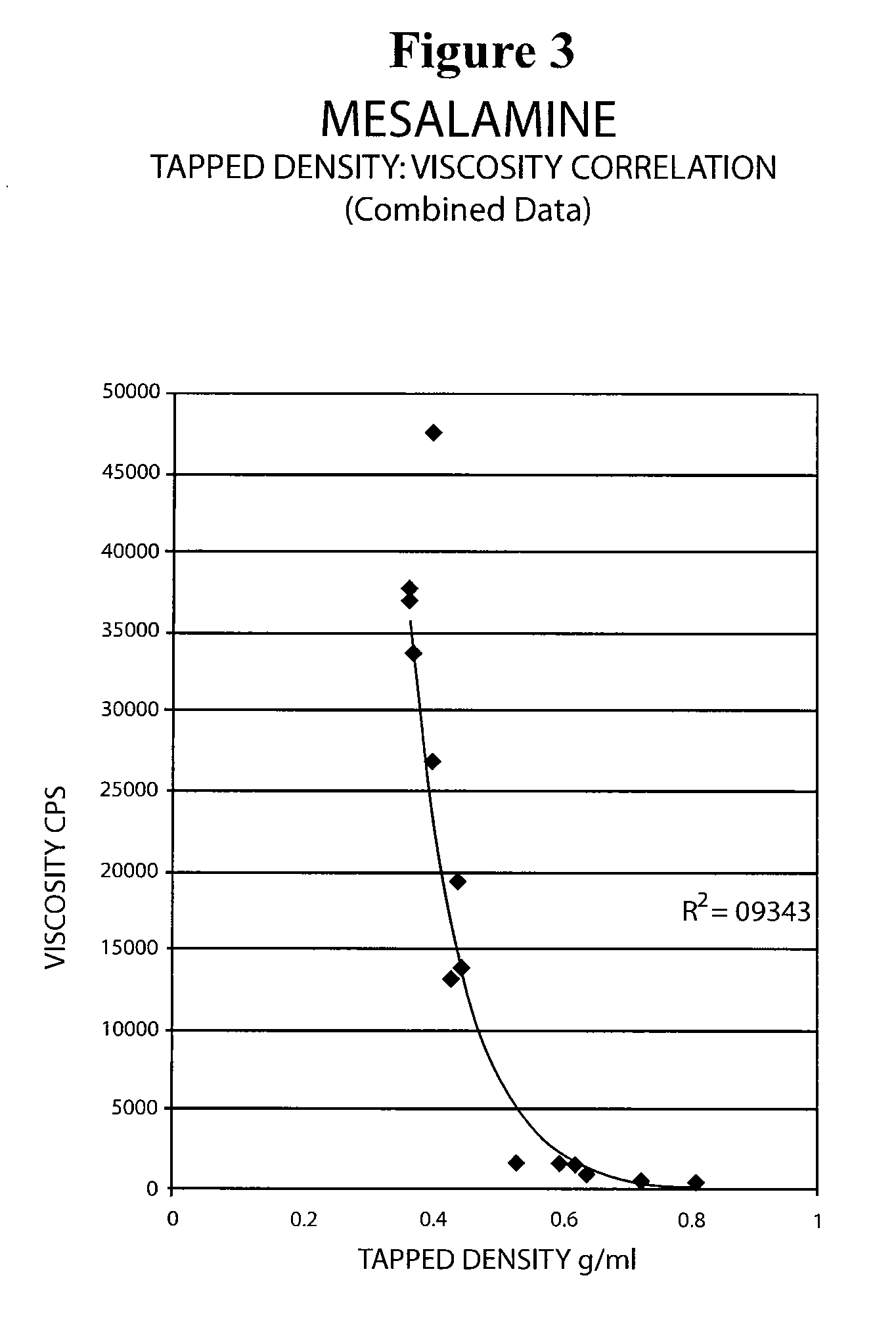
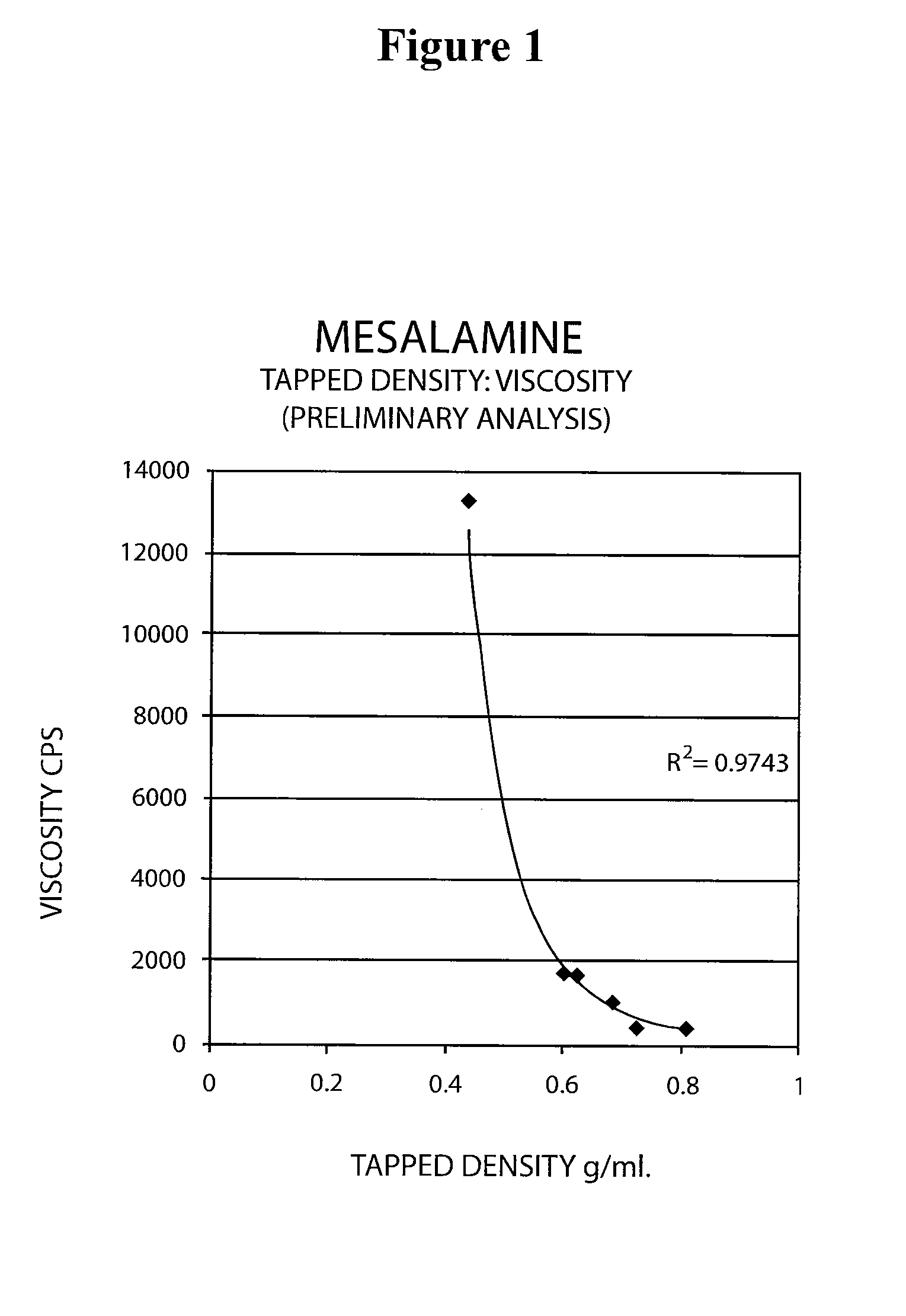
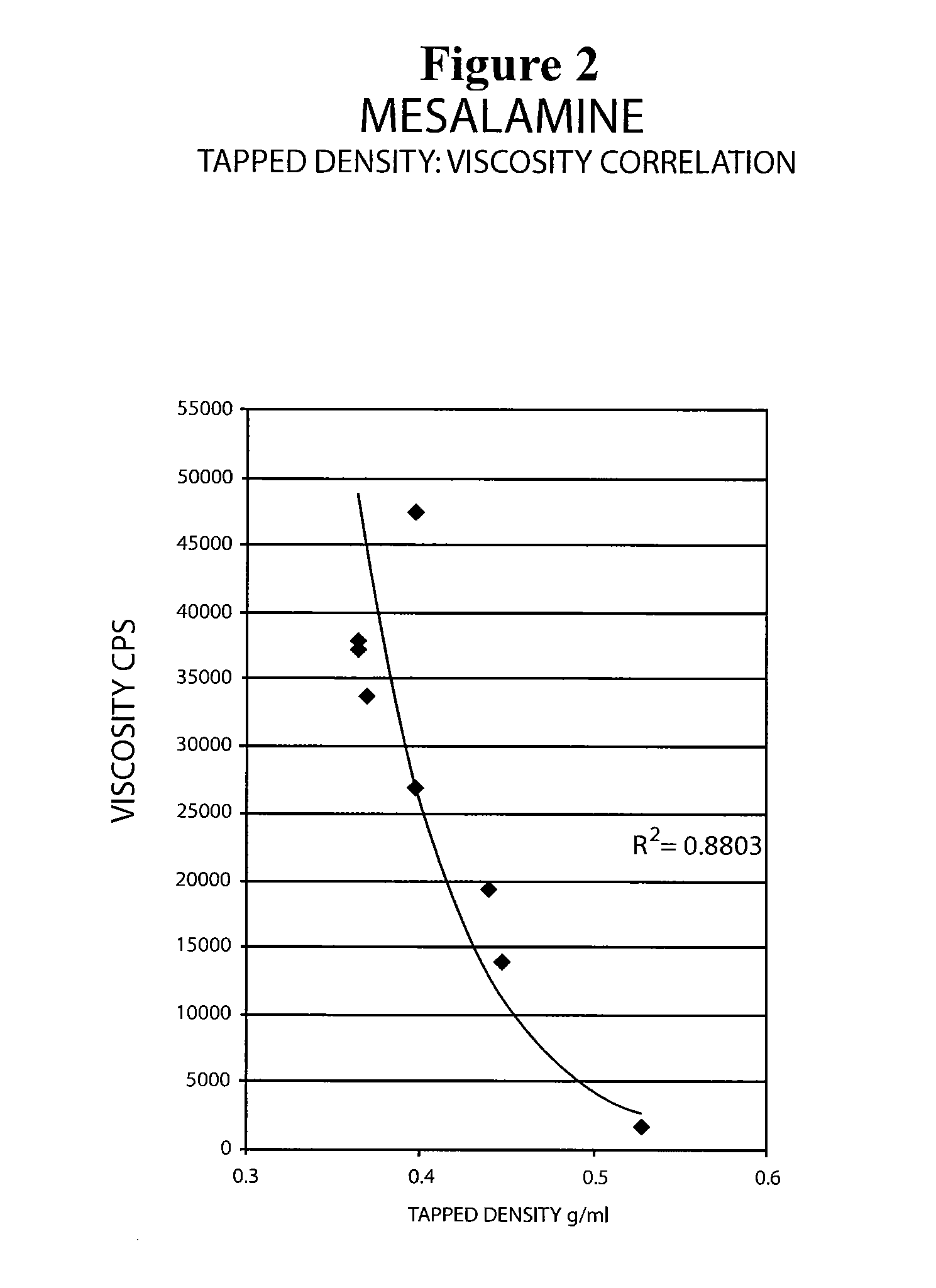
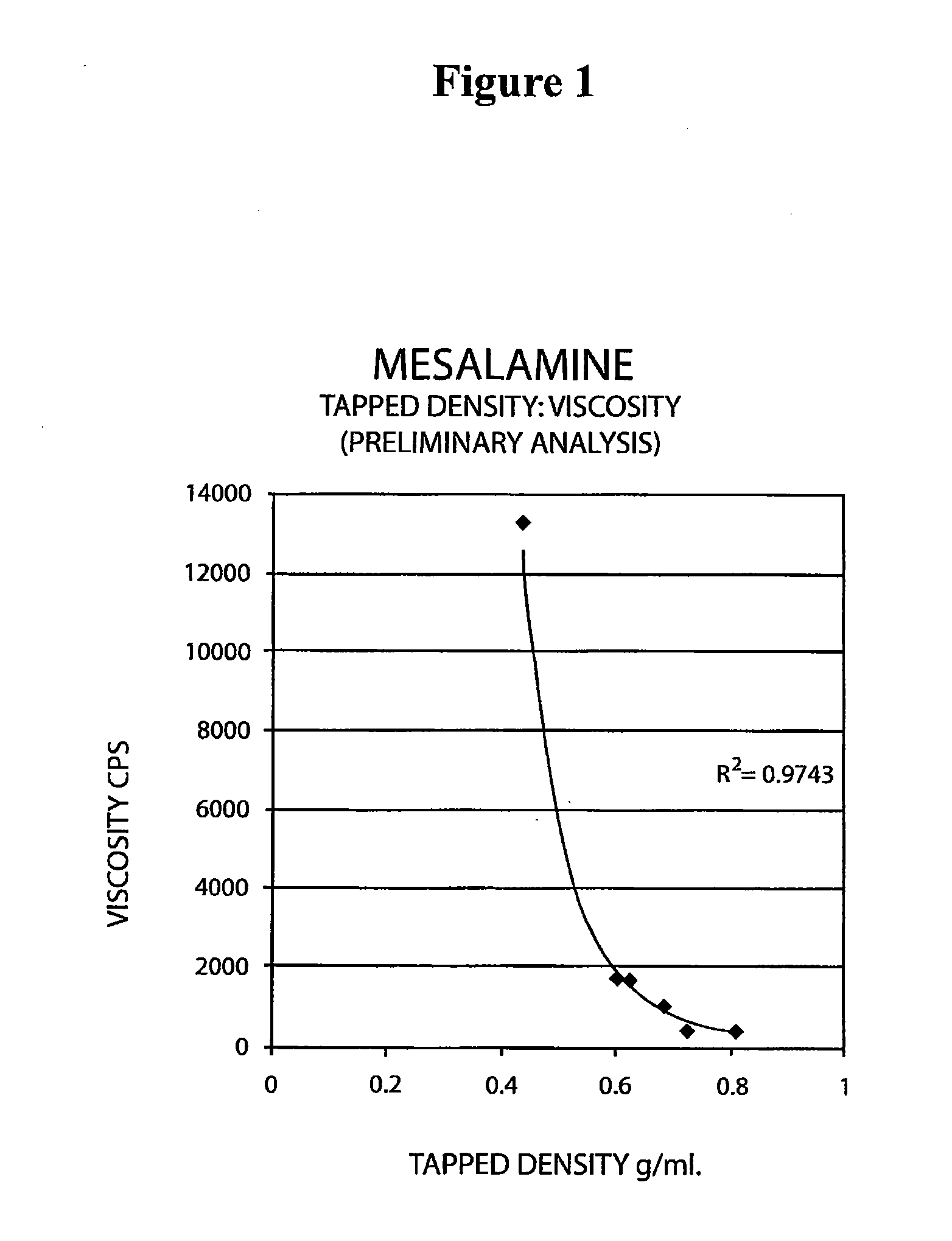
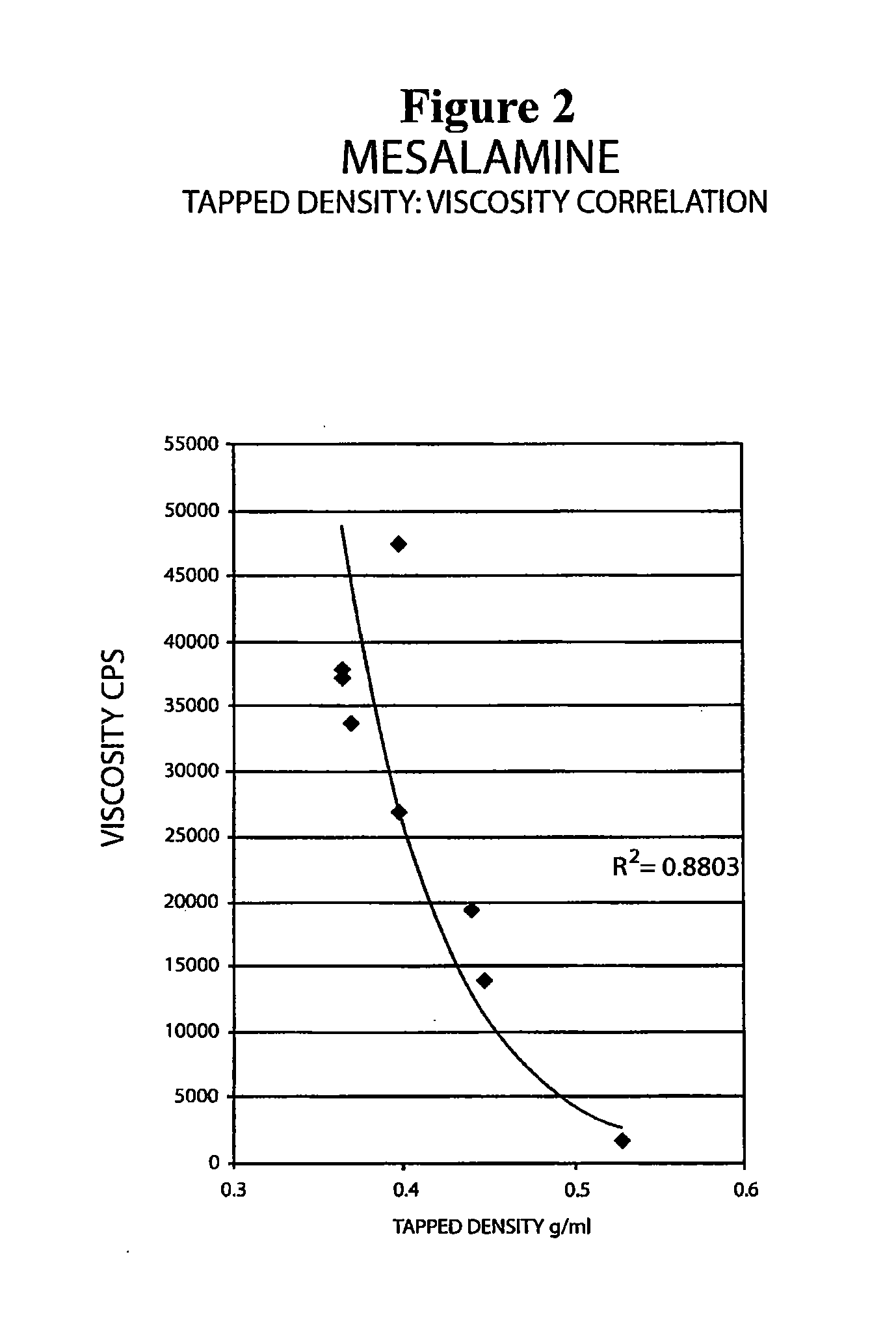
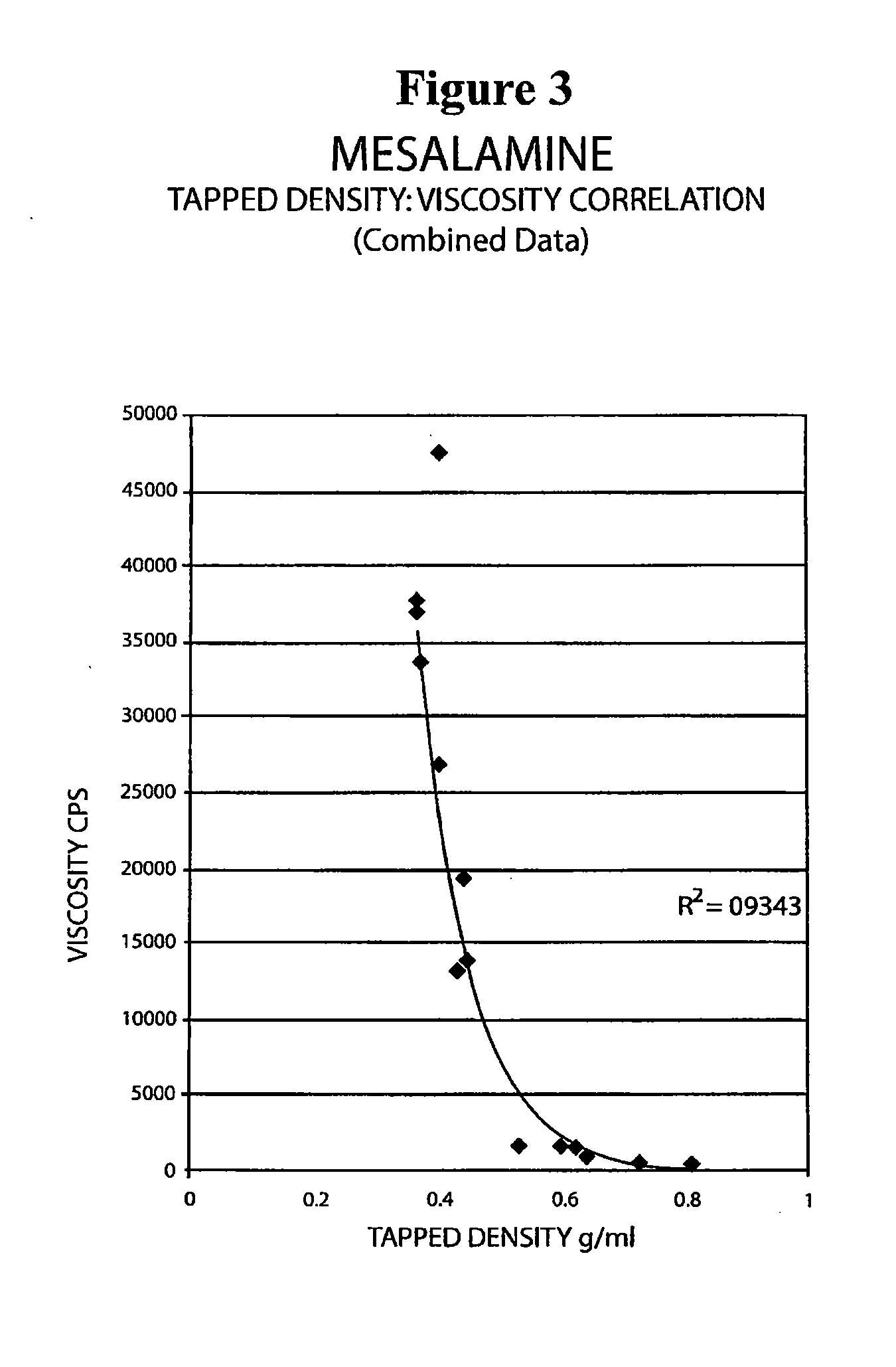
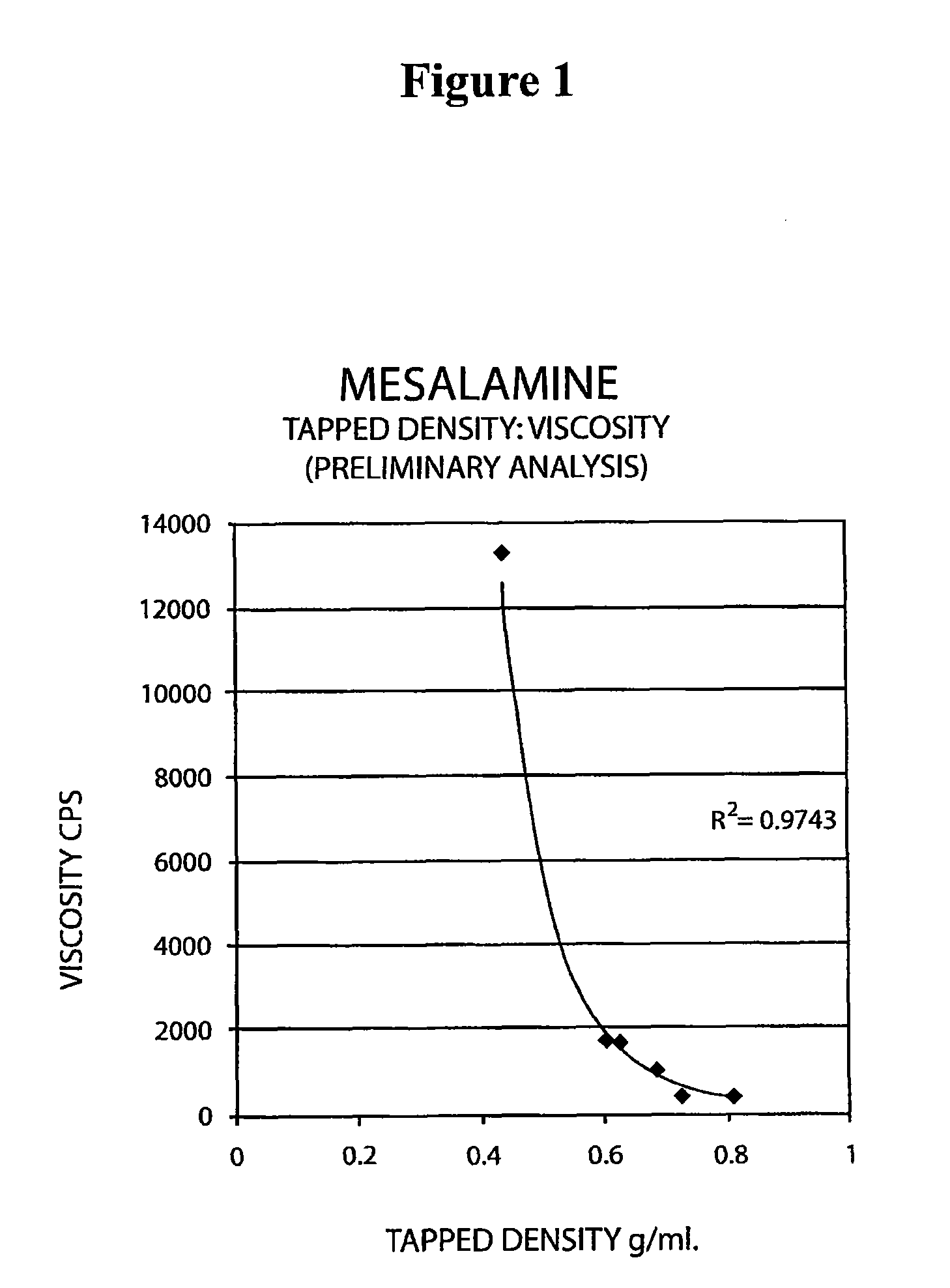
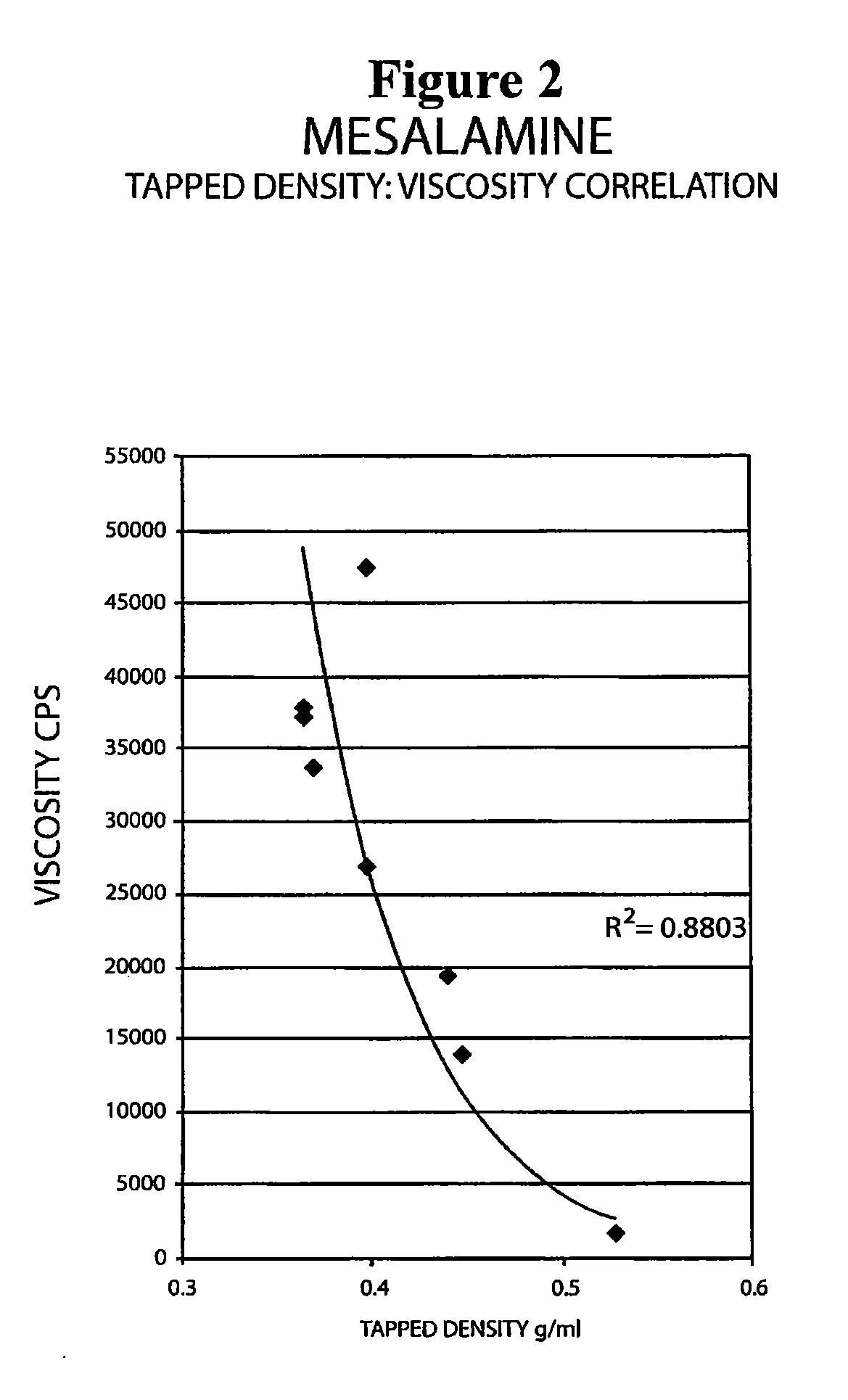
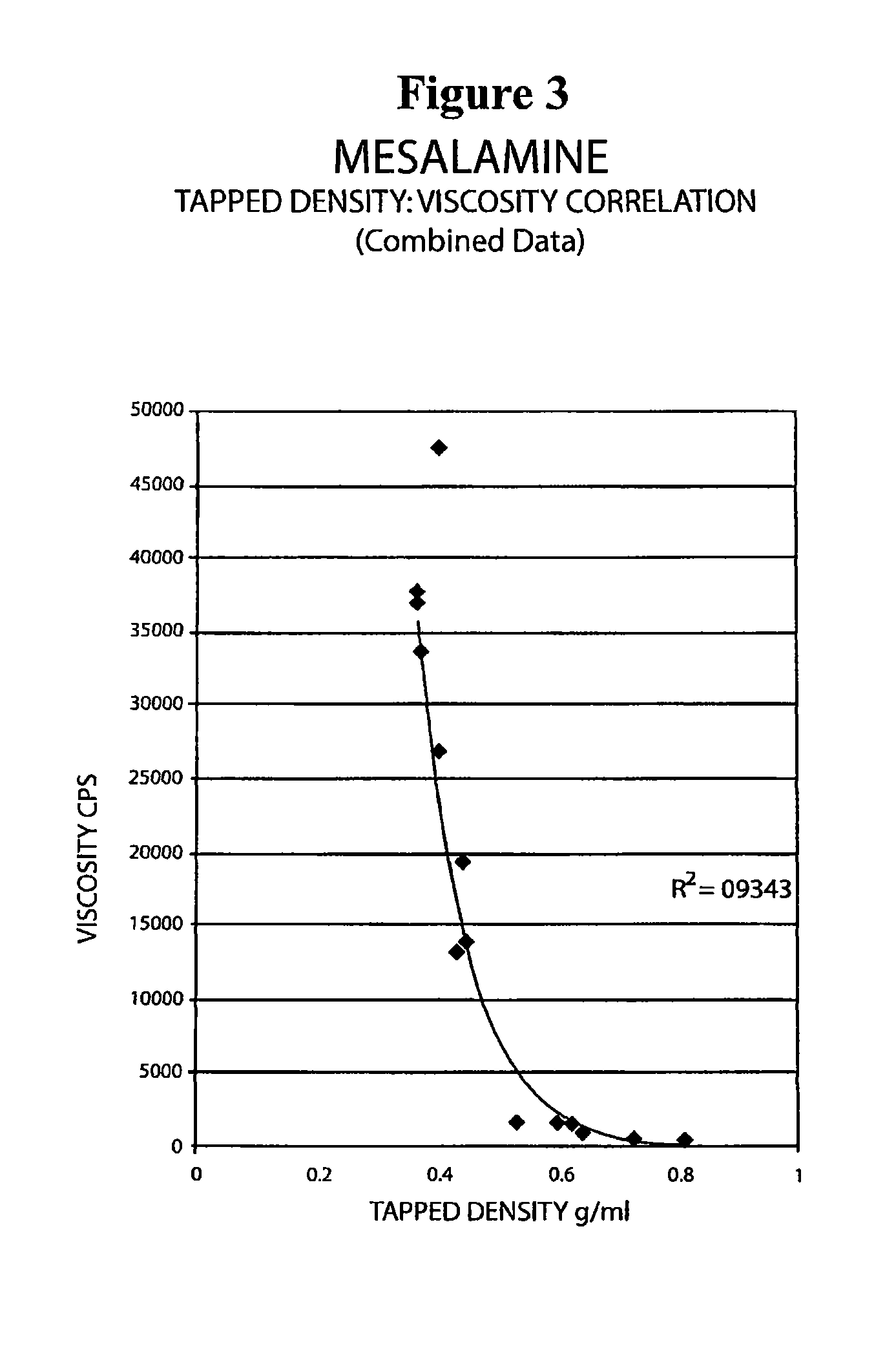
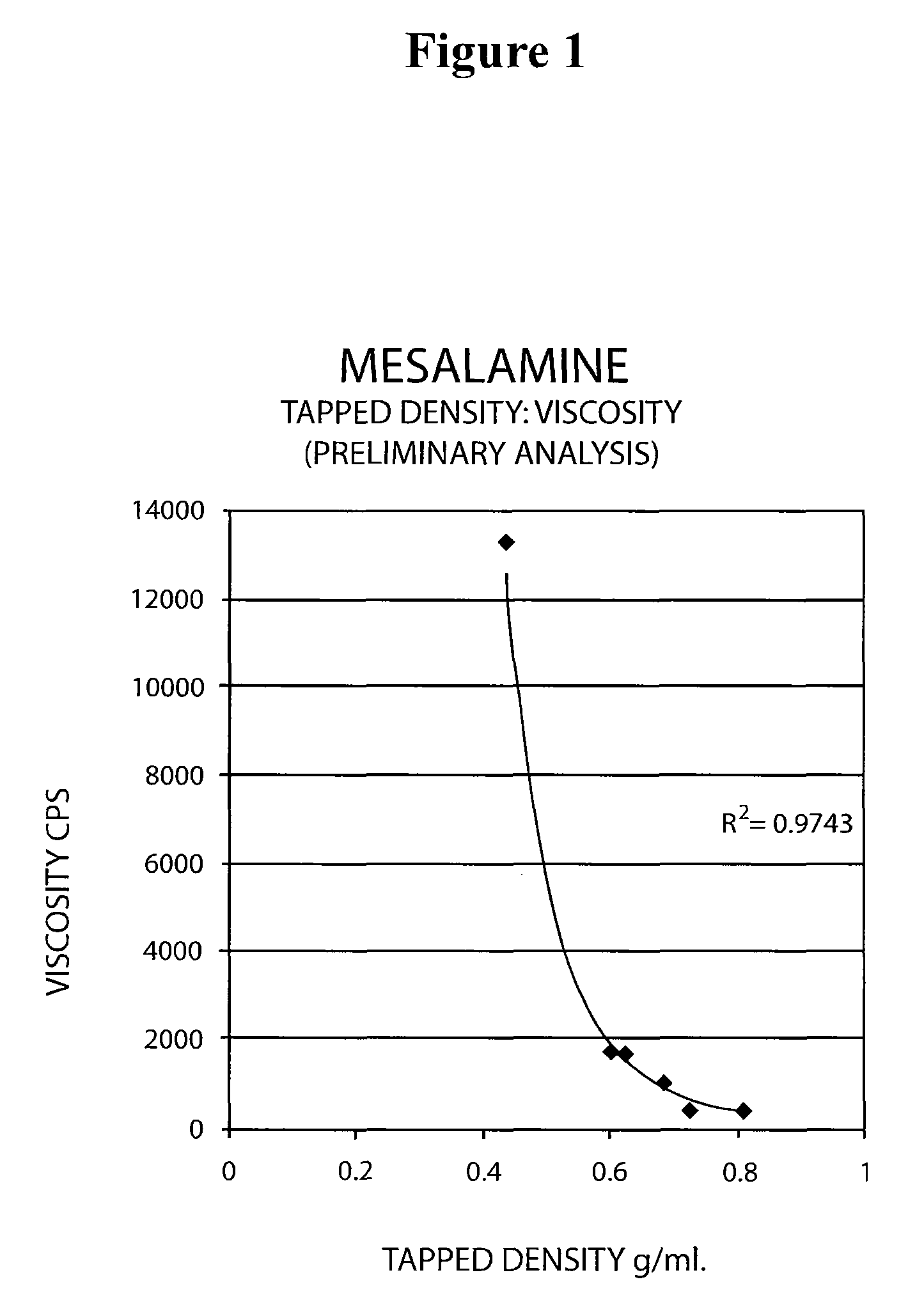
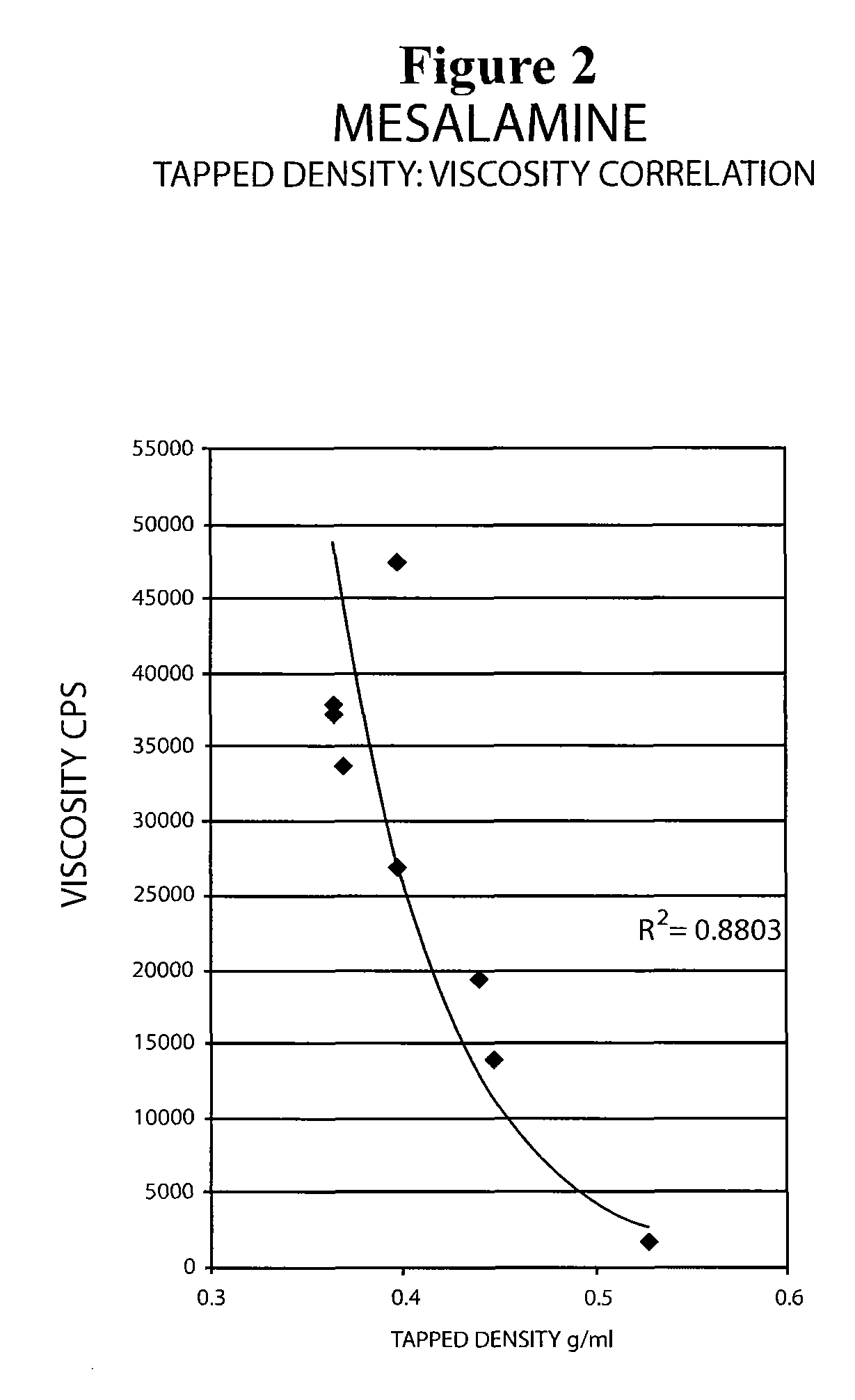
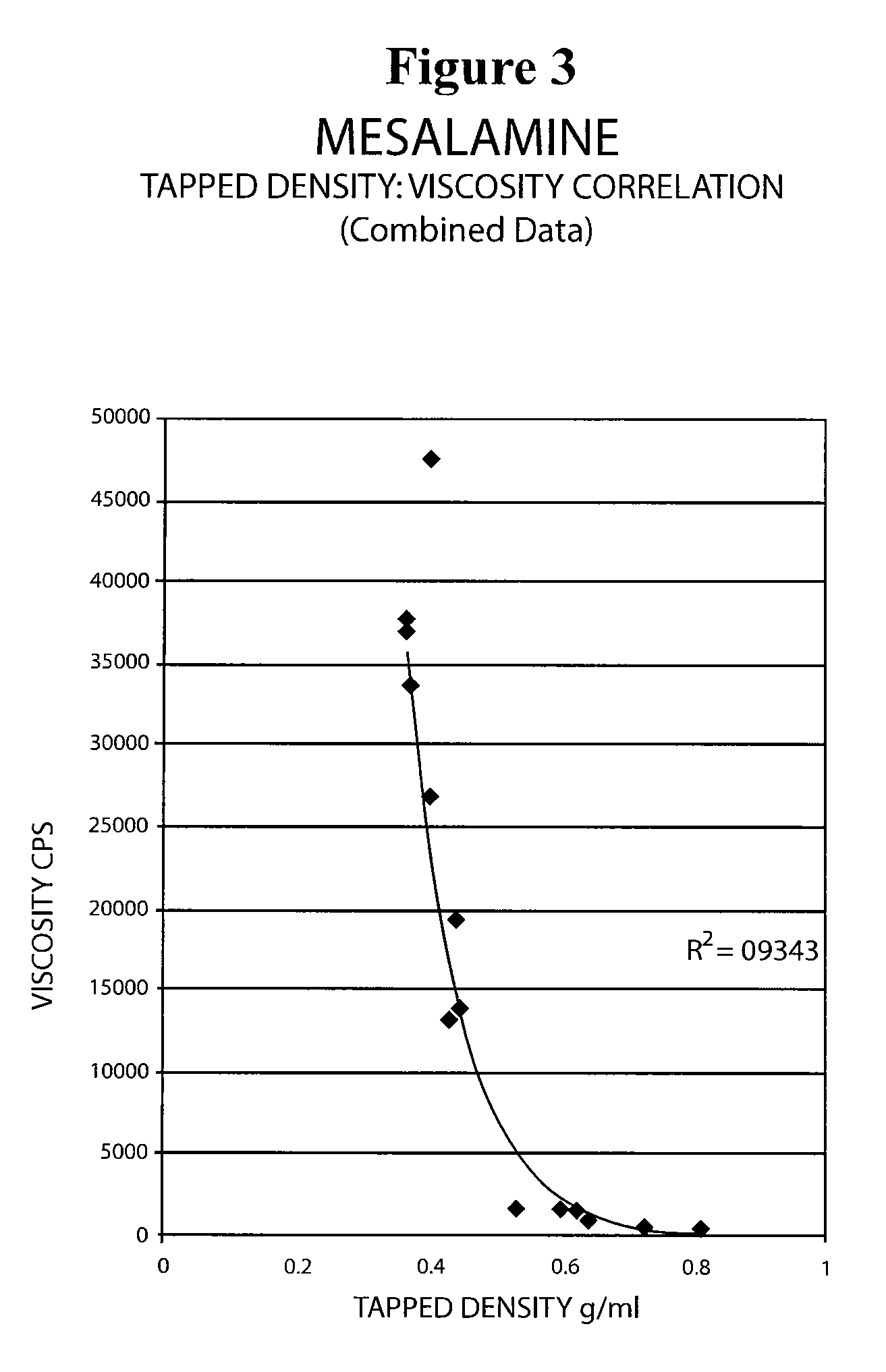
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 850 to about 1150 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 2250 to about 2700 mg. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:APTALIS PHARMA CANADA
