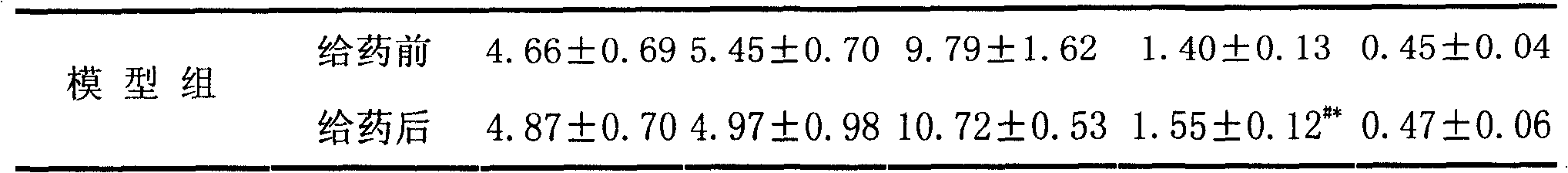Patents
Literature
35 results about "Intravenous route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytokine antagonists for the treatment of sensorineural hearing loss
InactiveUS6423321B2Improve hearingReduce developmentPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsIntravenous routeSensorineural hearing loss
Specific Cytokine Antagonists, including TNF antagonists and / or Interleukin-1 antagonists, are used as novel therapeutic agents for the treatment of hearing loss, including presbycusis and other forms of sensorineural hearing loss. The present invention provides a method for inhibiting the action of TNF and / or IL-1 antagonists for treating hearing loss in a human by administering a TNF antagonist and / or an IL-1 antagonist for reducing the inflammation affecting the auditory apparatus of said human, or for modulating the immune response affecting the auditory apparatus of said human, by administering a therapeutically effective dosage level to said human of a TNF antagonist and / or an IL-1 antagonist. Administration may be systemic, through the subcutaneous, intramuscular, oral, or intravenous routes; or by delivering an anatomically localized application in the region of the head. The TNF antagonist is selected from the group consisting of etanercept, infliximab, D2E7, CDP 571, or thalidomide; and the IL-1 antagonist is either IL-1 RA or IL-1R type II receptor. Antiviral agents may be added for treating certain patients.
Owner:TACT IP
Asymmetric magnetic mesoporous silica rod supporting chemotherapeutic and gene drugs and application thereof to tumor diagnosis and treatment
InactiveCN104225599AGood treatment effectReal-time monitoring of treatment effectGenetic material ingredientsInorganic non-active ingredientsBiocompatibility TestingMesoporous silica
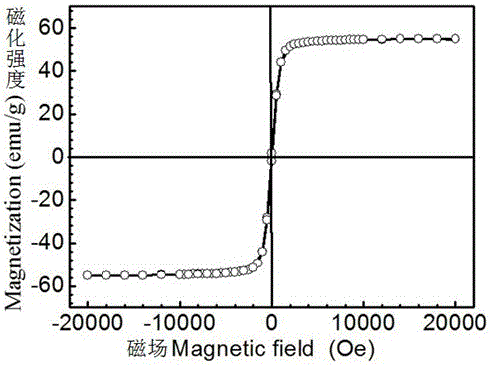
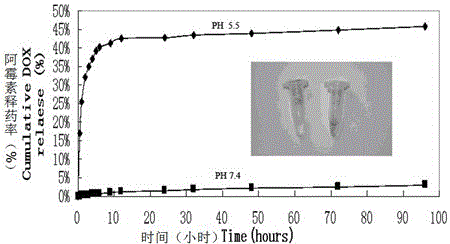
The invention relates to the field of nanometer drug carriers, and concretely relates to an asymmetric magnetic mesoporous silica rod supporting chemotherapeutic and gene drugs and application thereof to tumor diagnosis and treatment. The asymmetric magnetic mesoporous silica rod is prepared by employing spherical magnetic ferrite nanoparticles and ethyl orthosilicate through a sol-gel method, and the asymmetric magnetic mesoporous silica rod is subjected to surface functionalization modification, and is successively loaded with a chemotherapeutic drug, coated by a positive high-molecular polymer and loaded with a gene drug, so that a target product is obtained. The chemotherapeutic drug is connected with the silica rod through functionalization of the mesoporous surface, and the silica rod is endowed with the pH-responsive drug release characteristic, also the biocompatibility of the composite material is increased and the in-vivo cycling time is prolonged, and gene is supported in an electrostatic adsorption mode. The composite material is injected into a living body via an intravenous route, the characteristics of nanoparticle in-vivo passive targeting, gene guiding and pH-responsive drug release of the composite material are utilized, also the cooperativity of the multidrug resistant gene and the chemotherapeutic drug is utilized, and in-vitro magnetic targeting, NMR imaging and other technologies are applied to diagnosis and treatment of malignant tumors.
Owner:JILIN UNIV
NRP-1 ligand polypeptide-polyethylene glycol-phosphatide compound, active targeting liposome drug delivery system mediated thereby and preparation method thereof
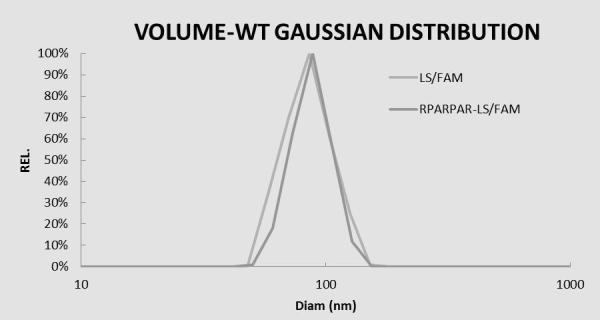
