Diclofenac composition
A technology of diclofenac and diclofenac potassium, applied in the directions of drug combination, drug delivery, antidote, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
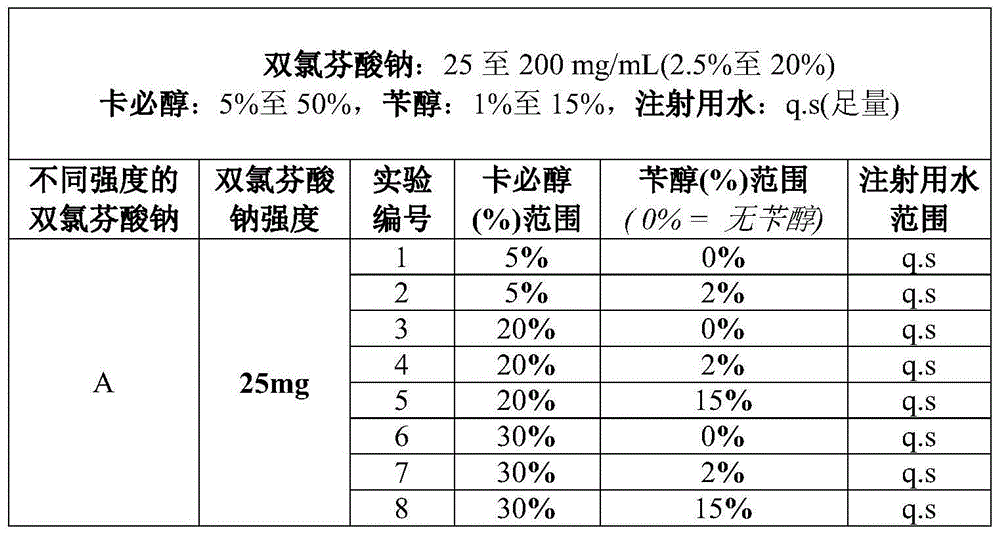
[0082] In a suitable vessel, prepare a solution containing diclofenac sodium at a concentration of 7.5% by adding 50% carbitol and 2% benzyl alcohol to previously boiled and cooled water and stirring well until the API is fully dissolved. If necessary, the pH of the solution is adjusted to the desired range by a suitable buffer such as Tris buffer, phosphate buffer such as sodium phosphate and optionally other alkalizing agents such as sodium hydroxide may be used. The solution was further diluted to final volume with sufficient water. Final volumes of solution are decanted into ampoules and multi-dose vials aseptically and under a continuous nitrogen blanket.
[0083] The prepared solution consisted of about 52% non-aqueous solution and 48% aqueous solution. A solution containing 20% diclofenac sodium, corresponding to a 200 mg / mL solution, was similarly prepared.
Embodiment 2
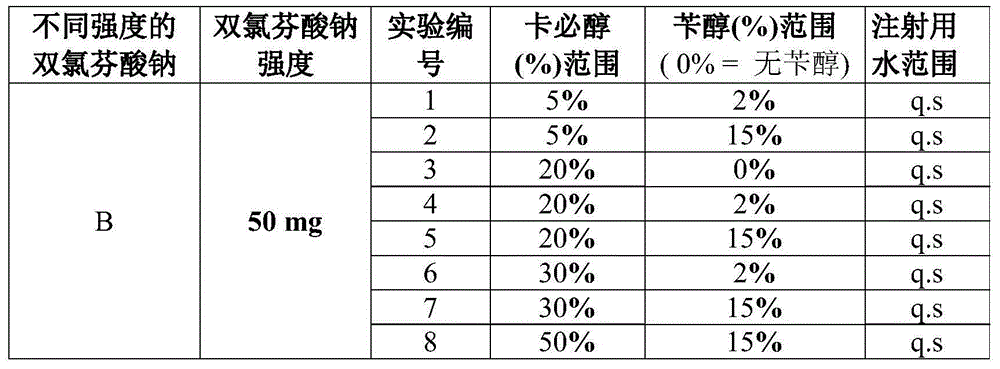
[0085] Heat water in a suitable vessel at 80 to 85°C and cool to room temperature. A solution was prepared by dissolving 7.5% diclofenac sodium together with 30% carbitol and 2% benzyl alcohol in 50% water with continuous vigorous stirring until a clear solution was obtained. The pH is adjusted within the desired range of 7.5 to 9 by a suitable buffer such as Tris buffer, phosphate buffer such as sodium phosphate or alternatively by other alkalizing agents. The solution was further diluted to volume by qs. water. The final volume of the solution is sterilized by sterile filtration using a 0.22 μm filter and poured into ampoules and multi-dose vials. This process was all done under a constant nitrogen purge.
[0086] The prepared solution contained approximately 32% non-aqueous solution and 68% aqueous solution. The resulting liquid containing 75 mg of diclofenac sodium in 1 ml can be administered by injection by I.M and I.V routes.
Embodiment 3
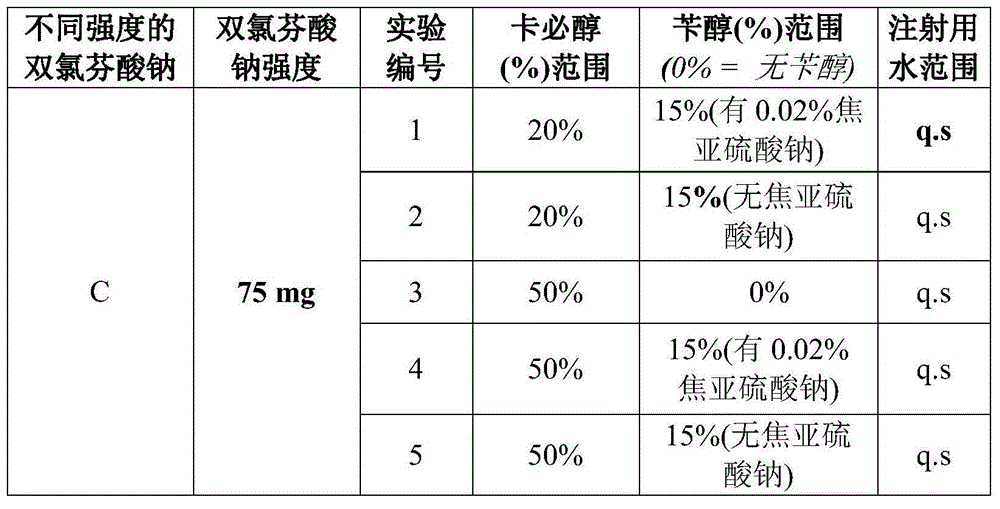
[0088] In a suitable vessel, 30% of the carbitol is added to 50% of the water which is initially heated at 80 to 85°C and allowed to cool at room temperature. The solution was then stirred well with the addition of diclofenac sodium at a concentration of 7.5%. After mixing the ingredients in solution, it was further diluted with sufficient water to the desired final volume such that the final liquid consisted of 75 mg of diclofenac sodium in 1 ml. The resulting liquid was sterile filtered by using a 0.2 μm membrane. The pH of the solution is maintained in the desired range of 7.5 to 9 by adding a suitable alkalizing agent or buffer such as Tris buffer or phosphate buffer such as sodium phosphate, potassium phosphate. The entire process is carried out under a nitrogen blanket to remove oxygen.
[0089] The prepared solution contained about 30% non-aqueous solution and 70% aqueous solution. The resulting solution is ready to be filled into ampoules and multi-dose vials. It w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com