Mesalamine suppository
ActiveUS20090264386A1Improve the comfort of useHigh tap densitySalicyclic acid active ingredientsBiocideRectal SuppositoryPhosphate
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 850 to about 1150 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 2250 to about 2700 mg. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 400 to about 600 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 870 to about 1715 mg. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:AXCAN PHARM CANADA INC
Wintergreen barberry root suppository and preparation method thereof
InactiveCN101618077AEnriched Litectin medicinal productsDigestive systemSuppositories deliveryAdditive ingredientPh regulation
The invention relates to a wintergreen barberry root suppository and a sustained and controlled release wintergreen barberry root suppository, belonging to the technical field of pharmaceuticals. The preparation is prepared by the following steps: using wintergreen barberry roots as raw materials and carrying out steps of pulverization, reflux extraction, decompression concentration, pH regulation, and the like to obtain a tan wintergreen barberry root extract; using the tan wintergreen barberry root extract as an active pharmaceutical ingredient and adding a fat-soluble suppository matrix orsustained and controlled release macromolecular materials to prepare the wintergreen barberry root suppository or the sustained and controlled release wintergreen barberry root suppository. The preparation has obvious anti-inflammatory effect and can be prepared into a rectal suppository used for treating internal hemorrhoids and rectal inflammations and a vaginal suppository used for treating vaginitis and acute vaginitis according to different application and administration routes.