The invention relates to an NRP-1 ligand polypeptide-polyethylene glycol-phosphatide compound, an active targeting liposome drug delivery system mediated thereby and a preparation method thereof. A carrier system comprises; a. phosphatide; b. cholesterol; c. methoxy polyethylene glycol-phosphatide compound; and d. polypeptide-polyethylene glycol-phosphatide compound containing an RPARPAR sequence. The above components are in a molar ratio of: a:b=5:1-1:5, a:c=1000:0.1-1000:100, and a:d=1000:0.1-1000:100. The system can carry out intravenous route drug administration and target the drug to a tumour position according to tumour EPR effect and RPARPAR mediation effect. The liposome drug delivery system can be applied to tumour diagnosis or targeting delivery of a treatment medicament.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Diagnosis of blood clots using fibrin-binding proteins bound with contrast agents
InactiveUS20060257389A1Quickly and easily interpretedEliminate side effectsPeptide/protein ingredientsMicrobiological testing/measurementVeinIntravenous route
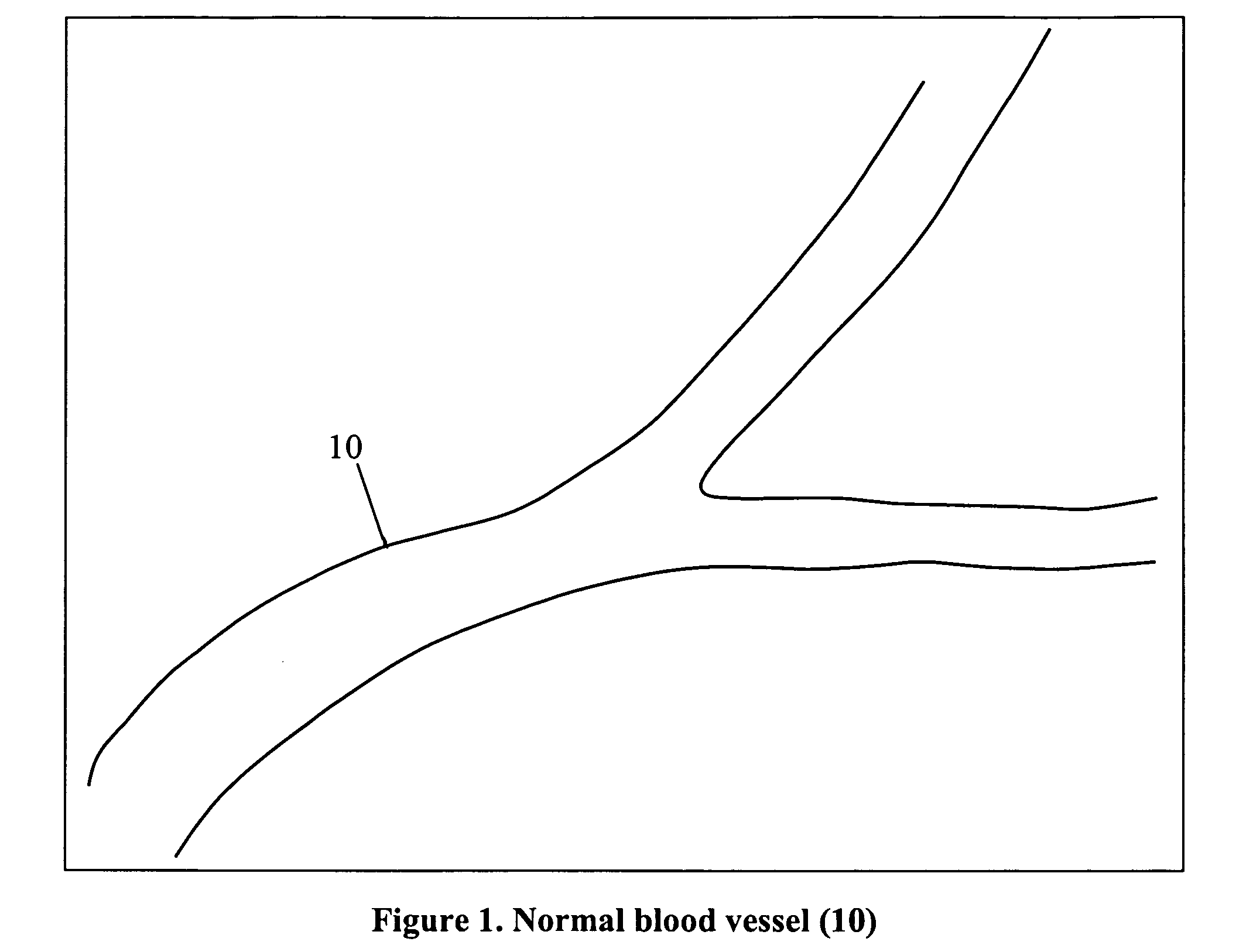
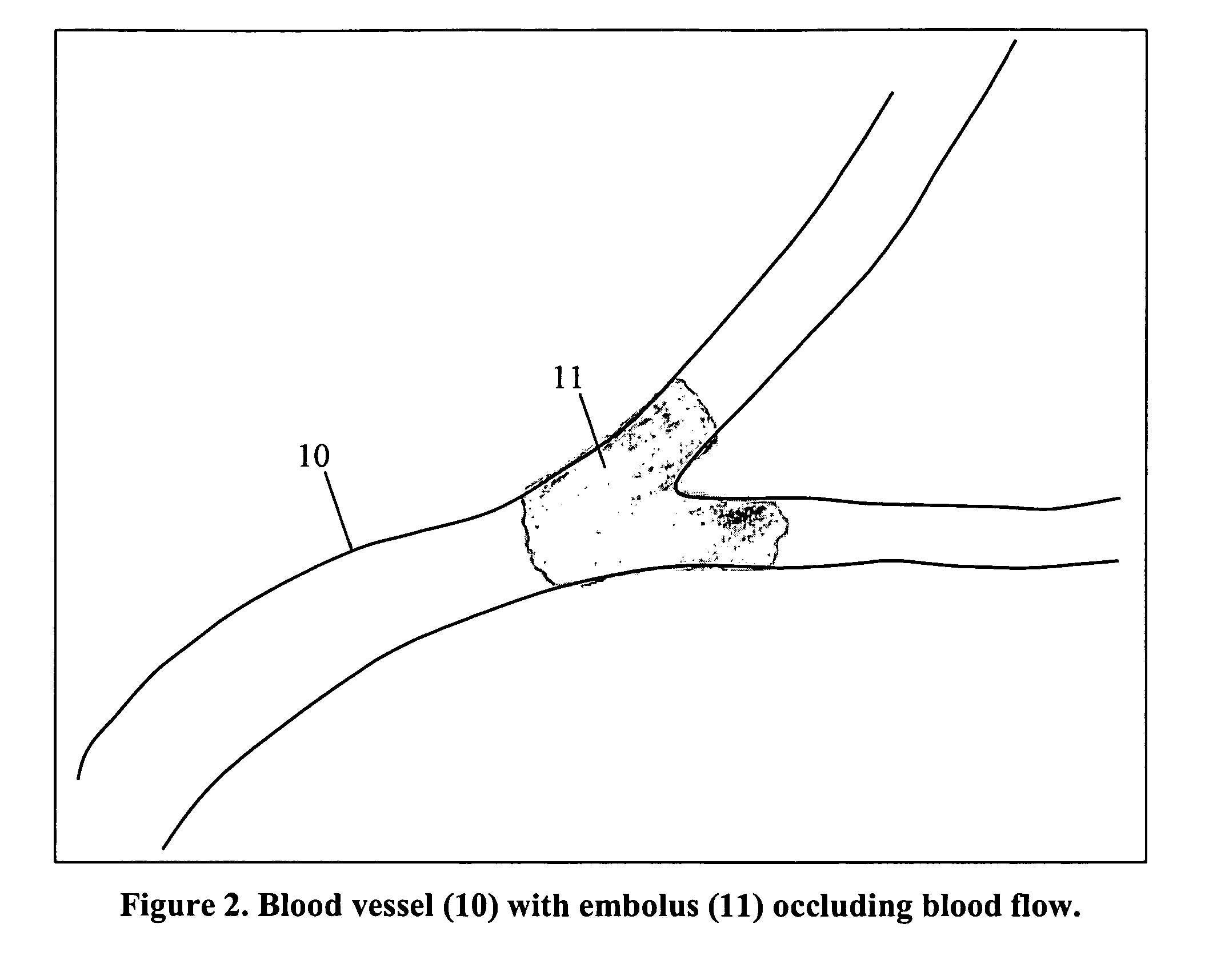
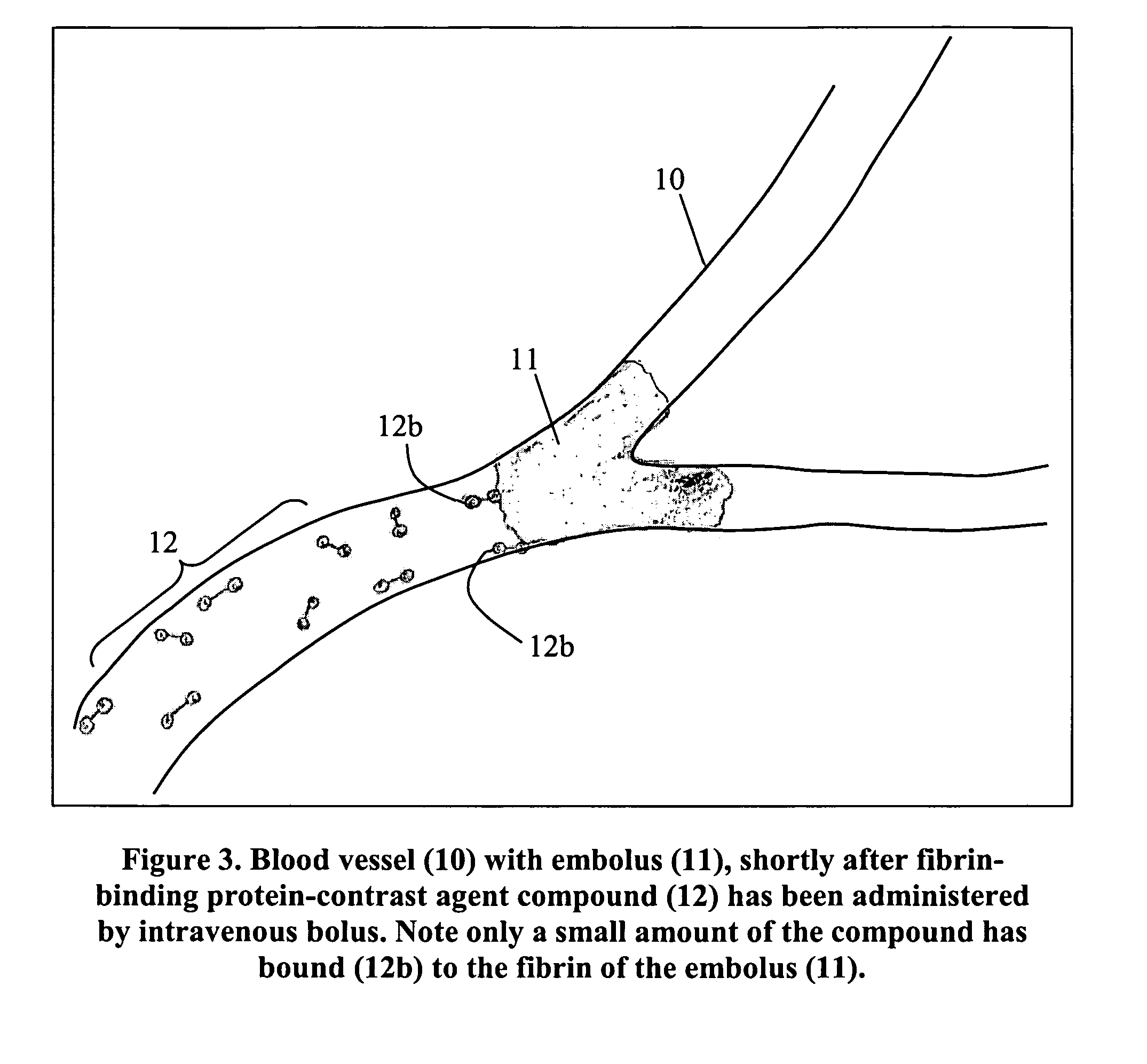
This invention describes how to modify a fibrin-binding protein, such as tPA, with a contrast agent, such as iodine. This substance could then be given to a patient suspected of having a blood clot by an intravenous route, and then detected by a radiographic study at a short pre-determined time later. Appropriate therapy is started immediately as determined by the radiographic study.
Owner:BENFORD JACOB
Injectable Preparations Of Diclofenac And Its Pharmaceutically Acceptable Salts
ActiveUS20080153914A1Relieve painHigh viscosityOrganic active ingredientsBiocideInjection siteIntravenous route
The present invention provides injectable formulations of water-soluble salts of diclofenac in single doses of less than 2 ml, which cause significantly less pain at the site of injection and can be administered by intradeltoid route, in addition to intragluteal and slow intravenous route. More specifically the injectable preparations contain 75 mg to 100 mg of water-soluble salts of diclofenac, in about 1 ml injection solution without significantly raising the viscosity of the injection solution without the use surfactants. The formulations are adjusted to pH 6 to 10 containing up to 100 mg of diclofenac salt in a medium comprising of water, along with one or more co-solvent(s) / solubiliser(s), antioxidants, preservatives, buffers, alkali and stabilizers.
Owner:TROIKAA PHARMA
Rafamycin analogs and methods for making same
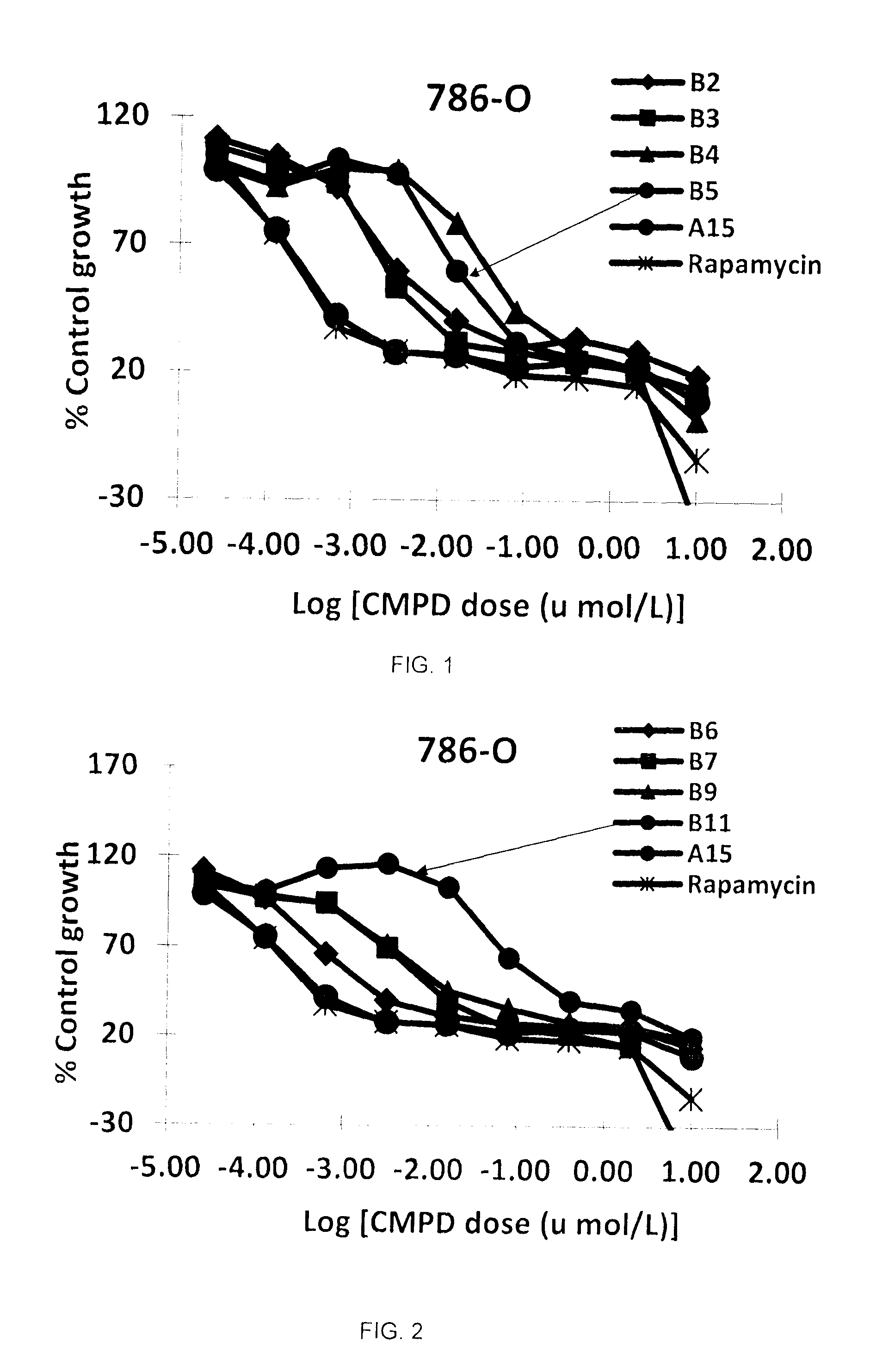
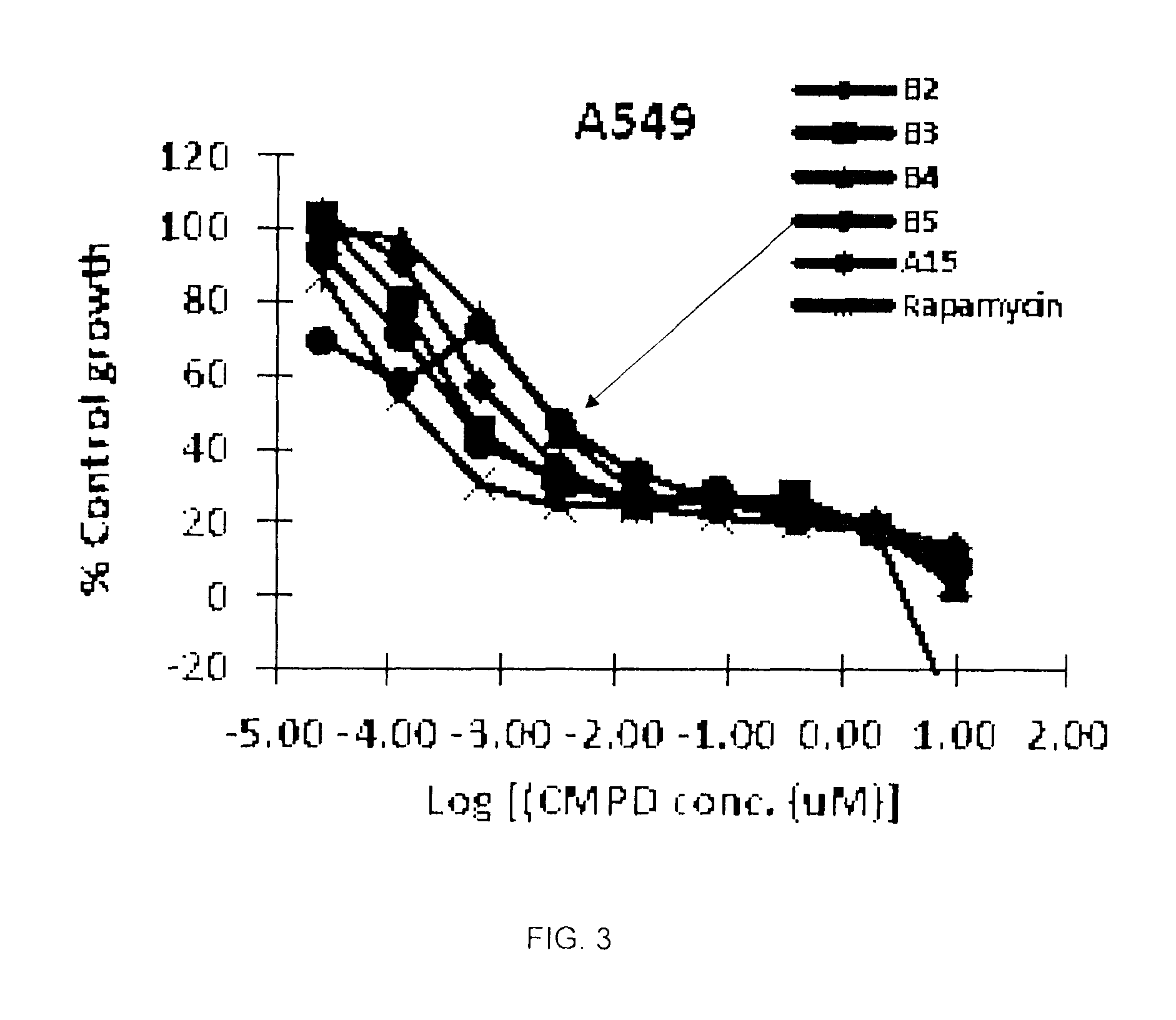
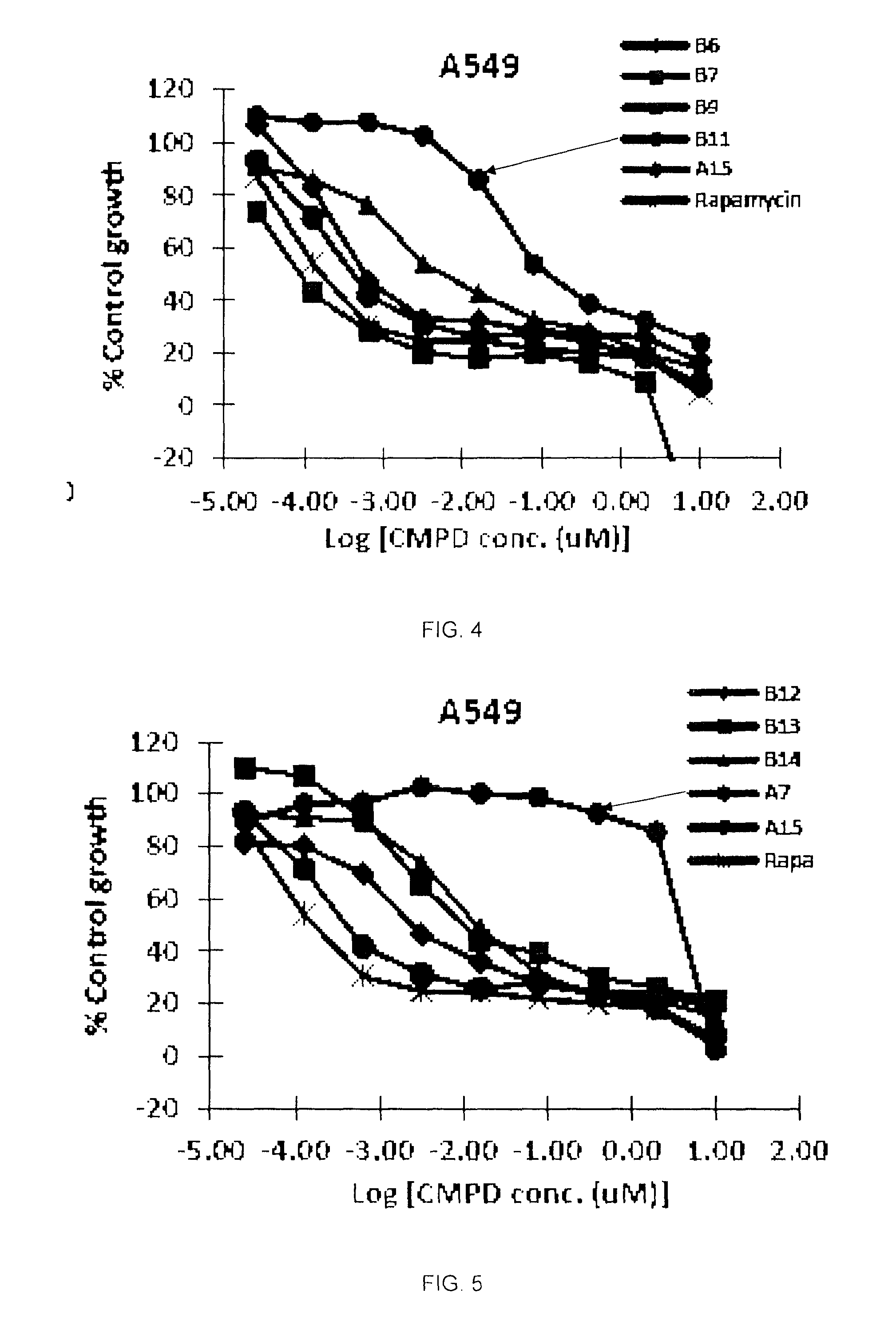
A semi-synthetic rapamycin analog with a triazole moiety or a pharmaceutically acceptable salt or prodrug thereof, is a broad-spectrum cytostatic agent and a mTOR inhibitor, and is useful in the treatment of various cancers, or tumors in organs such as kidney, liver, breast, head and neck, lung, prostate, and restenosis in coronary arteries, peripheral arteries, and arteries in the brain, immune and autoimmune diseases. Also disclosed are fungal growth-, restenosis-, post-transplant tissue rejection- and immune- and autoimmune disease-inhibiting compositions and a method of inhibiting cancer, fungal growth, restenosois, post-transplant tissue rejection, and immune and autoimmune disease in a mammal. One particular preferred application of such triazole-moiety containing rapamycin analog is in treating renal carcinoma, lung cancer, colon cancer, and breast cancers wherein potency of the drug, its half-life, tissue distribution properties, and its pharmacokinetic properties including bioavailability through oral and intravenous routes are essential to the clinical outcomes.
