Iron deficiency in treatment of individuals at risk of cardiovascular adverse events and iron for treatment of atrial fibrillation
A technology for adverse events and atrial fibrillation, which can be used in cardiovascular system diseases, medical preparations with inactive ingredients, blood diseases, etc. It can solve problems such as occurrence and persistence, abnormal ion channel function, and prolongation of atrial conduction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0467] Two phase III randomized, open-label, controlled safety and efficacy studies involving the use of iron isomalttoside ("IIM", Product name ) and iron sucrose ("IS", trade name )the treatment. These studies can compare the incidence of treatment-emergent cardiovascular adverse events, especially in patients with cardiovascular risk factors.
[0468] Research design
[0469] These studies were randomized, open-label, comparative studies. Individuals with iron deficiency anemia (IDA) were randomized 1:1 to one course of two treatments. Iron Isomalttoside 1000 is administered as a single dose of 1000 mg elemental iron. According to its US label, iron sucrose is administered as a slow intravenous bolus of 200 mg repeated up to 5 times to achieve a cumulative dose of 1000 mg.
[0470] Target
[0471] This study allowed to compare the incidence of protocol-defined adverse cardiovascular events in individuals with IDA and nondialysis-dependent chronic kidney disease tre...
Embodiment 2
[0665] A study was performed to determine the effect of iron on P wave dispersion.
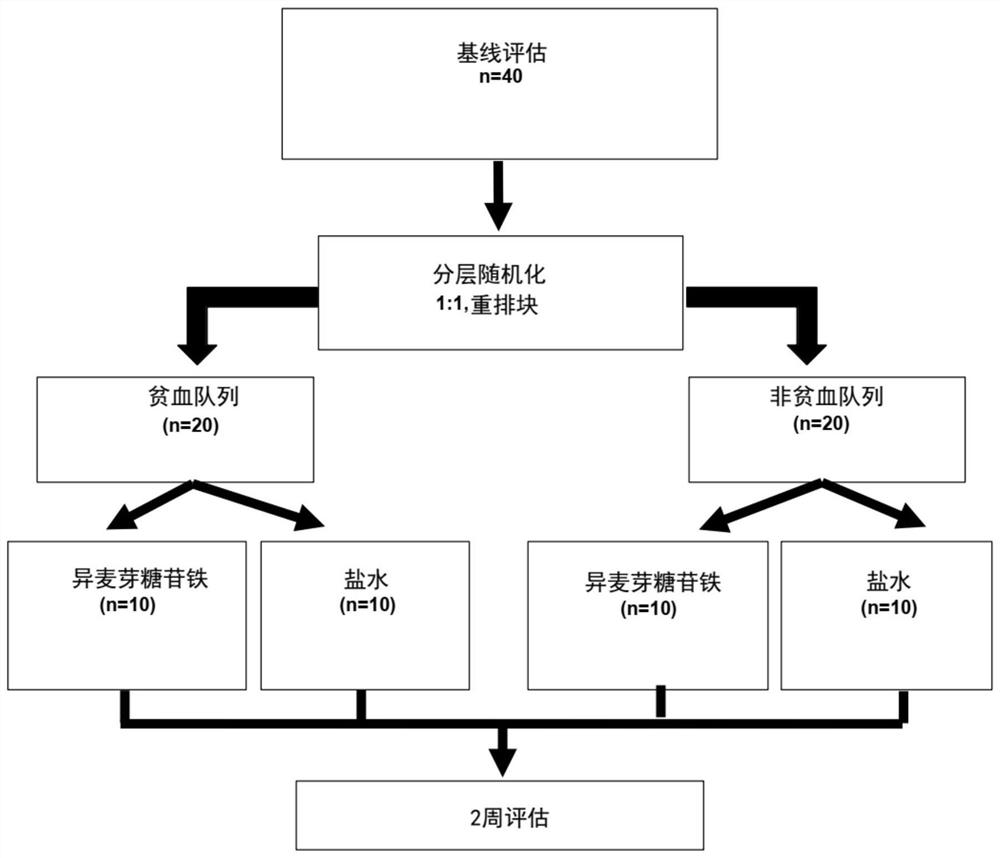
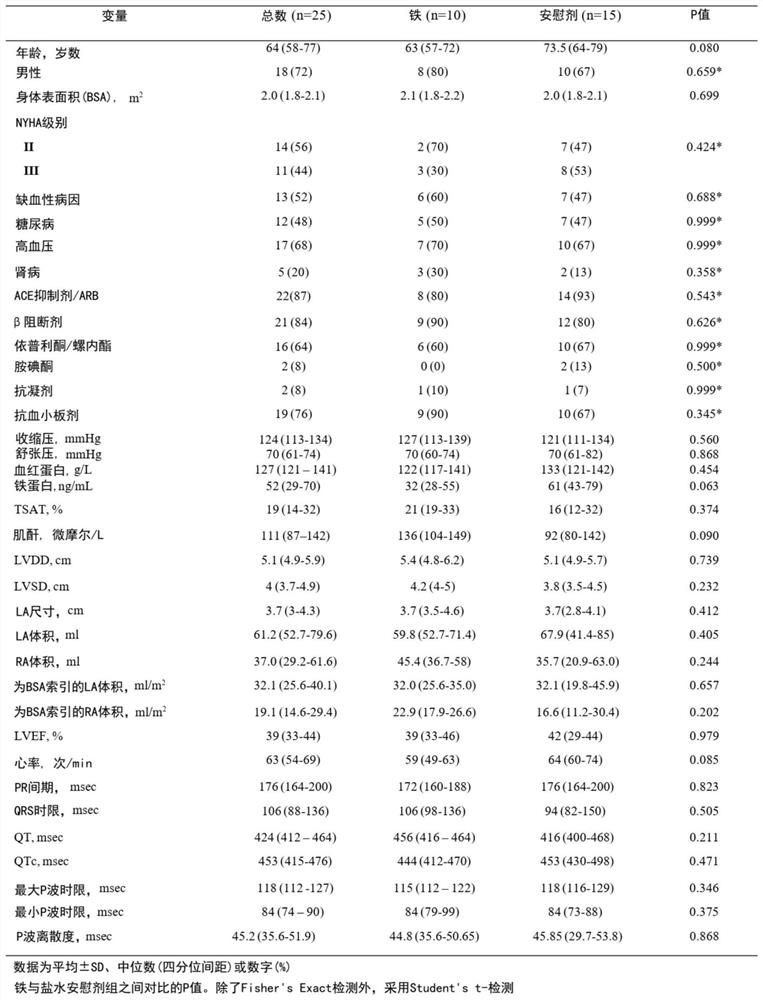
[0666] The study was a post-hoc analysis of a randomized, double-blind, placebo-controlled trial with independent research management from the South Central Berkshire Ethics Committee, the Medicines and Healthcare Products Regulatory Agency and King's College Hospital Committee Approval. King's College London and King's College Hospitals NHS Foundation are co-sponsors. The King’s Health Partners Office of Clinical Trials monitored the trial and ensured compliance with the International Conference on Harmonization guidelines for good clinical practice and the Declaration of Helsinki. All patients obtained written informed consent. figure 1 A flowchart representing the experiment. A total of 25 chronic heart failure patients with normal sinus rhythm were enrolled in this trial, 15 of whom were randomized to receive intravenous saline placebo and 10 were randomized to receive intravenous iron ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com