Owner:蒋伟哲
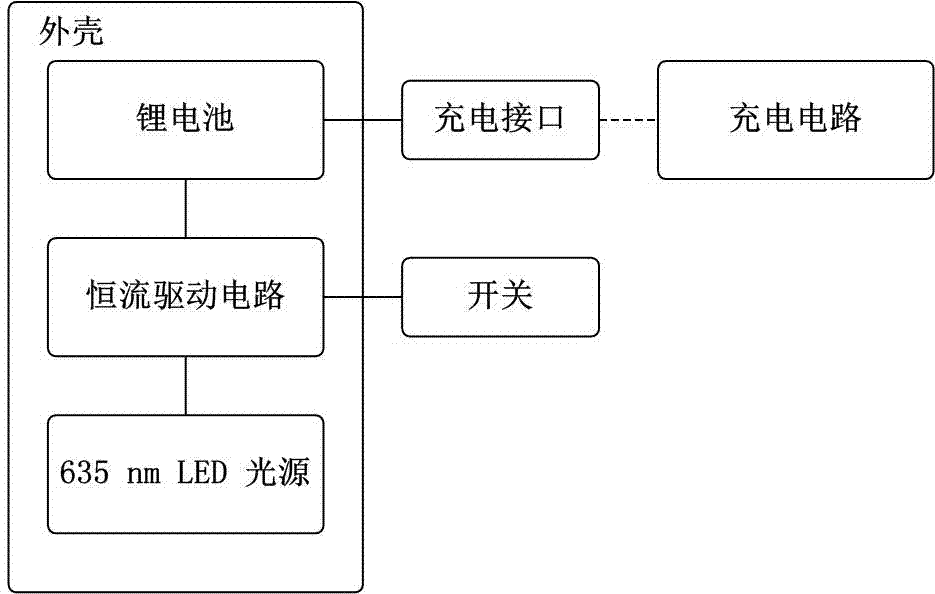
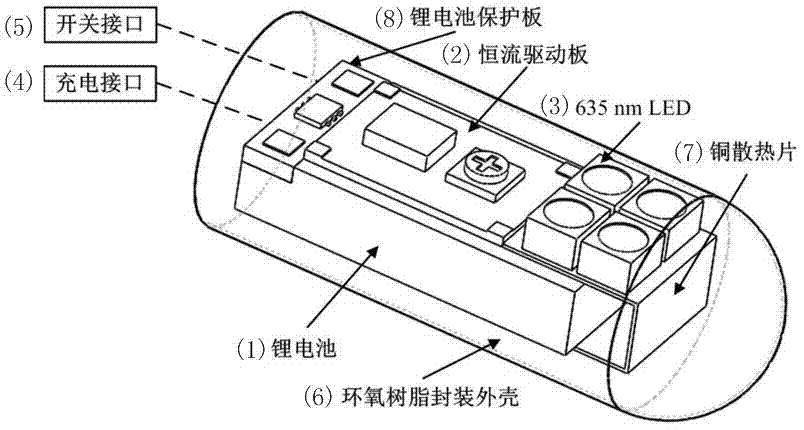
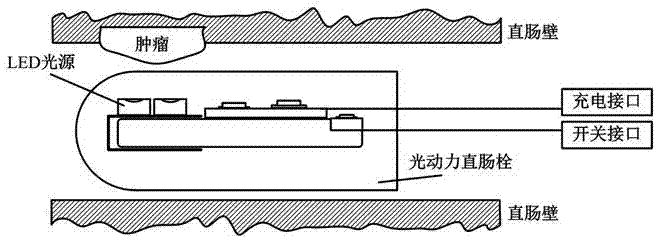
Rectal suppository LED (Light Emitting Diode) light source for photodynamic therapy of rectal cancers
ActiveCN106902468AUnrestricted activityLarge luminous areaLight therapyRectal SuppositoryPhotodynamic therapy
The invention discloses a rectal suppository irradiation light source for photodynamic therapy of rectal cancers, and belongs to a device for photodynamic therapy. A 635nm LED (Light Emitting Diode) light source, a lithium battery, a constant current control circuit and relevant circuits are packaged in a bullet-type rectal suppository having the length of about 3cm, the diameter of about 1cm and the front end slightly acute, wherein the packaging material is transparent epoxy resin adhesive. By such design, the overall light source can be placed in the rectum of a patient for photodynamic therapy, and is suitable for long-time and low-energy continuous irradiation. The photodynamic therapy process of rectal cancers is simplified, the compliance of patients is improved, the labor intensity of medical staffs is relieved, the cost of photodynamic therapy is reduced, the limitation of the photodynamic therapy space is broken, and patients can accept the therapy in clinics or at home. The light source involves in a scientific research experiment method as clinical reference data of rectal cancers.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
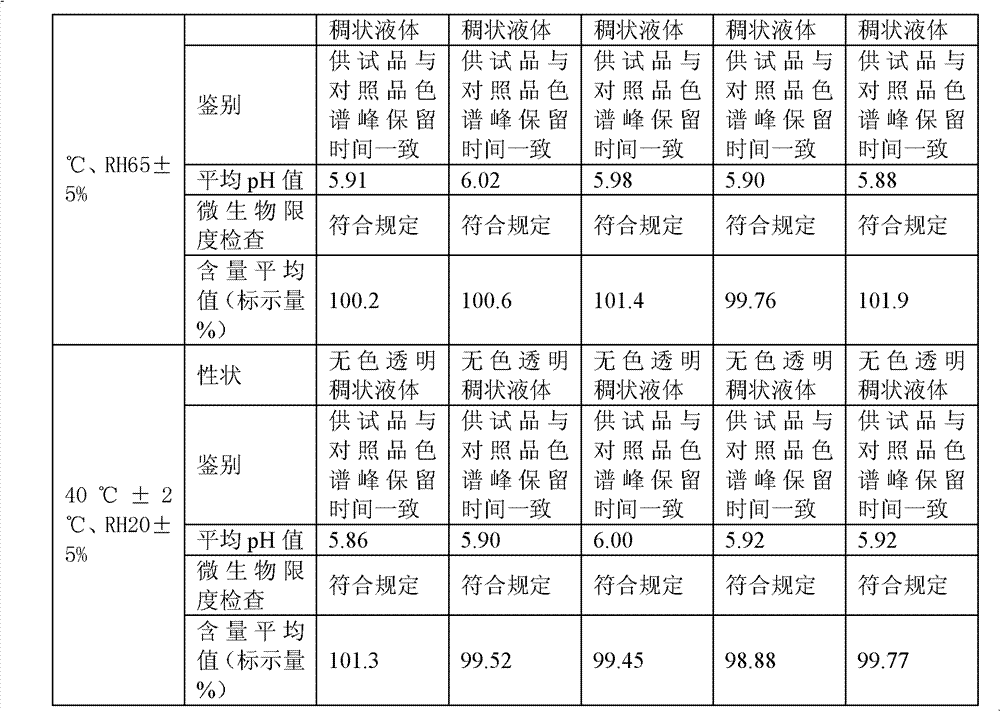
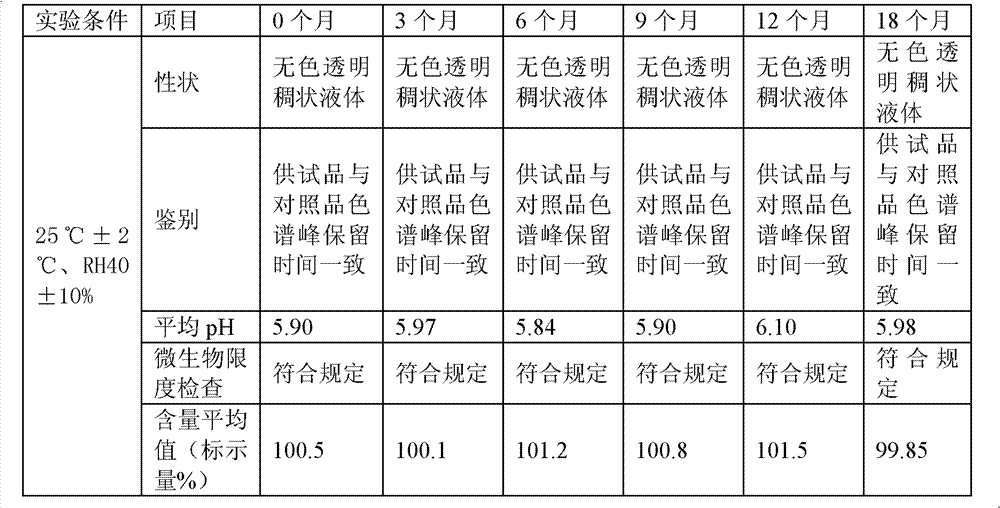
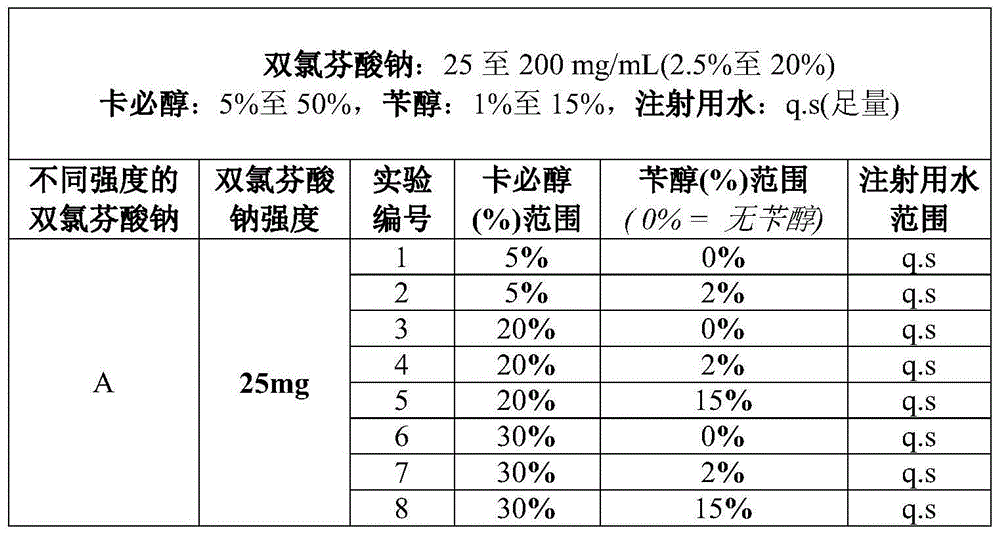
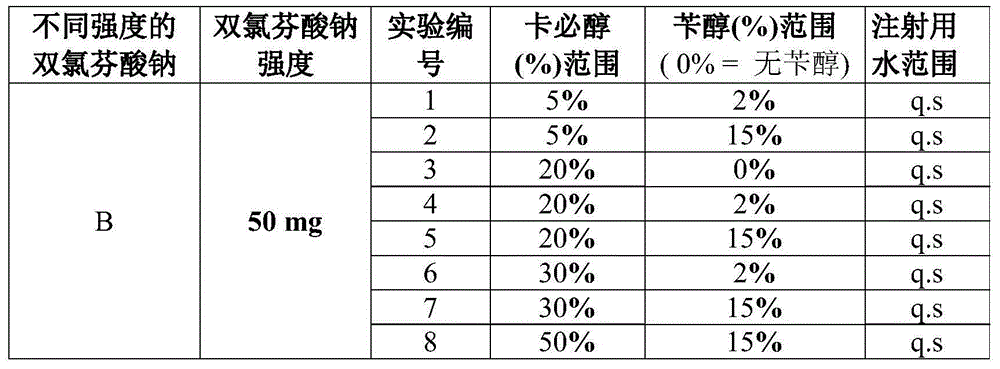
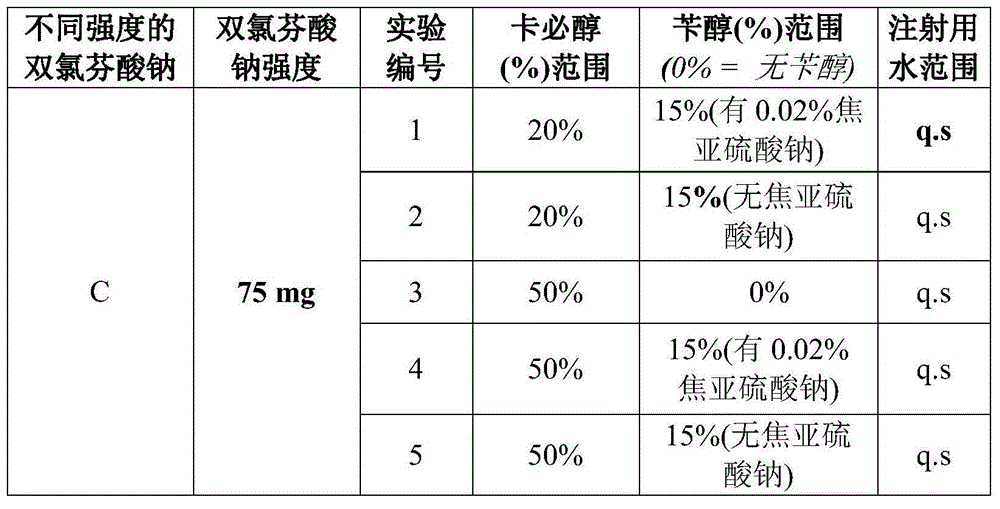
Diclofenac compositions
InactiveUS20140187635A1Not cause hemolysisNot irritatingBiocideOrganic active ingredientsNasal cavityRectal Suppository
The present invention relates a composition comprising Diclofenac and salts thereof wherein Diclofenac or its salts are present in an amount of 25-200 mg. The composition is suitable for the parenteral administration through intramuscular, intravenous route; also for oral, dermal, subcutaneous, cutaneous, nasal, ocular drops, as rectal suppository, vaginal pessaries, intra-articular, and otic delivery. The invention also provides compositions comprising a combination of Diclofenac and other drugs. The invention further provides a method for preparing said composition.
Owner:THEMIS MEDICARE LTD
Mesalamine suppository
ActiveUS20100105639A1Improve the comfort of useHigh tap densityPowder deliverySalicyclic acid active ingredientsRectal SuppositoryPhosphate
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Yet another embodiment is a mesalamine rectal suppository comprising mesalamine particles and one or more pharmaceutically acceptable excipients, where the mesalamine particles have a surface area of from about 0.1 m2 / g to about 2.8 m2 / g (e.g., from about 0.1 m2 / g to about 1.3 m2 / g). Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:AXCAN PHARM CANADA INC
Mesalamine suppository
InactiveUS20160175329A1Small sizeEasy to keepOrganic active ingredientsBiocideRectal SuppositoryGynecology
A mesalamine rectal suppository having improved comfort of use and better retention in lower rectum for a long period of time is provided. The suppository contains a suppository base, at least one surfactant and at least one mucoadhesive agent. A method for manufacturing the suppository and methods for treating ulcerative colitis, such as active ulcerative proctitis, using such suppository is also provided.
Owner:LUPIN ATLANTIS HLDG
Mesalamine suppository
ActiveUS8436051B2Improve the comfort of useHigh tap densityBiocideSalicyclic acid active ingredientsRectal SuppositorySuppository drug
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Yet another embodiment is a mesalamine rectal suppository comprising mesalamine particles and one or more pharmaceutically acceptable excipients, where the mesalamine particles have a surface area of from about 0.1 m2 / g to about 2.8 m2 / g (e.g., from about 0.1 m2 / g to about 1.3 m2 / g). Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:AXCAN PHARM CANADA INC
Mesalamine suppository