Owner:HANGZHOU ZYLOX PHARMA
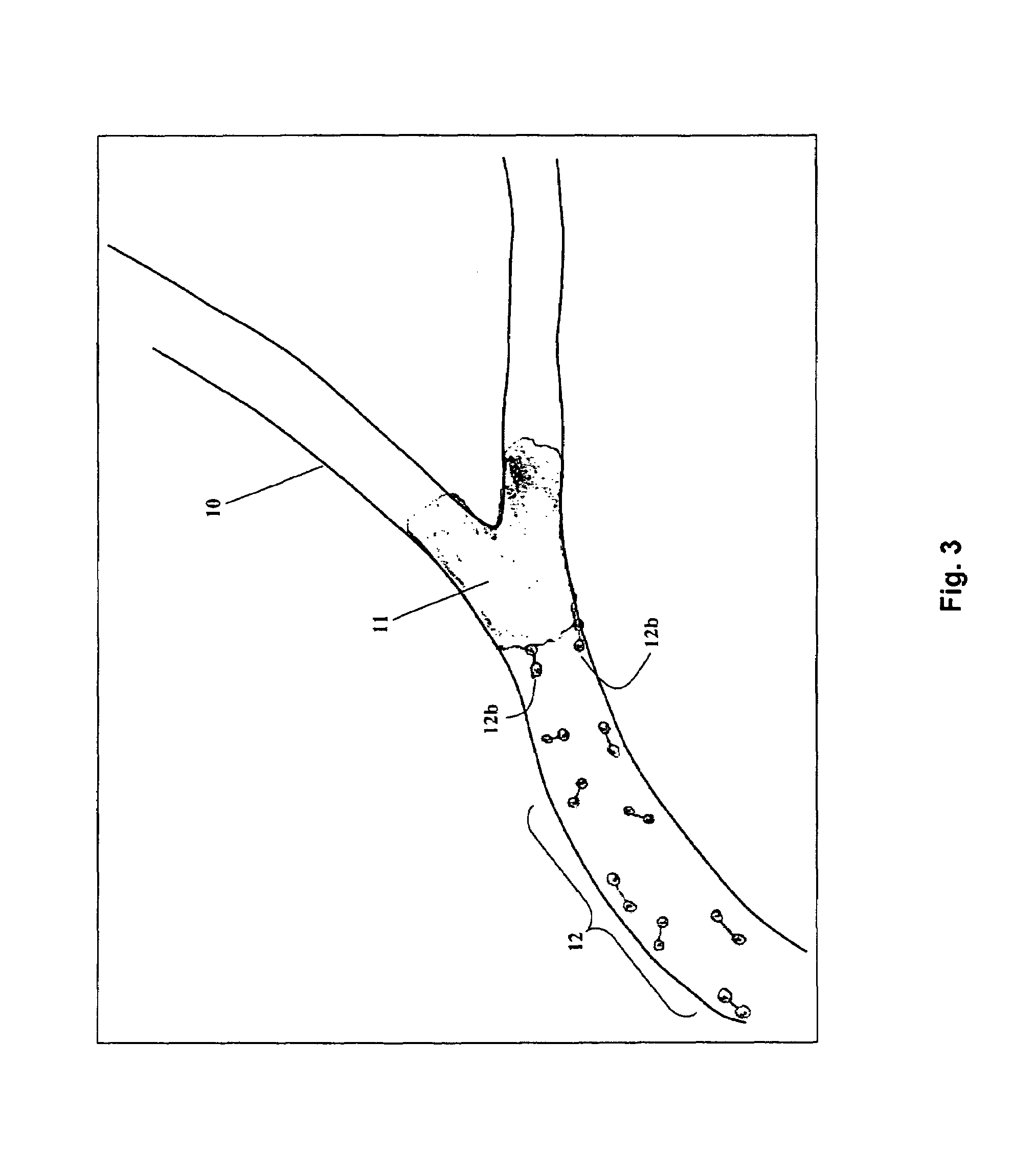
Flashing flow blood collecting needle
ActiveCN103393427APrevent leakageDiagnostic recording/measuringSensorsIntravenous routeRelative Volume
Owner:BECTON DICKINSON & CO
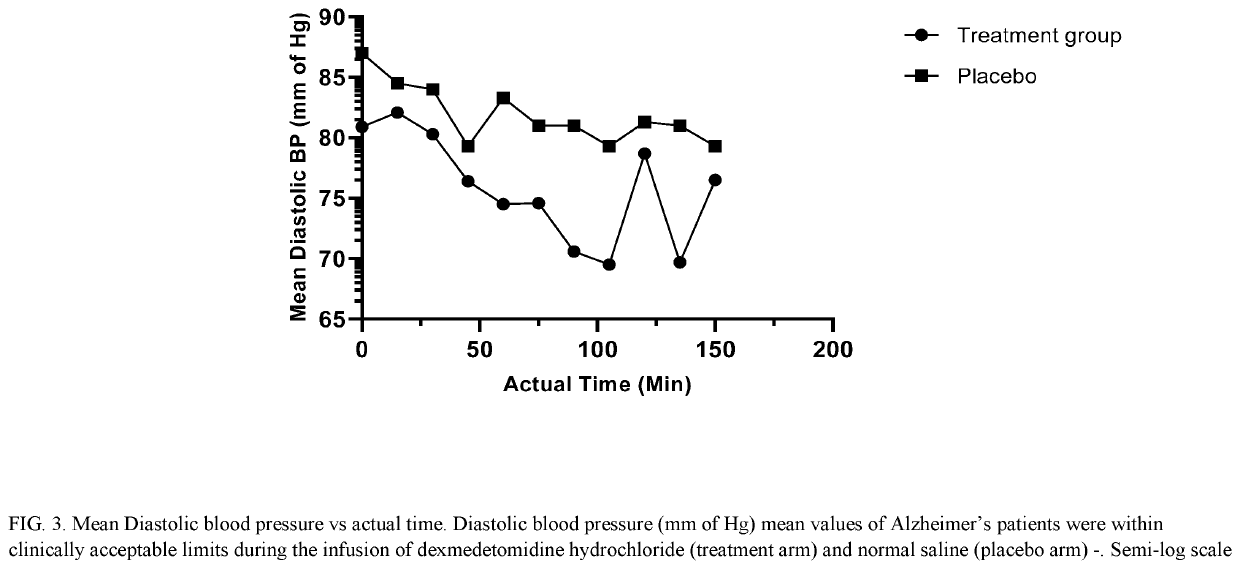
Methods for treating agitation using dexmedetomidine hydrochloride
PendingUS20210267944A1Reduce agitationReduce signOrganic active ingredientsNervous disorderIntravenous routePharmaceutical drug
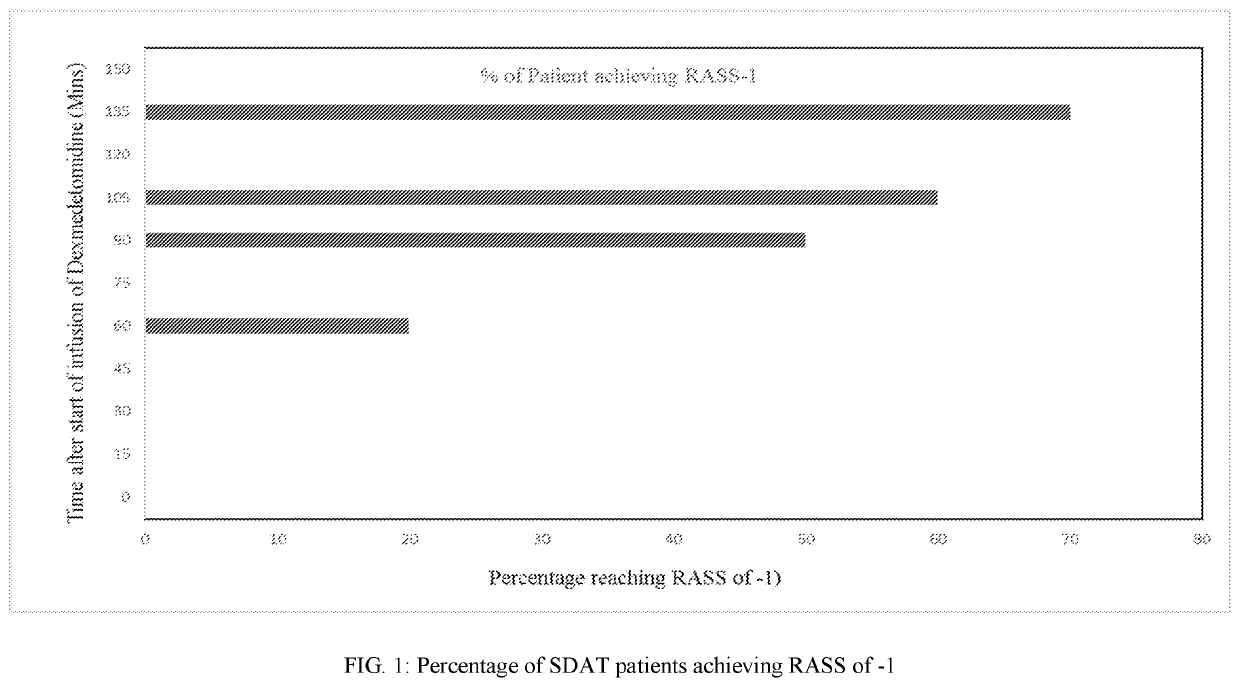
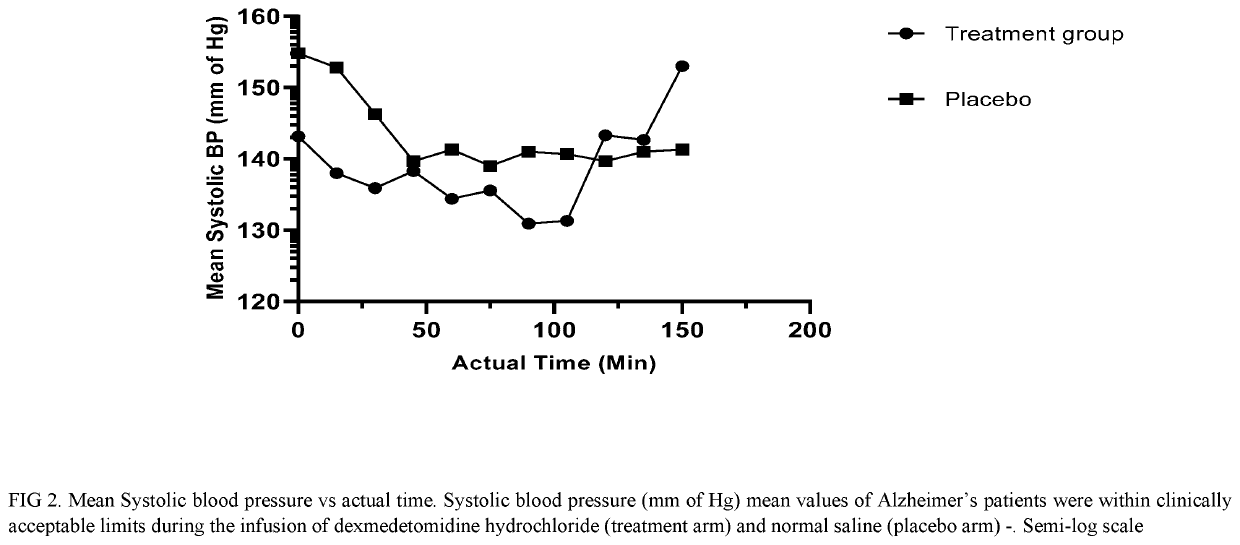
The present disclosure relates to the treatment of agitation or signs of agitation in certain human subjects, including subjects with a neurodegenerative, neuropsychiatric or opioid withdrawal disorder, by administering dexmedetomidine hydrochloride by the intravenous route.
Owner:BIOXCEL THERAPEUTICS INC
Diclofenac compositions
InactiveUS20140187635A1Not cause hemolysisNot irritatingBiocideOrganic active ingredientsNasal cavityRectal Suppository
The present invention relates a composition comprising Diclofenac and salts thereof wherein Diclofenac or its salts are present in an amount of 25-200 mg. The composition is suitable for the parenteral administration through intramuscular, intravenous route; also for oral, dermal, subcutaneous, cutaneous, nasal, ocular drops, as rectal suppository, vaginal pessaries, intra-articular, and otic delivery. The invention also provides compositions comprising a combination of Diclofenac and other drugs. The invention further provides a method for preparing said composition.
Owner:THEMIS MEDICARE LTD
Injectable preparations of diclofenac and its pharmaceutically acceptable salts
ActiveUS9211251B2Relieve painHigh viscosityBiocideOrganic active ingredientsInjection siteIntravenous route
Injectable formulations of water-soluble salts of diclofenac, which cause significantly less pain at the site of injection and can be administered by intradeltoid route, in addition to intragluteal and slow intravenous route.
Owner:TROIKAA PHARMA
Chonsurid for venous injection administration and its preparing method
The present invention discloses chonsurid preparation capable of being used for venous injection and its preparation process. The purified chonsurid preparation may be used for intramuscular injection and intravenous injection after being added into glucose solution or physiological saline, and has convenient use. Owing to the raised purity of chonsurid, the chonsurid preparation may be injected directly into blood circulation system resulting in high blood medicine concentration, fast acting, high curative effect and less side effect.
Owner:NANJING GRITPHARMA CO LTD
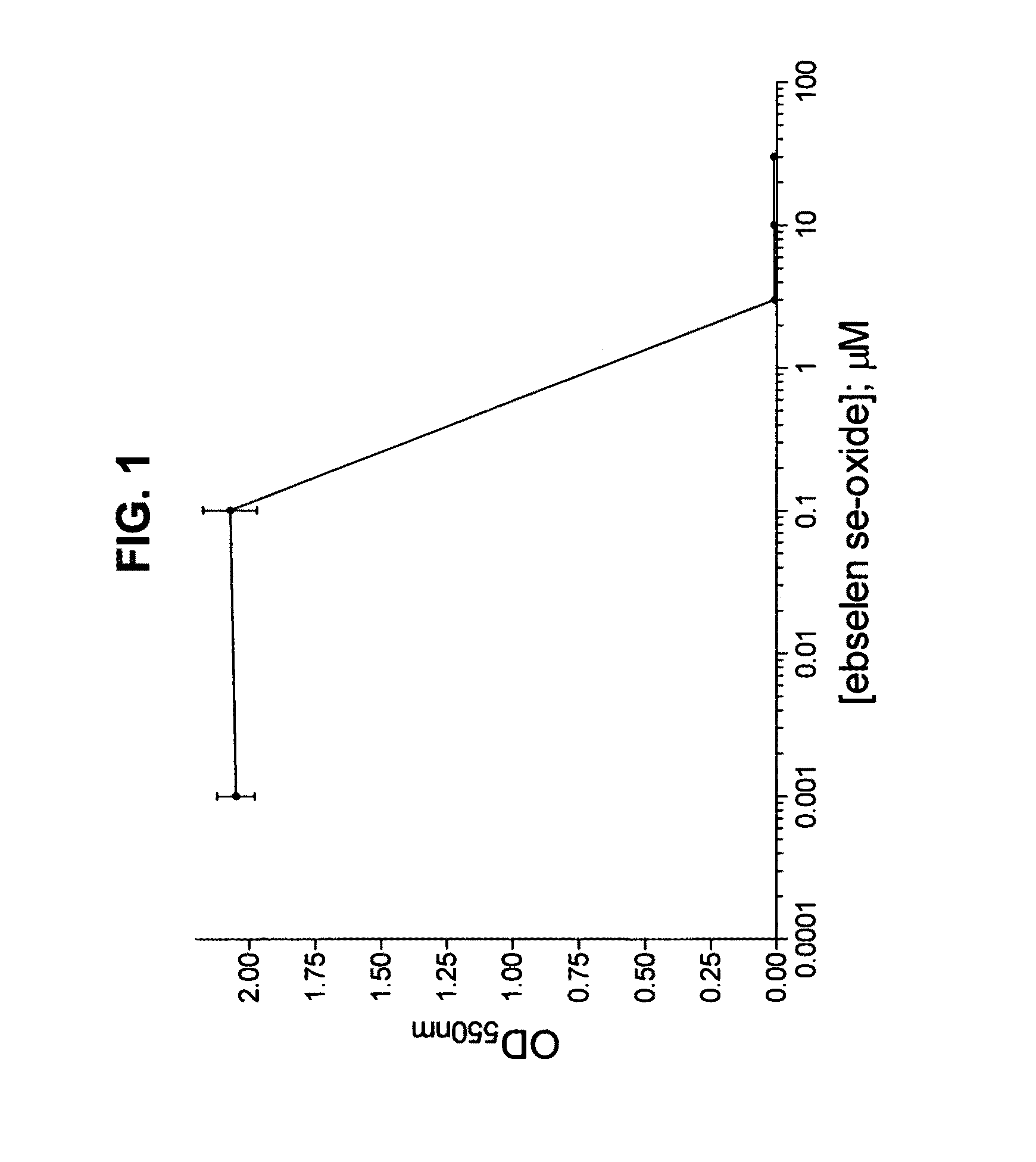
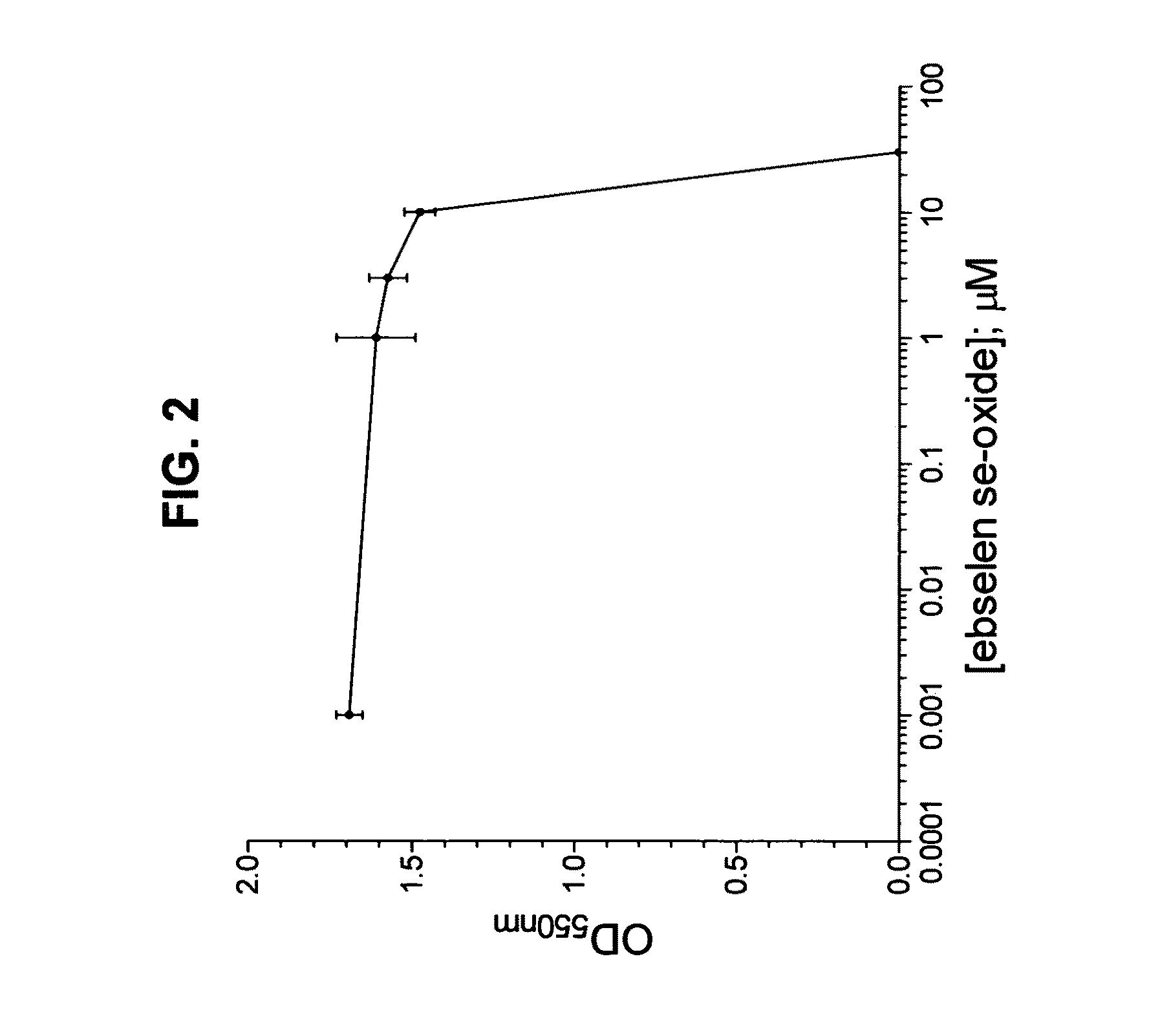
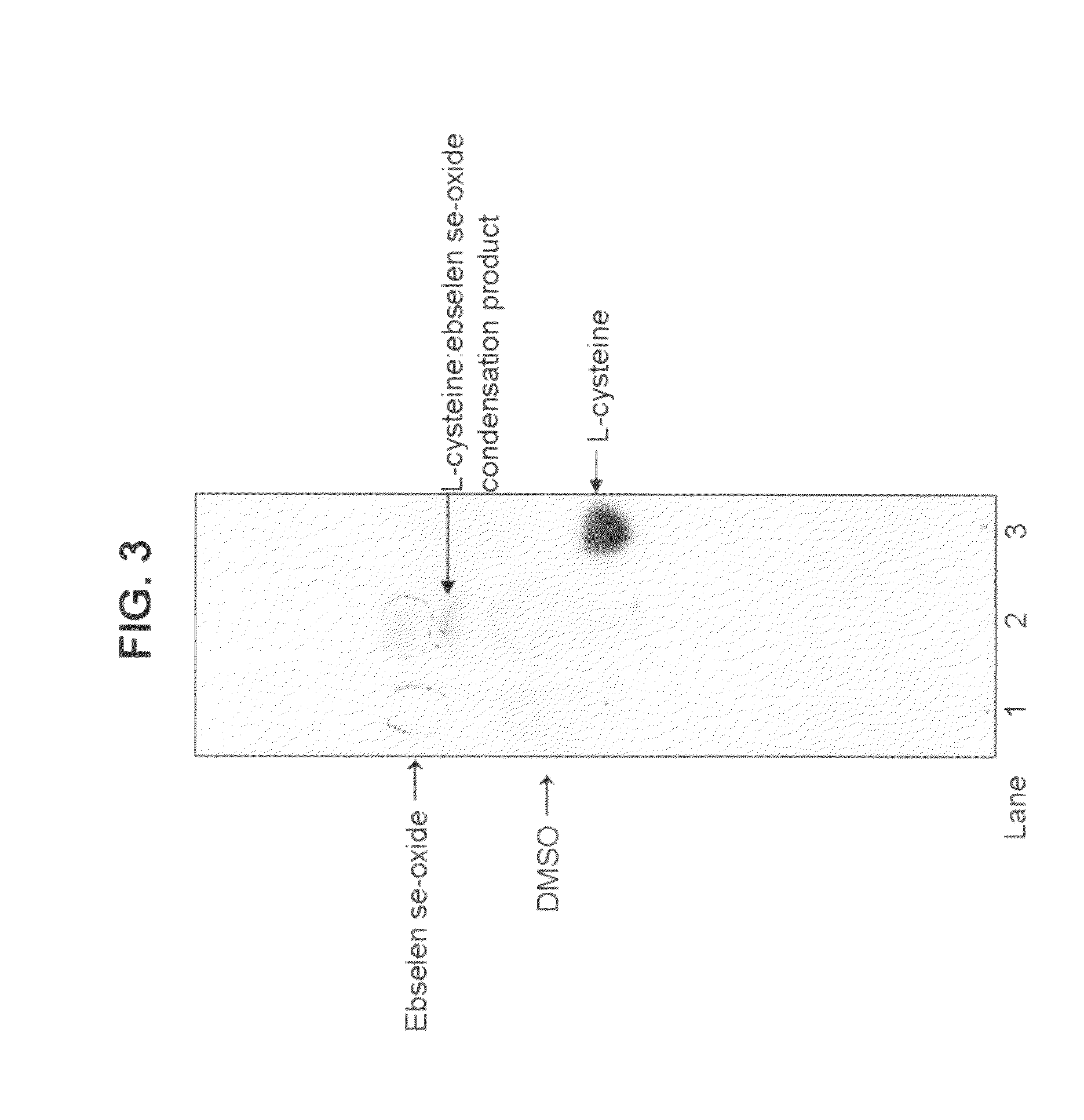
Process for the treatment of bacterial infections using 2-phenyl-1,2-benzisoselenazol-3(2H)-one 1-oxide
Owner:BILLACK BLASE CHRISTOPHER +1
Injectable preparations of diclofenac and its pharmaceutically acceptable salts
ActiveUS8809393B2Relieve painHigh viscosityBiocideOrganic active ingredientsIntravenous routeAntioxidant
The present invention provides injectable formulations of water-soluble salts of diclofenac in single doses of less than 2 ml, which cause significantly less pain at the site of injection and can be administered by intradeltoid route, in addition to intragluteal and slow intravenous route. More specifically the injectable preparations contain 75 mg to 100 mg of water-soluble salts of diclofenac, in about 1 ml injection solution without significantly raising the viscosity of the injection solution without the use surfactants. The formulations are adjusted to pH 6 to 10 containing up to 100 mg of diclofenac salt in a medium comprising of water, along with one or more co-solvent(s) / solubilizer(s), antioxidants, preservatives, buffers, alkali and stabilizers.
Owner:TROIKAA PHARMA
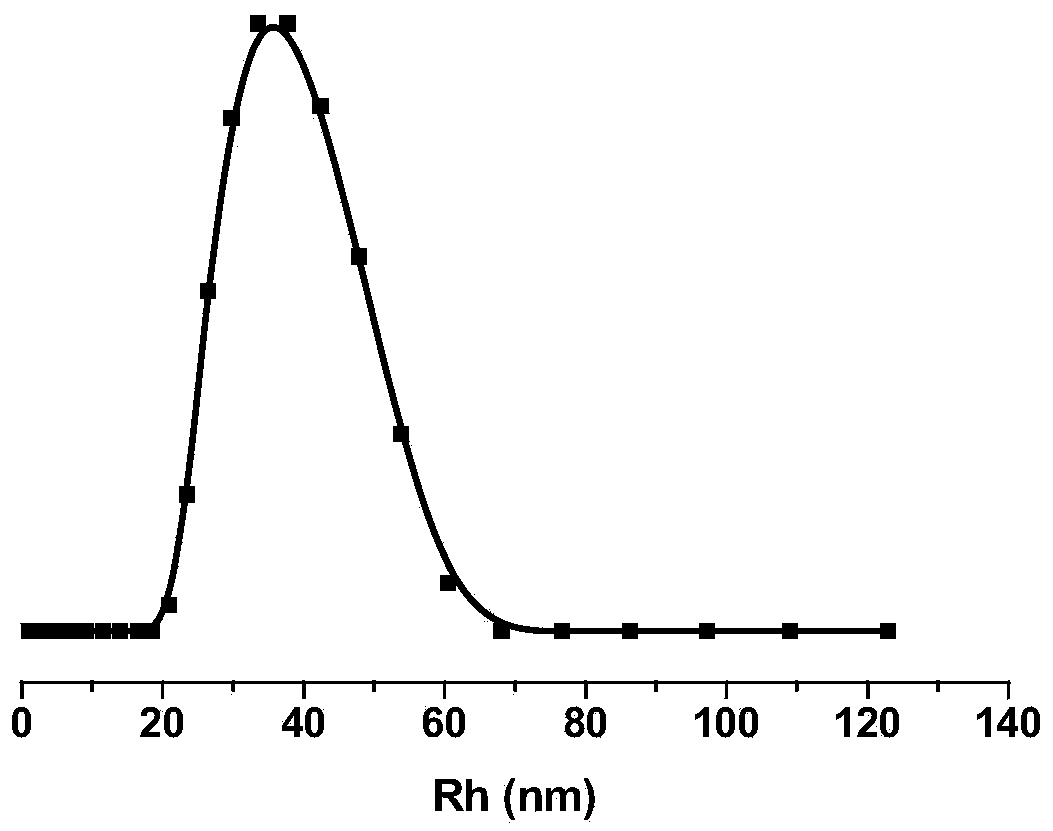
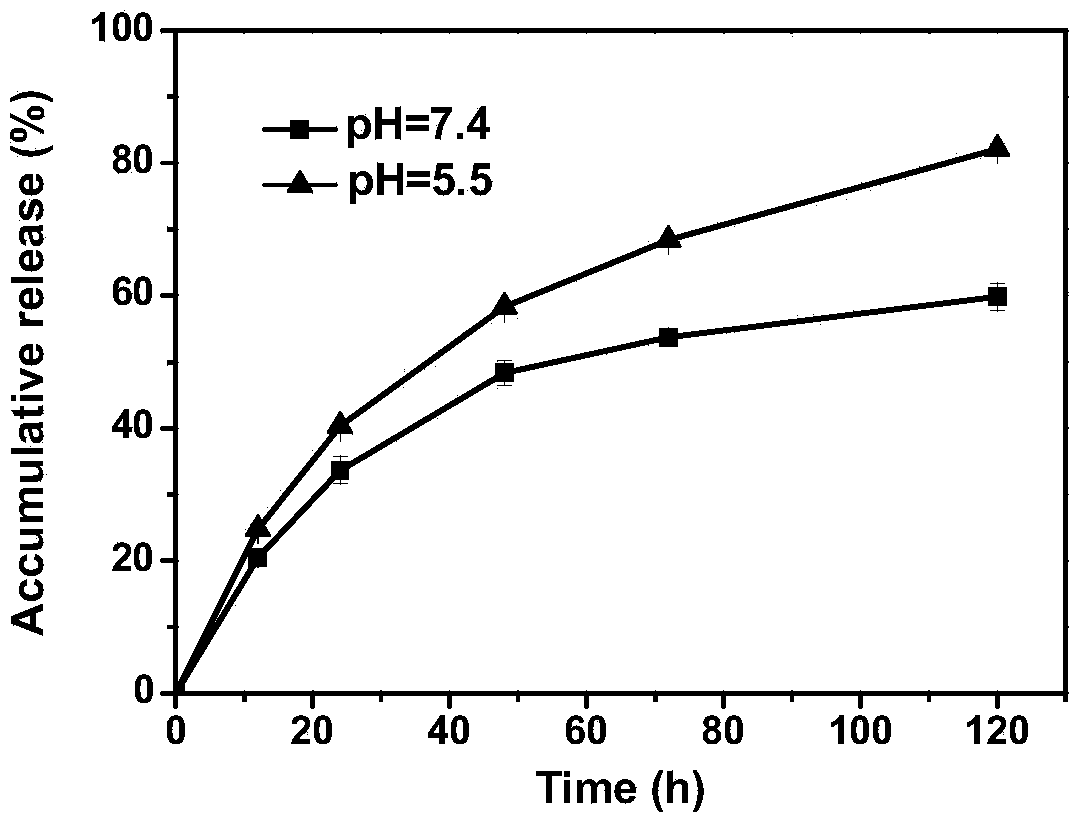
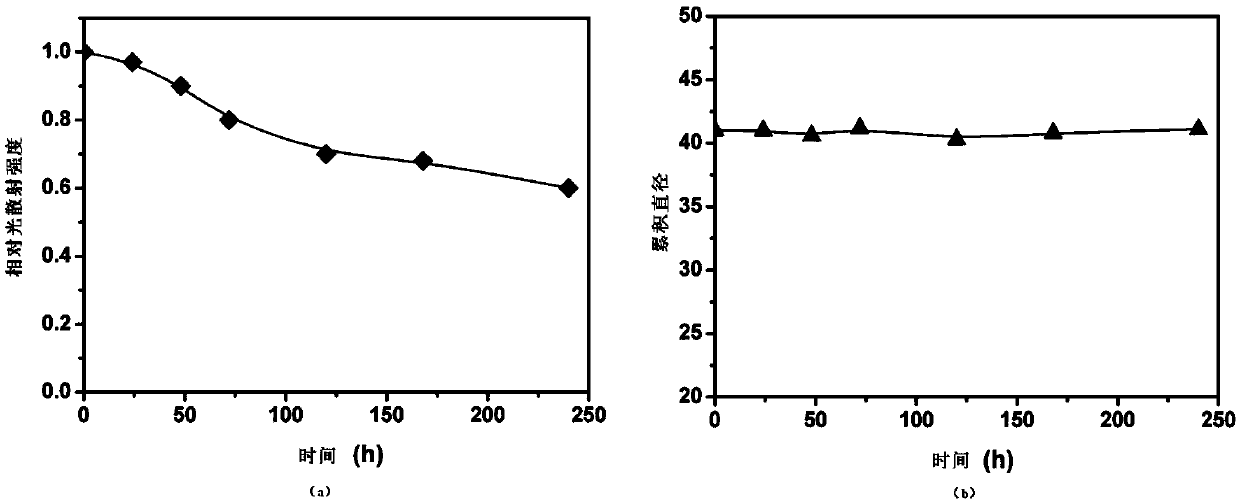
[Dichloro-1,2-cyclohexanediamine]platinum complex and preparation method thereof
ActiveCN103394095AGood biocompatibilityImprove solubilityPharmaceutical non-active ingredientsAntineoplastic agentsSolubilityTreatment effect
The invention provides a [dichloro-1,2-cyclohexanediamine]platinum complex, and the complex is obtained from the complex reactions between dichloro-1,2-cyclohexanediamine and a polymer which has the structure of the formula (I). The [Dichloro-1,2-cyclohexanediamine]platinum has a cyclohexanediamine structure, a stable inner-core structure is formed because of the accumulation and hydrophobic effect after the [Dichloro-1,2-cyclohexanediamine]platinum cooperate with a polymer, and the stability of the [Dichloro-1,2-cyclohexanediamine]platinum is good under physiology conditions. The complex can effectively avoid the problems of sudden release and non-specific interaction of [Dichloro-1,2-cyclohexanediamine]platinum after [Dichloro-1,2-cyclohexanediamine]platinum enters the blood circulation system through intravenous route. The complex can be assembled to form a micelle structure; accumulation of the complex on the tumor locations can be achieved through strengthening osmotic and retention effects, and thus the goals of reducing toxic and by effects and improving curative effects are achieved. Furthermore, the adopted complex has a good biological compatibility, is capable of degrading in human bodies, and thus the prepared [Dichloro-1,2-cyclohexanediamine]platinum has a good solubility.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Asymmetric magnetic mesoporous silica rods co-loading chemotherapy and gene drugs and their application in tumor diagnosis and treatment
InactiveCN104225599BGood treatment effectReal-time monitoring of treatment effectGenetic material ingredientsInorganic non-active ingredientsMesoporous silicaBiocompatibility
The invention relates to the field of nanometer drug carriers, and concretely relates to an asymmetric magnetic mesoporous silica rod supporting chemotherapeutic and gene drugs and application thereof to tumor diagnosis and treatment. The asymmetric magnetic mesoporous silica rod is prepared by employing spherical magnetic ferrite nanoparticles and ethyl orthosilicate through a sol-gel method, and the asymmetric magnetic mesoporous silica rod is subjected to surface functionalization modification, and is successively loaded with a chemotherapeutic drug, coated by a positive high-molecular polymer and loaded with a gene drug, so that a target product is obtained. The chemotherapeutic drug is connected with the silica rod through functionalization of the mesoporous surface, and the silica rod is endowed with the pH-responsive drug release characteristic, also the biocompatibility of the composite material is increased and the in-vivo cycling time is prolonged, and gene is supported in an electrostatic adsorption mode. The composite material is injected into a living body via an intravenous route, the characteristics of nanoparticle in-vivo passive targeting, gene guiding and pH-responsive drug release of the composite material are utilized, also the cooperativity of the multidrug resistant gene and the chemotherapeutic drug is utilized, and in-vitro magnetic targeting, NMR imaging and other technologies are applied to diagnosis and treatment of malignant tumors.
Owner:JILIN UNIV
Diagnosis of blood clots using fibrin-binding proteins bound with contrast agents