ActiveUS8217083B2Improve the comfort of useHigh tap densitySalicyclic acid active ingredientsBiocideRectal SuppositoryPhosphate
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository comprising mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 850 to about 1150 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 2250 to about 2700 mg. Another embodiment of the invention is a mesalamine rectal suppository comprising from about 400 to about 600 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 870 to about 1715 mg. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:AXCAN PHARM CANADA INC
Rectal suppository for anaesthesia and preparation method thereof
InactiveCN107137398AAvoid first pass effectSmall incrementOrganic active ingredientsSuppositories deliveryGynecologyRectocutaneous
The invention discloses a rectal suppository for anaesthesia. The preparation method of the rectal suppository comprises the following steps: taking dexmedetomidinehydrochloride and ketamine hydrochloride as raw materials, adding a certain amount of matrix, a surfactant, a diluent, a humectant and a bacteriostat, and performing matrix melting, mixing, membrane injection, cooling shaping and packagingseparately. The rectal suppository does not require building a special administrationchannel, is directly dosed through arectum, avoids the liver first pass effect, takes effect quickly and is good in effect; the melting temperature of the rectal suppository is between 32EDG C to 35 EDG C, not only is beneficial to product storage, but also does not affect melting of the rectal suppository during use, meanwhile, the content of the rectal suppository is uniform and stable, A plus 1.8S of multiple products is smaller than 8, the product stability is good, the shelf life reaches 24 months, the increment of impurities is small during storage, the preparation process is simple and practical, and the rectal suppository is worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
Rectal mucosal administration preparation of pulsatilla chinensis saponin b4 and preparation method therefor
A rectal mucosal administration preparation of Pulsatilla chinensis (Bge.) Regel saponin B4 contains Pulsatilla chinensis (Bge.) Regel saponin B4 and a pharmaceutically acceptable substrate. The drug preparation is a rectal gel or a rectal suppository. Compared with oral administration, the rectal mucosal administration preparation of Pulsatilla chinensis (Bge.) Regel saponin B4 has a lower effective dose, and has a better medicinal effect at the same dose.
Owner:LIU QI
Rectal suppository
InactiveCN1502326AHeavy metal active ingredientsInorganic non-active ingredientsRectal SuppositoryMedicine
The present invention discloses a rectal suppository. It is characterized by that in the course of its production the tourmaline, wheat-ball stone and magnet can be added, and the long-term application of said suppository is beneficial to prostate.
Owner:鲁景远
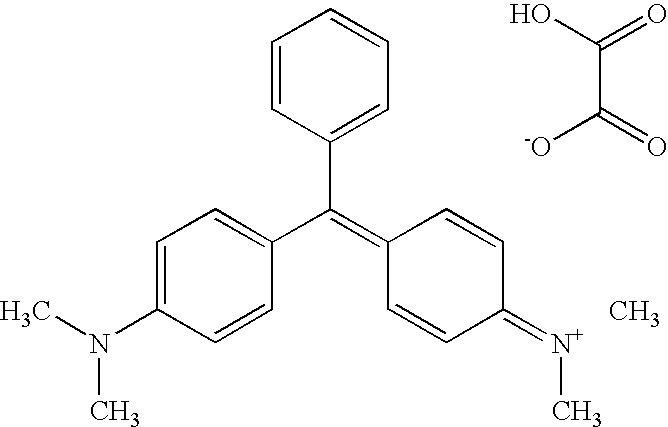
Use of malachite green oxalate for treating malignant neoplasms
InactiveUS8436195B2Shorten the durationGood curative effectBiocideOrganic chemistryDiseaseSide effect
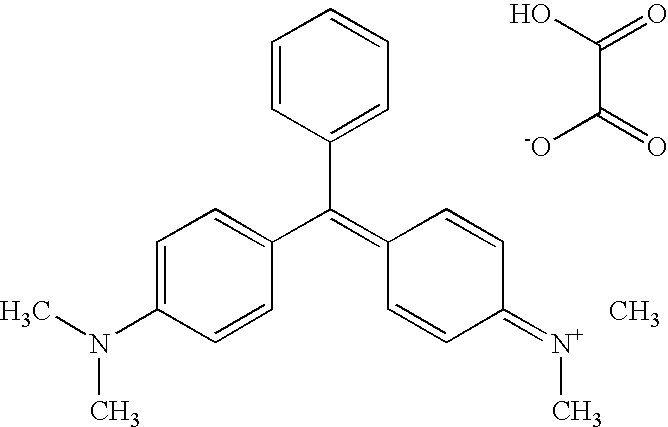
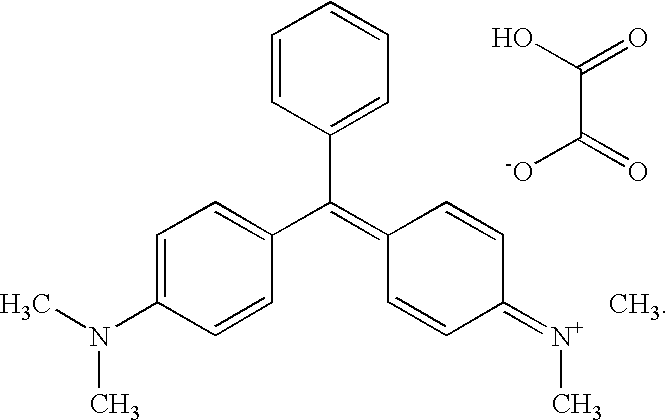
The use of malachite green in the form of a tetramethyldiamino-triphenyl-carbinol anhydro-oxalate [(C23H25N2)—(C2HO4)]2—C2H2O4 of the following structural formula as a drug for treating malignant neoplasms administered in a single dose of 1 to 2 g. Malachite green can be used when dissolved in an aqueous solution or in a physiological salt solution or in spirit. In different cases, the malachite green solution is perorally administered before and after a meal or is injected per rectum, or a 1% malachite green solution is intravenously introduced, or the malachite green is applied as a rectal suppository component or as a component of 1-5% ointment. The medicinal agent exhibits an extended range of therapeutic actions with respect to different oncological diseases, is freely available, not expensive, non-toxic, does not generate side effects when used in pharmaceutically acceptable doses, and makes it possible to reduce a treating time and to increase the efficiency of treatment.
Owner:GERMANOV EVGENY PAVLOVICH +1
Dose-controlled transdermal promethazine compositions and methods of use
InactiveUS20110092493A1Minimize side effectsMinimizing adverse reactionDigestive systemLiposomal deliverySide effectPromethazine
The current invention provides formulations for transdermal delivery of promethazine which achieve delivery of the drug with consistent plasma levels. The topical formulations of the invention are superior to both rectal suppositories and oral dosage forms in that variable blood levels, first-pass metabolism, unpredictable peaks in blood levels, and variable bioavailability are minimized. Formulations of the invention provide antiemetic and antipruritic relief to patients in need of treatment while minimizing side effects and adverse reactions known to occur with other routes of administration and other formulations.
Owner:LEVI CLARK +3
Rectal cancer photodynamic therapy mini-type LED illumination source based on wireless power supply
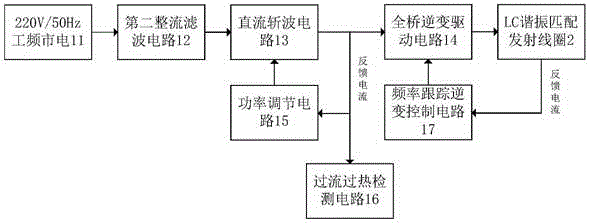
InactiveCN106422073AStable voltageEasy switch controlLight therapyElectric power transmissionVoltage regulator module
The present invention discloses a rectal cancer photodynamic therapy mini-type LED illumination source based on wireless power supply. The source comprises bullet-shaped housing, the housing is internally provided a voltage regulator module and a LED light source with the wavelength range of 630-650nm, the housing is internally provided with a LC resonance matching receiving coil and a first rectification filter circuit, the LC resonance matching receiving coil is 20*5*2mm, and the inductance value is 60[Mu]H; the mini-type LED illumination source also comprises an electric power emission unit consisting of a current generation current and a LC resonance matching sending coil; and the current generation circuit is configured to generate alternating current to allow the LC resonance matching sending coil to generate resonance. The Rectal cancer photodynamic therapy mini-type LED illumination source based on the wireless power supply can obtain electric energy at any moment so as to solve the power supply problem in the rectal suppository therapy and enhance the reliability of the rectal suppository. The contradictions of the mini-type implantable medical device power supply and the small size, and the attention of medical staff to the rectal suppository electric quantity is saved, and a condition is created for the product clinical expansion.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Mesalamine suppository
ActiveUS7541384B2Improve the comfort of useHigh tap densityBiocideSalicyclic acid active ingredientsRectal SuppositoryPhosphate
The present invention relates to a mesalamine rectal suppository designed to provide improved comfort of use. One embodiment of the invention is a mesalamine rectal suppository containing mesalamine and one or more pharmaceutically acceptable excipients, wherein the drug load of the suppository ranges from 35% to 50%. Another embodiment of the invention is a mesalamine rectal suppository containing from about 850 to about 1150 mg mesalamine and one or more pharmaceutically acceptable excipients, wherein the total weight of the suppository ranges from about 2250 to about 2700 mg. Yet another embodiment of the invention is a mesalamine rectal suppository comprising mesalamine having a tap density ranging from about 600 to about 800 g / L (as measured by USP <616>) and a hard fat having an ascending melting point of 32 to 35.5° C. Methods of preparing and methods of treatment with mesalamine suppositories are also provided. The invention further provides a method of determining a dissolution parameter (such as dissolution rate) of a mesalamine rectal suppository, such as a 1 g mesalamine suppository, by measuring its dissolution with USP Apparatus #2 at 40° C. and a paddle rotation speed of 125 rpm in 0.2 M phosphate buffer at a pH of 7.5.
Owner:APTALIS PHARMA CANADA
Rectal suppository for ulcerative colitis
A rectal suppository comprising mesalamine and a local anaesthetic agent is provided. The suppository contains a suppository base, at least one surfactant and at least one mucoadhesive agent. A method for manufacturing the suppository and methods for treating ulcerative colitis, such as active ulcerative proctitis, using such suppository is also provided.
Owner:OAKDENE HLDG LLC
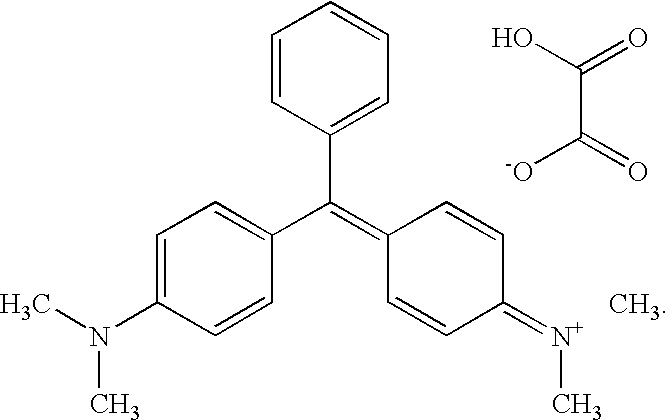
Use of malachite green in the form of drug for treating malignant neoplasms
InactiveUS20100105776A1Treatment duration is reducedGood curative effectBiocideOrganic chemistryMalachite greenDisease
The use of malachite green in the form of a tetramethyldiamino-triphenyl-carbinol anhydro-oxalate [(C23H25N2)—(C2HO4)]2—C2H2O4 of the following structural formula as a drug for treating malignant neoplasms administered in a single doze of 1 to 2 g. Malachite green can be used when dissolved in an aqueous solution or in a physiological salt solution or in spirit. In different cases, the malachite green solution is perorally administered before and after a meal or is injected per rectum, or a 1% malachite green solution is intravenously introduced, or the malachite green is applied as a rectal suppository component or as a component of 1-5% ointment. The medicinal agent exhibits an extended range of therapeutic actions with respect to different oncological diseases, is freely available, not expensive, non-toxic, does not generate side effects when used in pharmaceutically acceptable doses, and makes it possible to reduce a treating time and to increase the efficiency of treatment.
Owner:GERMANOV EVGENY PAVLOVICH +1
Suppository containing periplaneta americana and preparation method of suppository
ActiveCN107929325AEasy to useEasier to use than enemaAnthropod material medical ingredientsDigestive systemRectal SuppositoryOral medication
The invention belongs to the field of pharmaceutical preparations and particularly relates to a periplaneta americana rectum drug preparation. The periplaneta americana rectum drug preparation is a rectum suppository taking various forms of periplaneta americana extracts as active ingredients. The periplaneta americana suppository provided by the invention has the effect of prolonging the drug release and action time or quickly releasing to take effects, can be used for treating ulcerative colitis-related diseases and indications, significantly relieves intestinal ulcer and inflammatory reaction and overcomes the defects of worse oral administration effect and slow effect, the drug action efficiency is improved, a better treatment mode is provided for clinical patients, and clinical medication forms of the periplaneta americana preparation are enriched.
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
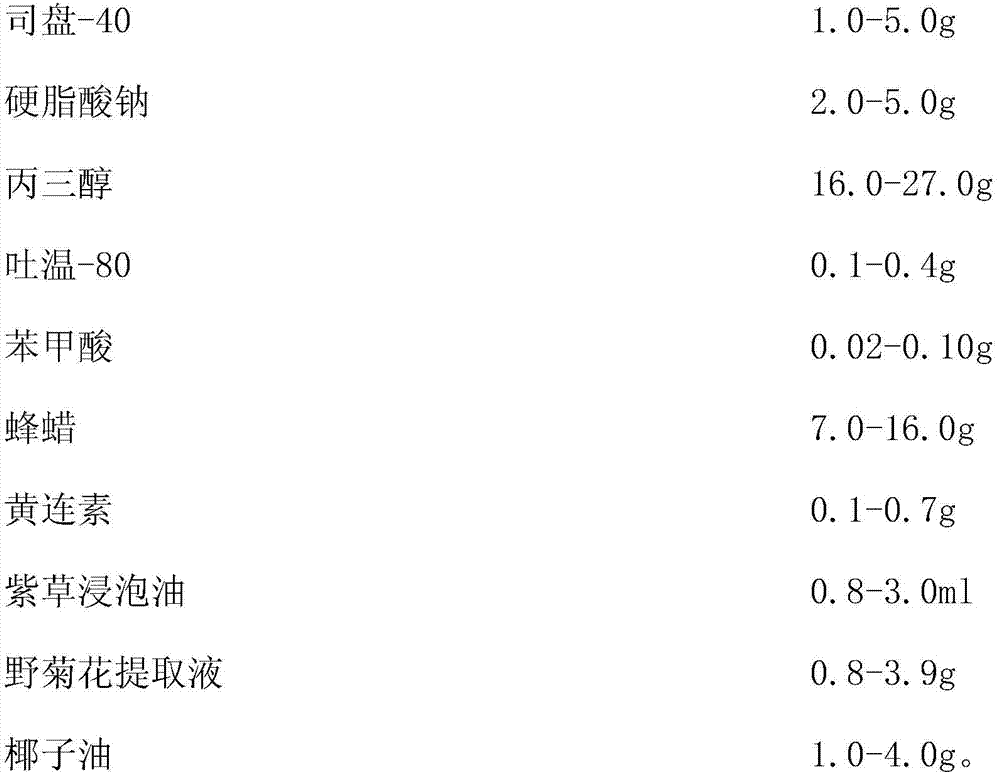
Anal plug for gynecological pelvic inflammatory disease and preparation method thereof
ActiveCN104147291BGood curative effectModerate hardnessAntibacterial agentsOrganic active ingredientsBenzoic acidSodium stearate
The invention discloses a gynecological pelvic inflammatory disease rectal suppository and a preparation method thereof. The suppository includes: 1.0-5.0g of span-40, 2.0-5.0g of sodium stearate, 16.0-27.0g of glycerol, 0.1-0.4g of tween-80, 0.02-0.10g of benzoic acid, 7.0-16.0g of bee wax, 0.1-0.7g of berberine, 0.8-3.0 ml of lithospermum soaked oil, 0.8-3.9g of chrysanthemum indicum extracted liquid and 1.0-4.0g of coconut oil. The gynecological pelvic inflammatory disease rectal suppository is light yellow in color, is moderate in hardness, is uniform in texture and is free of irritation. A weight difference and a melting time are in conformity with a requirement. The berberine, the lithospermum soaked oil and the chrysanthemum indicum extracted liquid and the like ingredients are good in antisepsis and anti-inflammation effect. The suppository is an effective drug for treating gynecological pelvic inflammatory disease and deserves to be further popularized and applicated.