InactiveUS7465434B2Quickly and easily interpretedEliminate side effectsPeptide/protein ingredientsMicrobiological testing/measurementVeinIntravenous route
This invention describes how to modify a fibrin-binding protein, such as tPA, with a contrast agent, such as iodine. This substance could then be given to a patient suspected of having a blood clot by an intravenous route, and then detected by a radiographic study at a short pre-determined time later. Appropriate therapy is started immediately as determined by the radiographic study.
Owner:BENFORD JACOB
Pharmaceutical Compositions Comprising Paracetamol and Process for Preparing The Same
InactiveUS20170165210A1Organic active ingredientsPharmaceutical delivery mechanismHigh concentrationAntifungal
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150 mg / ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent system comprising glycofurol, ethanol, polyethylene glycol, water. The viscosity of the said injectables is <28 cps. Further disclosed is the process for preparing the said injectables. The injectables can be administered by intramuscular route, intravenous route or as intravenous infusion after diluting in one of the routinely used intravenous fluids, infusion solutions of antibacterial, antifungal and amoebicidal drugs and along with anxiolytics (Midazolam injection) or narcotic analgesics (Fentanyl Citrate injection etc) as they remain stable, clear and transparent at least for 6 hours after dilution.
Owner:TROIKAA PHARMA
Pharmaceutical Compositions Comprising Paracetamol and Process for Preparing The Same
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150 mg / ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent system comprising glycofurol, ethanol, polyethylene glycol, water. The viscosity of the said injectables is <28 cps. Further disclosed is the process for preparing the said injectables. The injectables can be administered by intramuscular route, intravenous route or as intravenous infusion after diluting in one of the routinely used intravenous fluids, infusion solutions of antibacterial, antifungal and amoebicidal drugs and along with anxiolytics (Midazolam injection) or narcotic analgesics (Fentanyl Citrate injection etc) as they remain stable, clear and transparent at least for 6 hours after dilution.
Owner:TROIKAA PHARMA
Methods of inducing immune tolerance and reducing Anti-drug antibody response
PendingUS20210008183A1Reduce incidenceReduce intensityDispersion deliveryPeptide/protein ingredientsAntiendomysial antibodiesIntravenous route
The present invention disclosed methods for suppressing or preventing an immune response to a specific antigen in a subject, comprising administering to the subject, the specific antigen by an intravenous route followed by an inhalation route.
Owner:KAMADA
Injectable Preparations Of Diclofenac And Its Pharmaceutically Acceptable Salts
ActiveUS20130137769A1Relieve painHigh viscosityOrganic active ingredientsBiocideInjection siteIntravenous route
Injectable formulations of water-soluble salts of diclofenac, which cause significantly less pain at the site of injection and can be administered by intradeltoid route, in addition to intragluteal and slow intravenous route.
Owner:TROIKAA PHARMA
Iron deficiency in treatment of individuals at risk of cardiovascular adverse events and iron for treatment of atrial fibrillation
PendingCN114286682AHeavy metal active ingredientsPharmaceutical non-active ingredientsDiseaseHeart valvular disease
The present invention relates to the field of treating an individual at risk of cardiovascular adverse events with an intravenous iron carbohydrate complex, such as iron isomaltoside, where treatment of iron deficiency reduces the incidence or risk of cardiovascular adverse events in the individual. In another aspect, the present invention provides a method of reducing P-wave dispersion / time limit for treating an animal having atrial fibrillation or a disease associated with atrial fibrillation (e.g., heart failure, hypertension, heart valvular disease, coronary heart disease, obesity, and diabetes), the method comprises administering a therapeutically effective amount of an iron agent to the animal by oral, intramuscular or intravenous route.
Owner:PHARMACOSMOS HLDG
Dichloro-1,2-cyclohexanediamine platinum complex and its preparation method
ActiveCN103394095BGood biocompatibilityImprove solubilityPharmaceutical non-active ingredientsAntineoplastic agentsSolubilityTherapeutic effect
The invention provides a [dichloro-1,2-cyclohexanediamine]platinum complex, and the complex is obtained from the complex reactions between dichloro-1,2-cyclohexanediamine and a polymer which has the structure of the formula (I). The [Dichloro-1,2-cyclohexanediamine]platinum has a cyclohexanediamine structure, a stable inner-core structure is formed because of the accumulation and hydrophobic effect after the [Dichloro-1,2-cyclohexanediamine]platinum cooperate with a polymer, and the stability of the [Dichloro-1,2-cyclohexanediamine]platinum is good under physiology conditions. The complex can effectively avoid the problems of sudden release and non-specific interaction of [Dichloro-1,2-cyclohexanediamine]platinum after [Dichloro-1,2-cyclohexanediamine]platinum enters the blood circulation system through intravenous route. The complex can be assembled to form a micelle structure; accumulation of the complex on the tumor locations can be achieved through strengthening osmotic and retention effects, and thus the goals of reducing toxic and by effects and improving curative effects are achieved. Furthermore, the adopted complex has a good biological compatibility, is capable of degrading in human bodies, and thus the prepared [Dichloro-1,2-cyclohexanediamine]platinum has a good solubility.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Chonsurid for venous injection administration and its preparing method
The present invention discloses chonsurid preparation capable of being used for venous injection and its preparation process. The purified chonsurid preparation may be used for intramuscular injection and intravenous injection after being added into glucose solution or physiological saline, and has convenient use. Owing to the raised purity of chonsurid, the chonsurid preparation may be injected directly into blood circulation system resulting in high blood medicine concentration, fast acting, high curative effect and less side effect.
Owner:NANJING GRITPHARMA CO LTD
Pharmaceutical formulations comprising diclofenac
PendingUS20220280463A1Organic active ingredientsInorganic non-active ingredientsThrombophlebitisDiclofenac Acid
Conventional injectable formulations of Diclofenac are known to have excipients which cause irritation at the site of injection and are painful. Further, such conventional formulations also cause thrombophlebitis. Other injectable formulations of Diclofenac known in the prior art also contain excipients which are tissue irritants and may cause toxicity when administered through intravenous route. The cyclodextrin compounds used in Dyloject® may cause problems while elimination in the renal compromised patients. The present invention therefore provides injectable Diclofenac formulations which do not cause irritation and pain at the site of injection. Further, the formulations of the present invention do not include cyclodextrins, therefore can also be administered to the renal compromised patients.