Owner:刘红枚
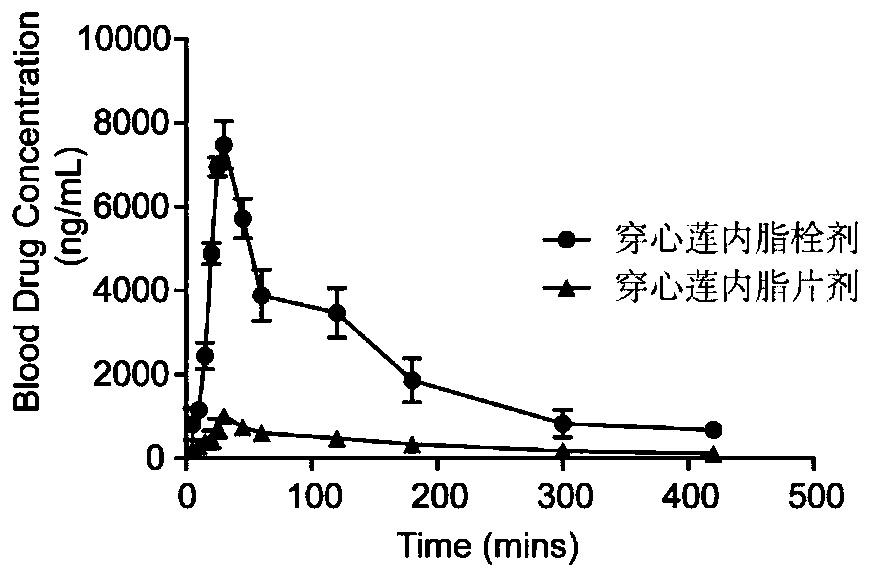
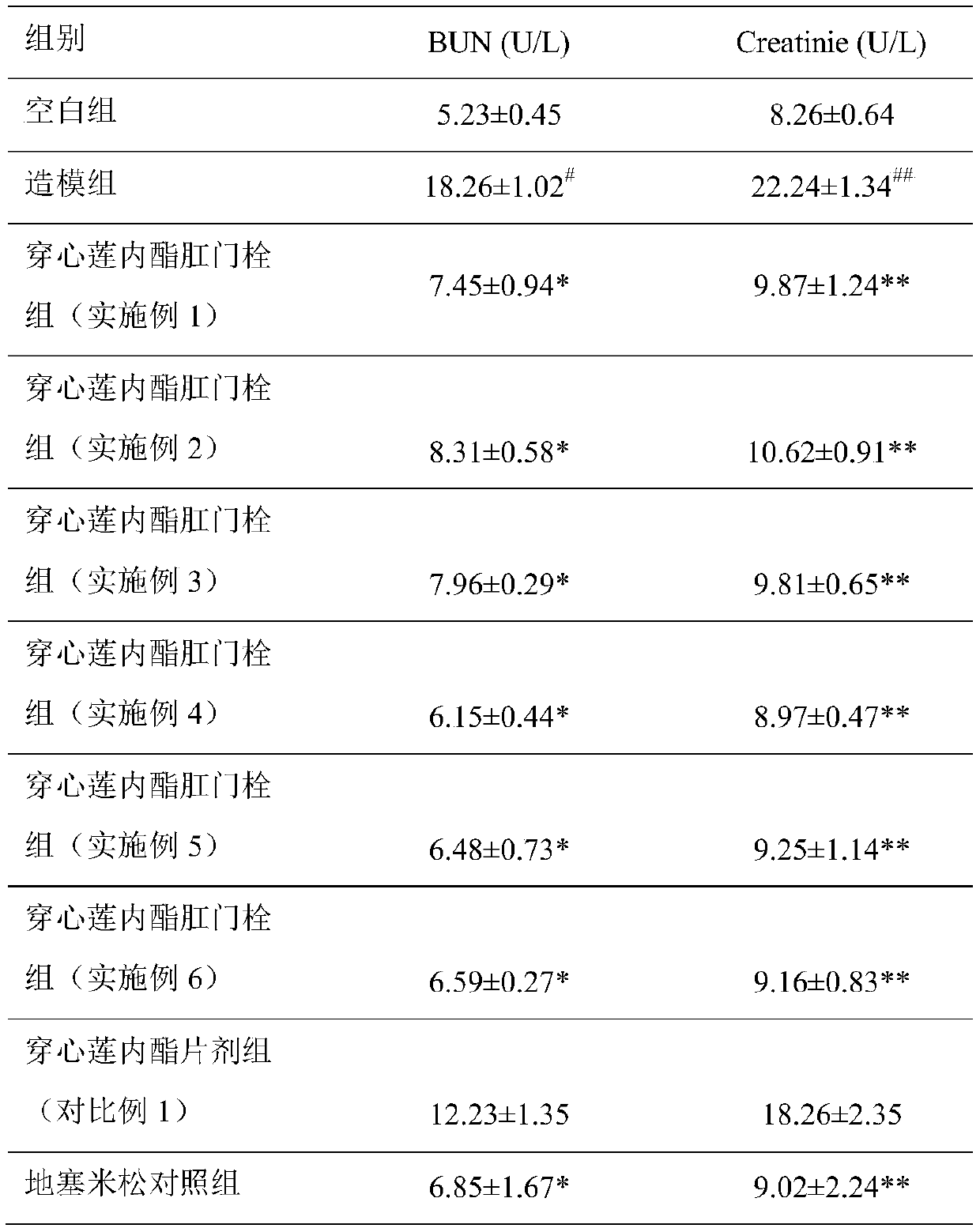
Application of andrographolide and preparation method of andrographolide rectal suppository
InactiveCN110101694AImprove bioavailabilityImprove complianceHydroxy compound active ingredientsSuppositories deliveryRectal SuppositoryMolten state
The invention discloses an application of andrographolide in preparation of medicines for treating acute kidney injury, and a preparation method of an andrographolide rectal suppository for treating the acute kidney injury. The method comprises the steps that 220-240 parts of semi-synthetic fatty glyceride is heated into a molten state, 5-15 parts of andrographolide powder is added and uniformly stirred, the mixture is cooled to room temperature and injecting a rectal suppository mould, and demoulding is carried out after the suppository is solidified. The effective components of the preparedandrographolide rectal suppository are easier to absorb, release is fast, and the efficacy time is long.
Owner:刘琦
Gynecological pelvic inflammatory disease rectal suppository and preparation method thereof
ActiveCN104147291AModerate hardnessSmooth textureAntibacterial agentsOrganic active ingredientsBenzoic acidSodium stearate
The invention discloses a gynecological pelvic inflammatory disease rectal suppository and a preparation method thereof. The suppository includes: 1.0-5.0g of span-40, 2.0-5.0g of sodium stearate, 16.0-27.0g of glycerol, 0.1-0.4g of tween-80, 0.02-0.10g of benzoic acid, 7.0-16.0g of bee wax, 0.1-0.7g of berberine, 0.8-3.0 ml of lithospermum soaked oil, 0.8-3.9g of chrysanthemum indicum extracted liquid and 1.0-4.0g of coconut oil. The gynecological pelvic inflammatory disease rectal suppository is light yellow in color, is moderate in hardness, is uniform in texture and is free of irritation. A weight difference and a melting time are in conformity with a requirement. The berberine, the lithospermum soaked oil and the chrysanthemum indicum extracted liquid and the like ingredients are good in antisepsis and anti-inflammation effect. The suppository is an effective drug for treating gynecological pelvic inflammatory disease and deserves to be further popularized and applicated.
Owner:刘红枚
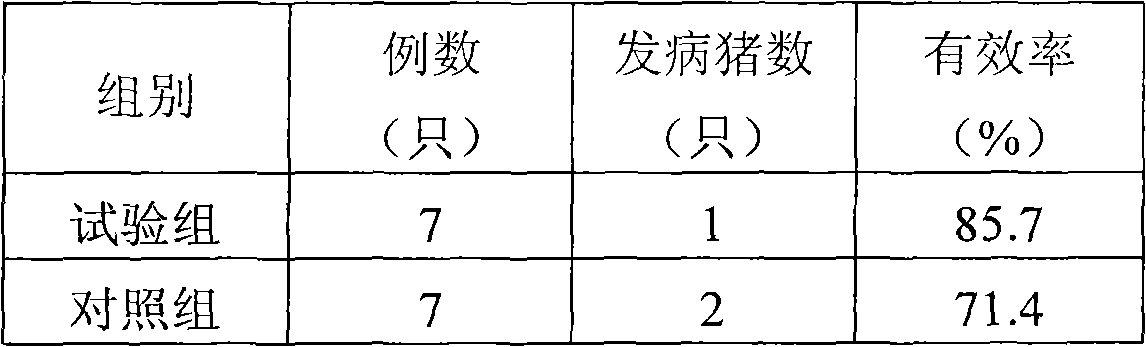
Preparation used for preventing and treating animal viral diseases
InactiveCN101933896AAvoid destructionQuick effectSuppositories deliveryAntiviralsPolymerIndividual animal
The invention relates to a compound preparation used for preventing and treating animal viral diseases. The compound preparation mainly comprises a polyinosinic acid-polycytidylic acid polymer and appropriate auxiliary materials. Polyinosinic-polytidylin acid has high-efficiency interferon induction and immune adjustment effects. A conventional polyinosinic-polytidylin acid preparation is widely used for preventing and treating human and animal viral diseases; and the preparation formulation is an injection, an eye drop and the like. The injection has the disadvantages of great stress, inconvenient use and the like. Therefore, in order to bring convenience to animal medication, the preparation is prepared into a rectal suppository which is suitable to be used for animals by special process treatment, and simultaneously provides a feasible way for industrial large-scale production and is suitable for popularization and application.
Owner:TIANJIN RINGPU BIO TECH
Promethazine hydrochloride microenema prescription and preparation method thereof
InactiveCN102204883BAvoid failureGood rectal absorption propertiesOrganic active ingredientsNervous disorderPediatric patientVitamin C
The invention discloses a promethazine hydrochloride microenema prescription and a preparation method thereof. The preparation method comprises the following steps of: dissolving hydroxypropyl methylcellulose, or polyethylene glycol 6000 or polyvinylpyrrolidone in purified water to form a solution A; dissolving sodium bisulfite, sodium sulfite, vitamin C and EDTA-2Na (Ethylene Diamine TetraaceticAcid-2Na) in purified water, then adding promethazine hydrochloride and dissolving to form a solution B; dissolving ethylparaben in a small amount of boiled water to form a solution C; and adding thesolution C to the solution B to form a solution D, adding the solution D to the solution A, stirring and adding purified water to form a solution and stirring evenly, inspecting and subpackaging. Thepromethazine hydrochloride microenema prescription is convenient to use, is easy to adjust the administration dose to adapt to sick children of different ages, and has better clinical applicability and convenience, thereby ensuring safe and reasonable medication; and the promethazine hydrochloride microenema prescription can overcome the defect in convenience of tablets and rectal suppositories in dose adjustment and the like, overcomes the defect of syrup in stability, is especially applicable to pediatric patients, and is easy to adjust the dose according to the condition of patients to meet the individual requirement on treatment.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Diclofenac composition
InactiveCN104968331ALow viscosityLow toxicityOrganic active ingredientsSenses disorderRectal SuppositoryIntravenous route
The present invention relates a composition comprising Diclofenac and salts thereof wherein Diclofenac or its slats are present in an amount of 25 - 200 mg. The composition is suitable for the parenteral administration through intramuscular, intravenous route; also for oral, dermal, subcutaneous, cutaneous, nasal, ocular drops, as rectal suppository, vaginal pessaries, intra-articular, and otic delivery. The invention also provides compositions comprising a combination of Diclofenac and other drugs. The invention further provides a method for preparing said composition.
Owner:THEMIS MEDICARE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com