Owner:FTF PHARMA
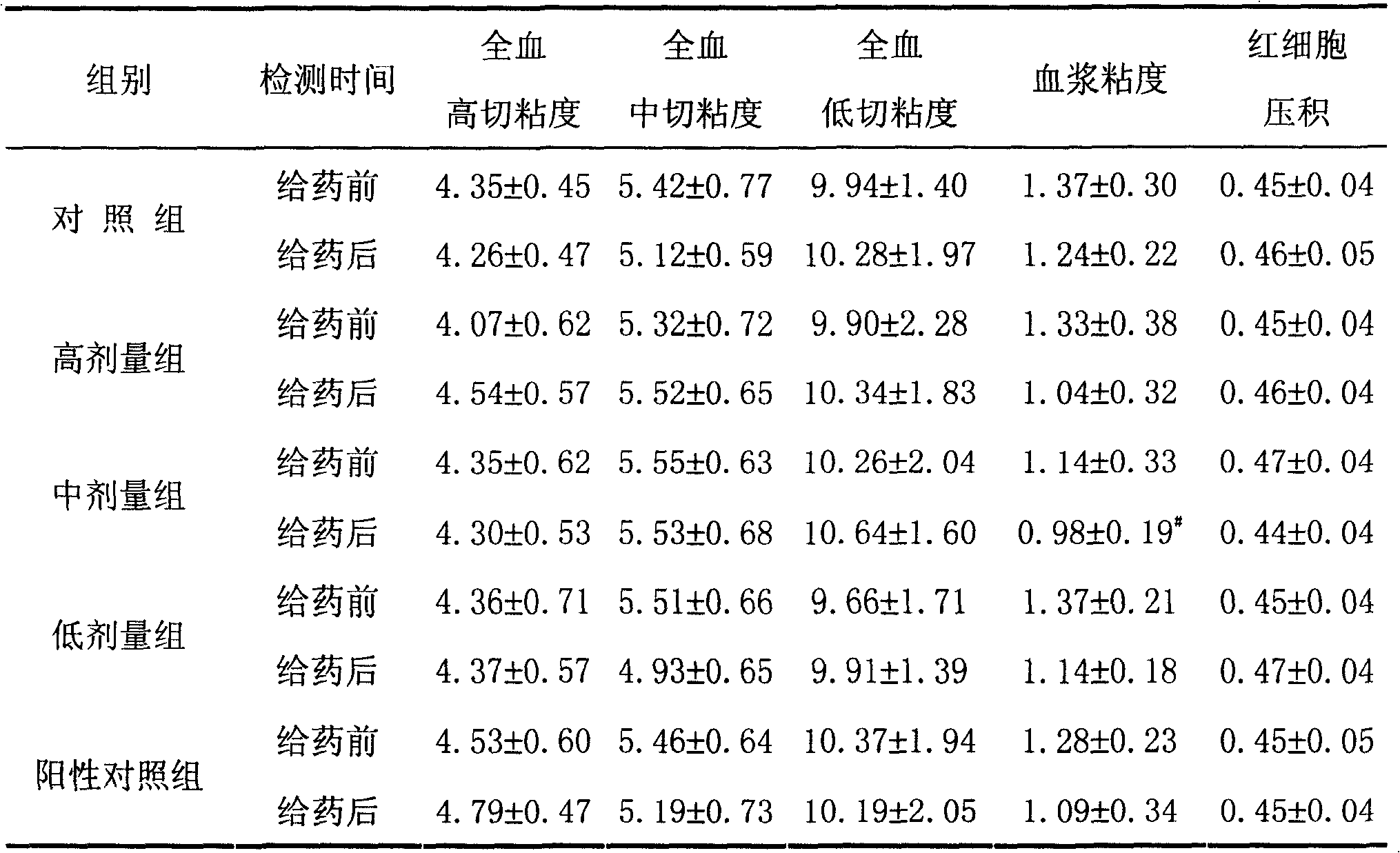
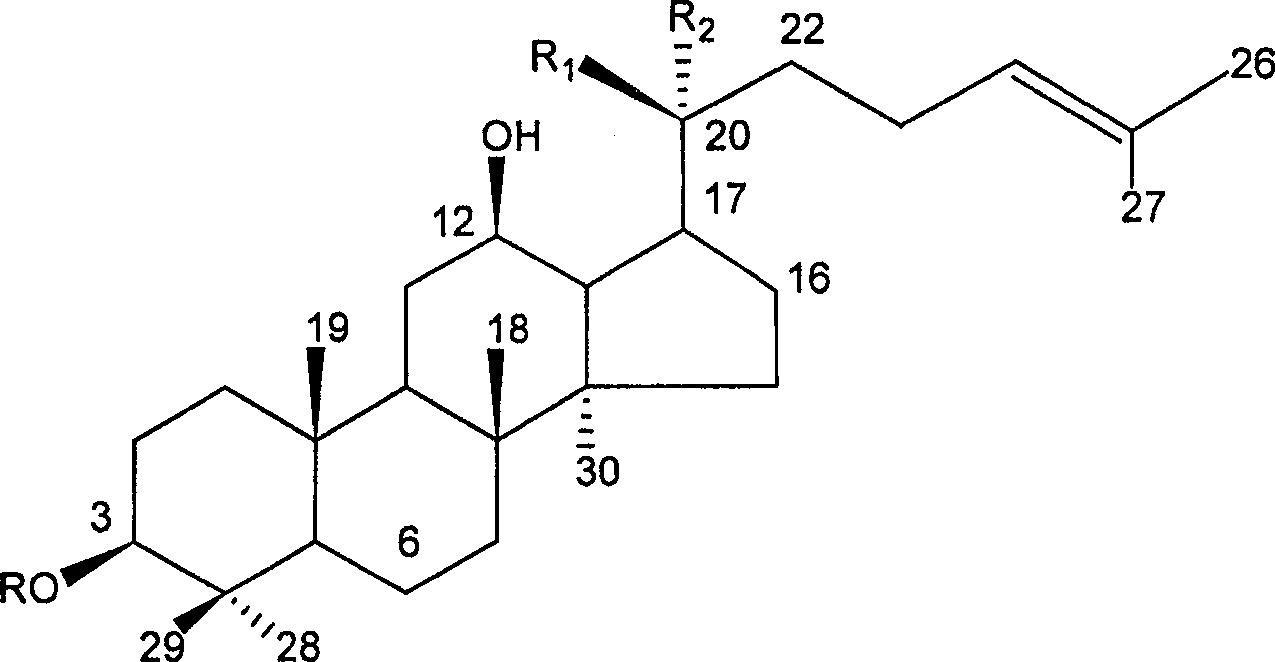
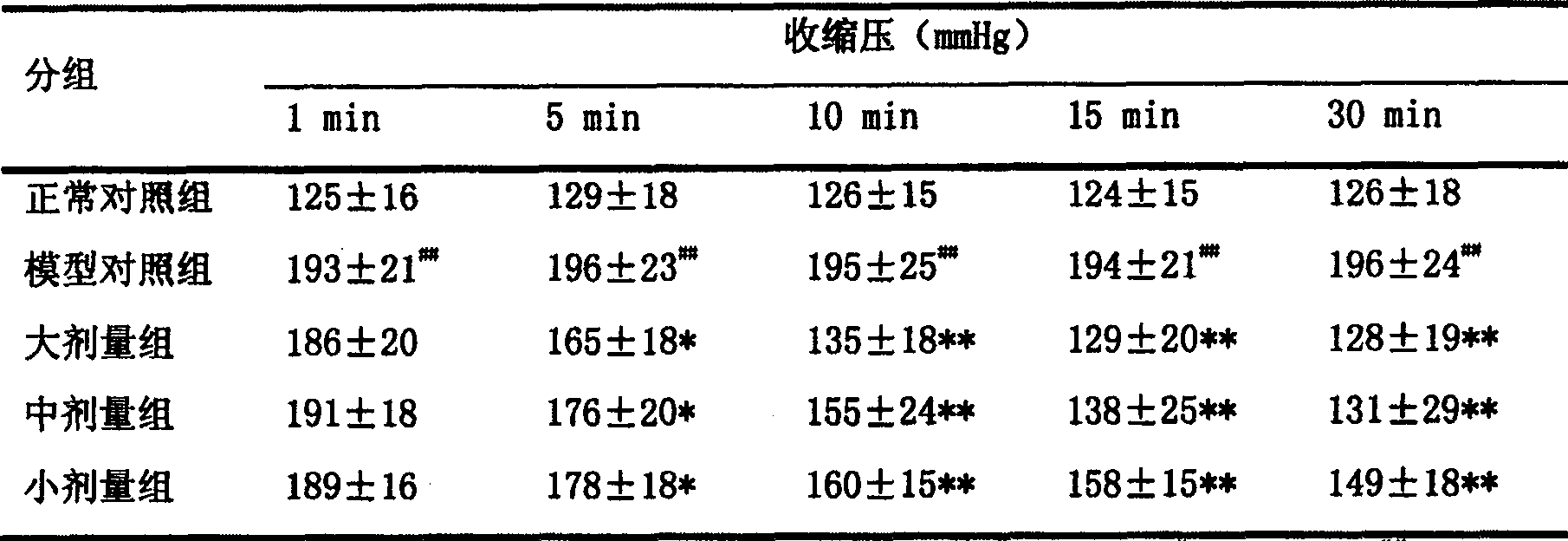
Application of 20(S)-panaxcoside -Rg3 in preparation of medicine for treating or preventing high blood pressure
InactiveCN100525772COrganic active ingredientsPharmaceutical delivery mechanismIntravenous routeHigh Blood Pressures
The invention provides the application of 20(S)-ginsenoside-Rg3 in the preparation of medicines for treating or preventing hypertension, and the invention also provides a pharmaceutical composition containing 20(S)-ginsenoside-Rg3, the pharmaceutical composition Administration can be by oral, sublingual, transdermal, intramuscular, subcutaneous or intravenous routes.
Owner:山东同余堂人参科技有限公司
Pharmaceutical compositions comprising paracetamol and process for preparing the same
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150 mg / ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent system comprising glycofurol, ethanol, polyethylene glycol, water. The viscosity of the said injectables is <28 cps. Further disclosed is the process for preparing the said injectables. The injectables can be administered by intramuscular route, intravenous route or as intravenous infusion after diluting in one of the routinely used intravenous fluids, infusion solutions of antibacterial, antifungal and amoebicidal drugs and along with anxiolytics (Midazolam injection) or narcotic analgesics (Fentanyl Citrate injection etc) as they remain stable, clear and transparent at least for 6 hours after dilution.
Owner:TROIKAA PHARMA
Diclofenac composition
InactiveCN104968331ALow viscosityLow toxicityOrganic active ingredientsSenses disorderRectal SuppositoryIntravenous route
The present invention relates a composition comprising Diclofenac and salts thereof wherein Diclofenac or its slats are present in an amount of 25 - 200 mg. The composition is suitable for the parenteral administration through intramuscular, intravenous route; also for oral, dermal, subcutaneous, cutaneous, nasal, ocular drops, as rectal suppository, vaginal pessaries, intra-articular, and otic delivery. The invention also provides compositions comprising a combination of Diclofenac and other drugs. The invention further provides a method for preparing said composition.
Owner:THEMIS MEDICARE LTD
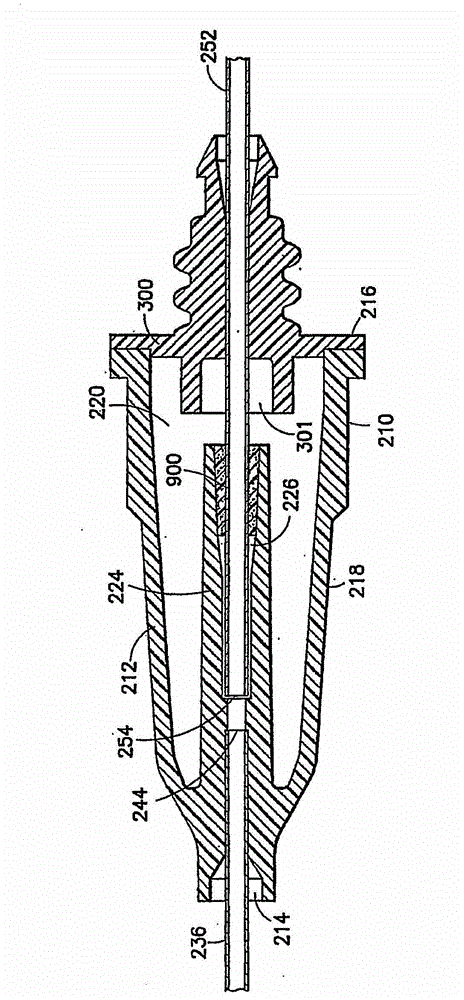
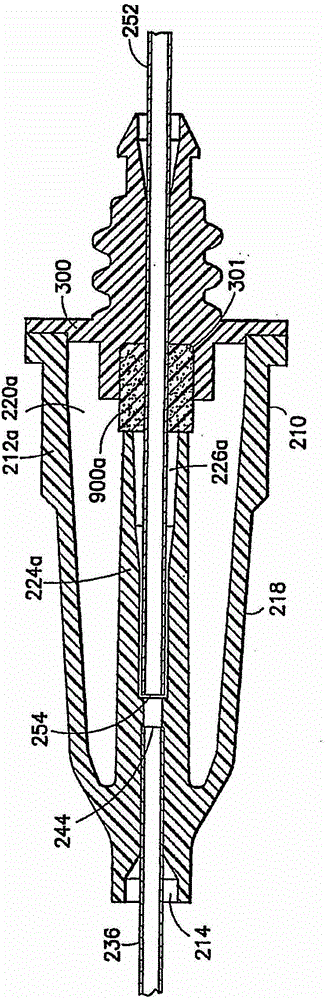
Blunt needle safety drug delivery system
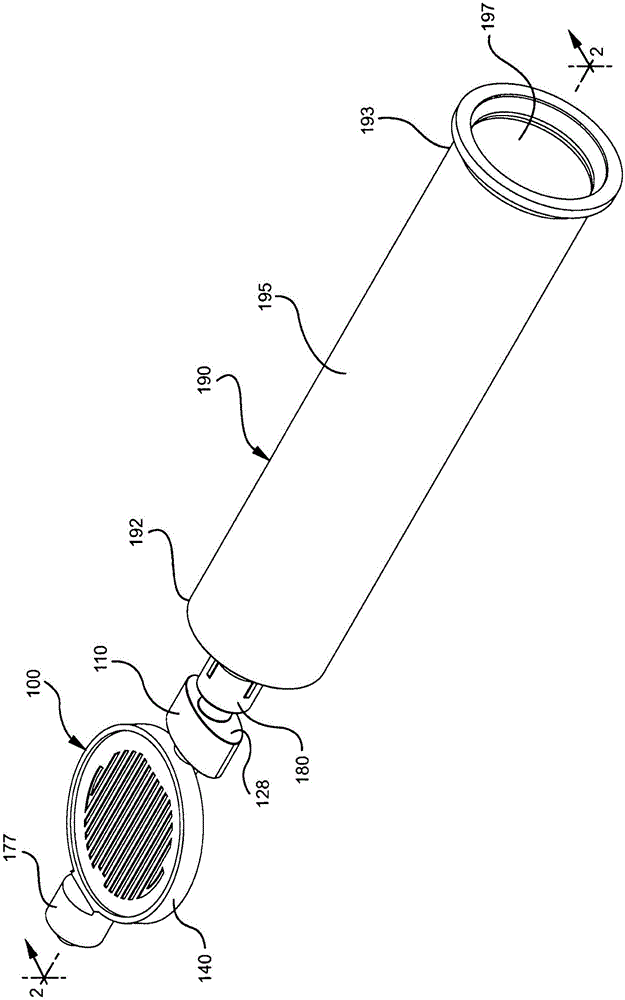
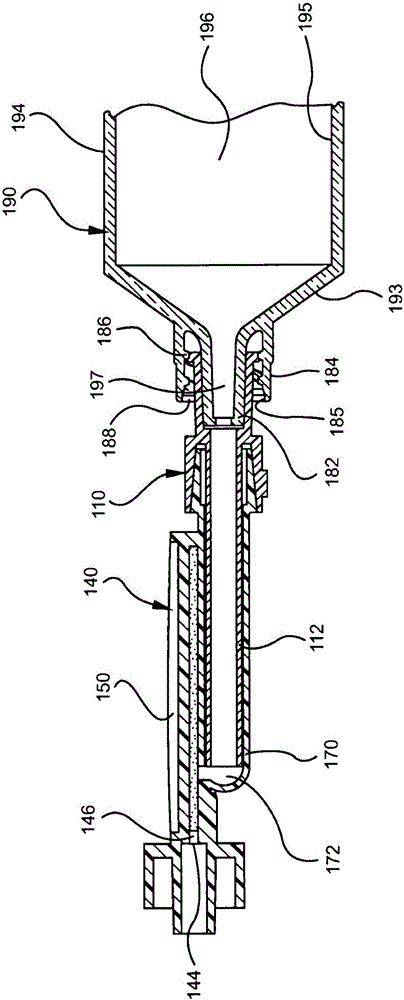
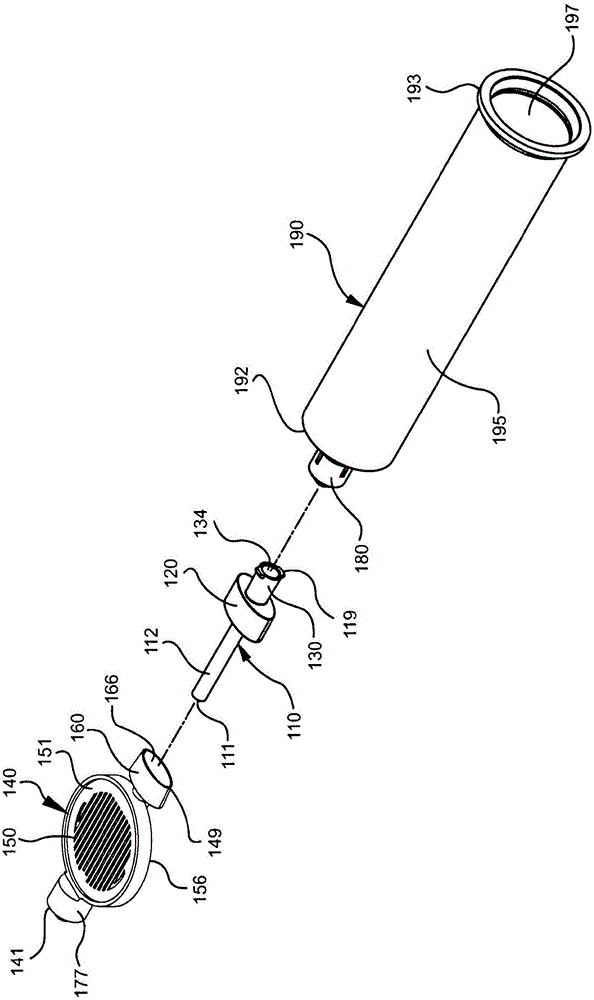
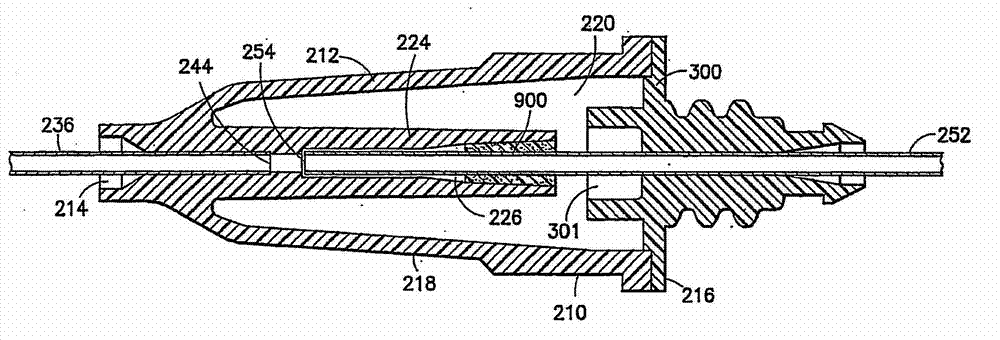
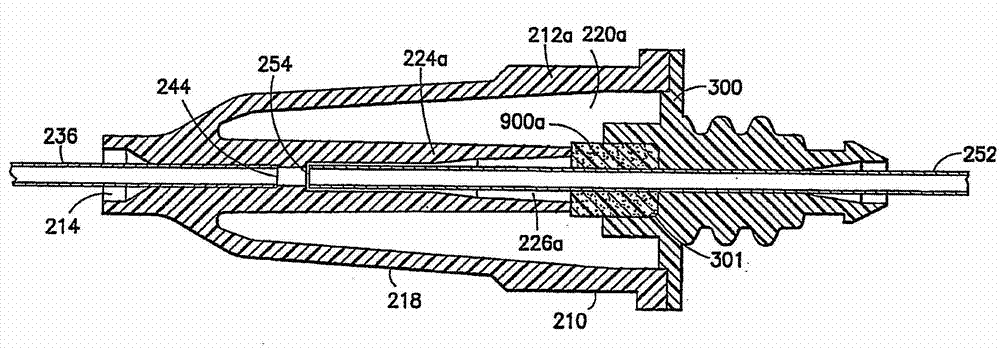
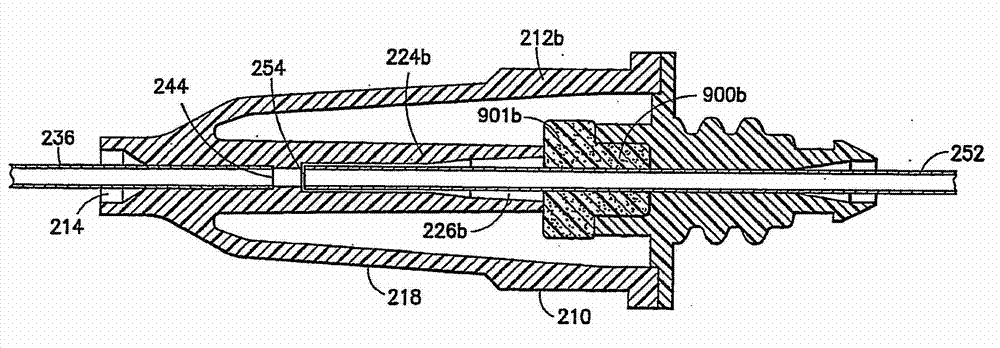
Drug delivery devices having non-luer connections and adapters and adaption connectors for providing non-luer connections to drug delivery devices are provided. An exemplary drug delivery device for use with a catheter connection includes a blunt needle component (110) with a non-luer connection (120) and a filter component (140) with a non-luer connection (160), wherein the non-luer connections (120, 160) of the blunt needle component and filter component are incompatible with standard luer fitting and intravenous route-accessing devices.
Owner:BECTON DICKINSON & CO
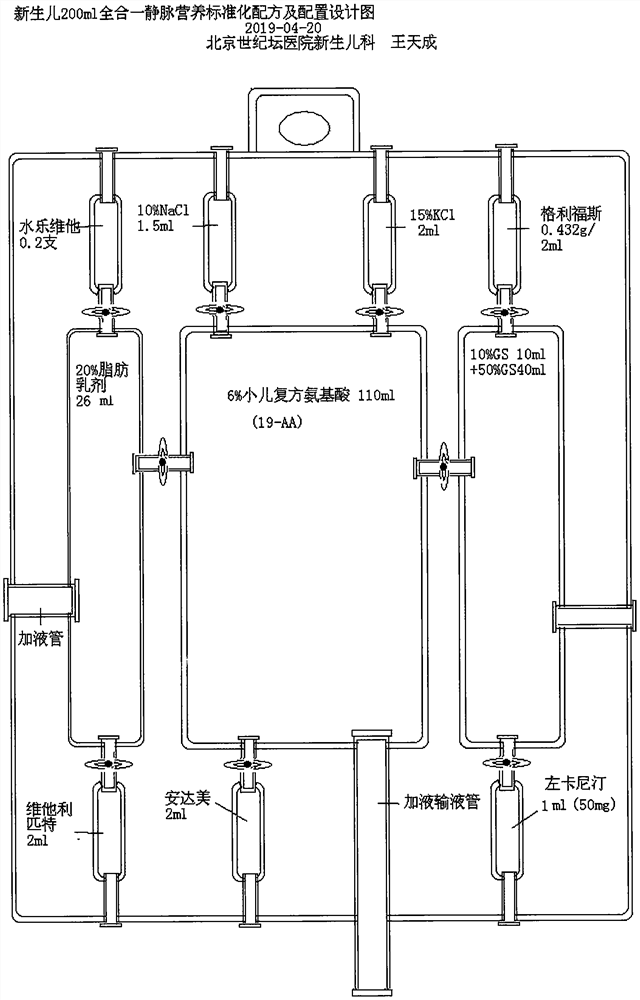
Intravenous nutrition formula for newborns
InactiveCN113908116AAvoid double countingReduce work intensityHeavy metal active ingredientsHydroxy compound active ingredientsFormularyNutrition
The invention discloses an intravenous nutrition formula for newborns, which is prepared from water levistat, 10% of NaCl, 15% of NaCl, Grifors, 20% of fat emulsion, 6% of pediatric compound amino acid, 10% of GS, 50% of GS, Vitalipiride, Andamex and levocarnitine. The intravenous nutrition formula is suitable for newborns which cannot be fed by mouth or are partially fed by mouth, the insufficient part of newborns are fed by vein, various daily required nutrient substances are input through an intravenous way, and the traditional Chinese medicine is free of toxic and side effects, quick in effect, simple and easy to implement.
Owner:李世红
Flashing flow blood collecting needle
Owner:BECTON DICKINSON & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![[Dichloro-1,2-cyclohexanediamine]platinum complex and preparation method thereof [Dichloro-1,2-cyclohexanediamine]platinum complex and preparation method thereof](https://images-eureka.patsnap.com/patent_img/09f8f797-80e3-45df-9b38-aceb6c8b06ab/HDA0000368705880000011.PNG)
![[Dichloro-1,2-cyclohexanediamine]platinum complex and preparation method thereof [Dichloro-1,2-cyclohexanediamine]platinum complex and preparation method thereof](https://images-eureka.patsnap.com/patent_img/09f8f797-80e3-45df-9b38-aceb6c8b06ab/HDA0000368705880000012.PNG)
![[Dichloro-1,2-cyclohexanediamine]platinum complex and preparation method thereof [Dichloro-1,2-cyclohexanediamine]platinum complex and preparation method thereof](https://images-eureka.patsnap.com/patent_img/09f8f797-80e3-45df-9b38-aceb6c8b06ab/HDA0000368705880000021.PNG)