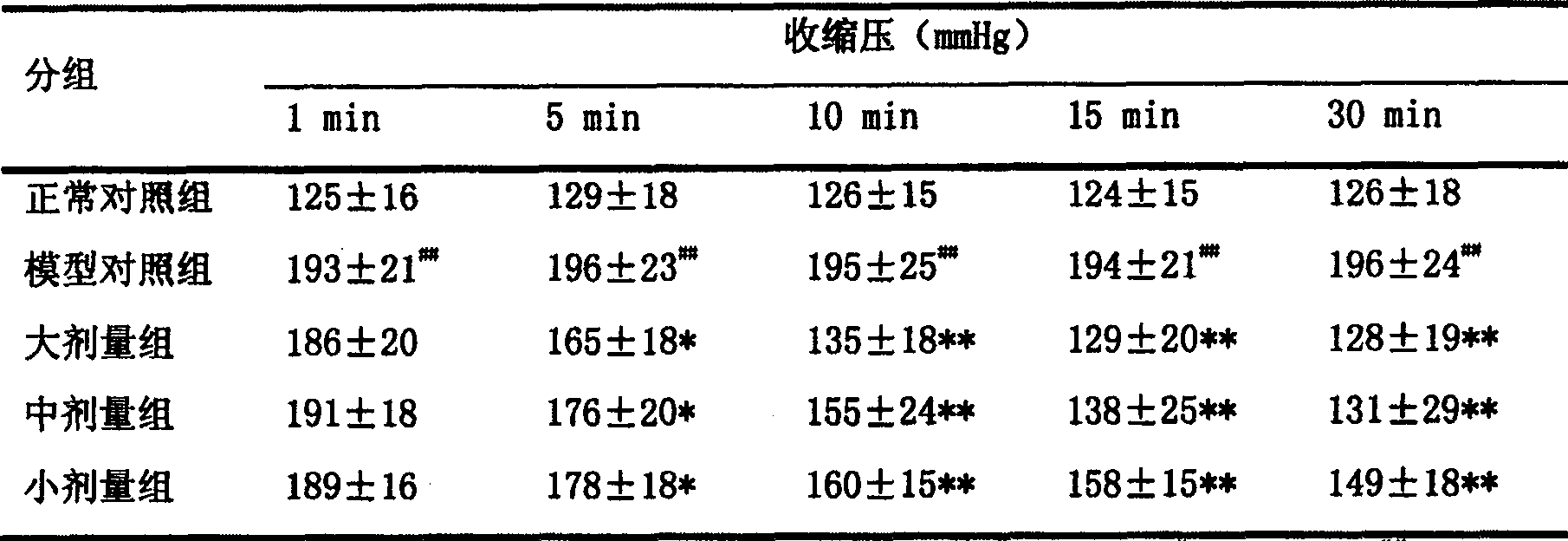
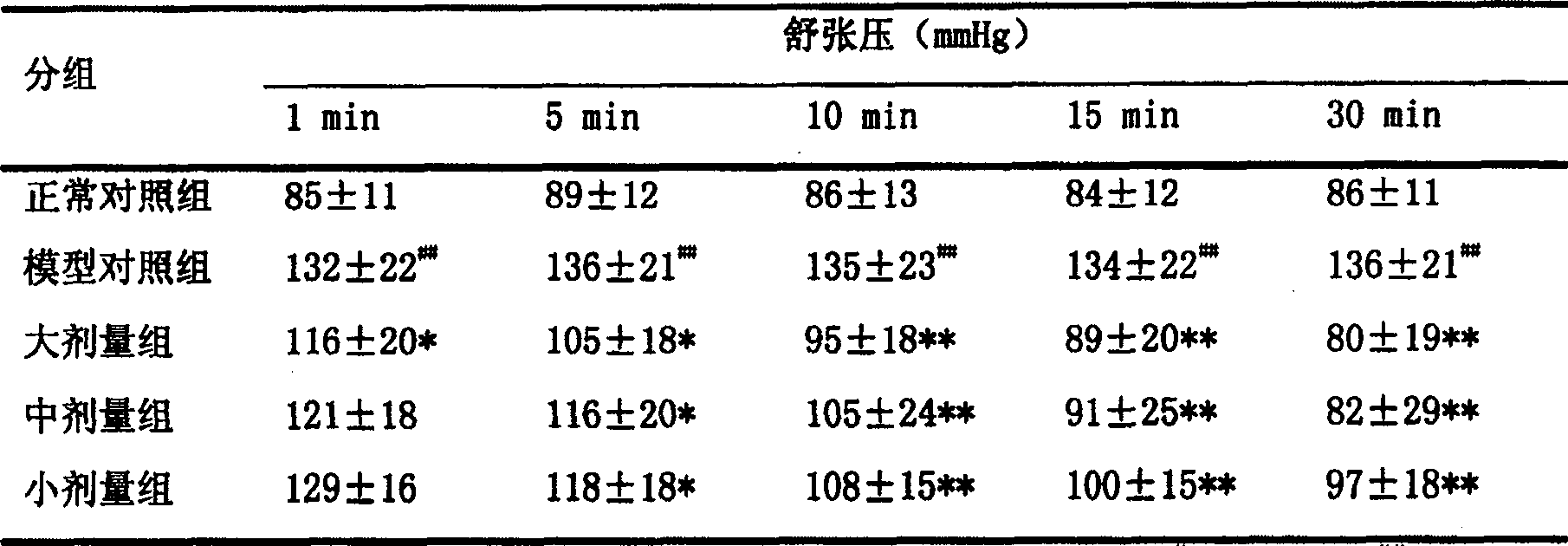
Application of 20(S)-panaxcoside -Rg3 in preparation of medicine for treating or preventing high blood pressure
A technology of ginsenoside and hypertension, applied in the field of application of 20(S)-ginsenoside-Rg3 in the preparation of medicines for treating or preventing hypertension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
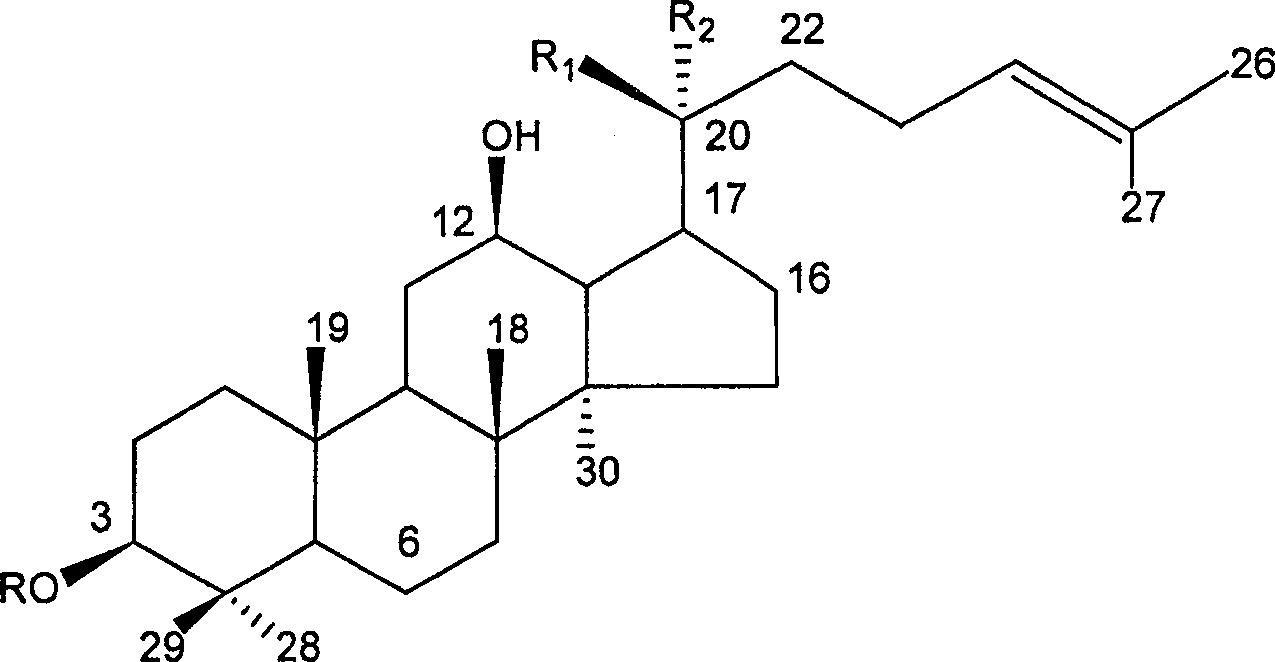
[0012] Preparation Example 1: Preparation of 20(S)-ginsenoside Rg3
[0013] Take Panax notoginseng saponins (or ginsenosides) as raw material, add 50% acetic acid aqueous solution, heat in a water bath at 70°C for 6 hours, cool, add 5% sodium carbonate solution, extract the solution with n-butanol, evaporate the extract to dryness, and put Silica gel column, using chloroform-methanol as eluent, gradient elution to obtain 20(S)-ginsenoside Rg3, and repeated silica gel column chromatography to obtain 20(S)-ginsenoside Rg3.
preparation example 2
[0014] Preparation Example 2: Preparation of Injection
[0015] Weigh 0.50g 20(S)-ginsenoside Rg3, add 50g PEG-400 to dissolve the saponin; prepare 50g pH5.5 phosphate buffer, weigh 0.02g sodium bisulfite and add to the above phosphate buffer ; Add the buffer solution to the PEG solution of saponin, mix evenly, add 0.01% activated carbon for needles and incubate at 100°C for 30 minutes, G 3 Filtration with a vertically fused glass funnel and a 0.22um microporous membrane. The filtered medicinal solution is packaged into 50 injections of 2ml each.
preparation example 3
[0016] Preparation Example 3: Preparation of Freeze-dried Powder for Injection
[0017] Weigh 0.50g20(S)-ginsenoside Rg 3 , add 50g PEG-400 to dissolve the saponin. Prepare 50g of pH5.5 phosphate buffer, weigh 0.02g of sodium bisulfite and add it to the above phosphate buffer; add the buffer to the PEG solution of saponin, mix well, add 0.01% activated carbon for needles, 100°C Keep warm for 30 minutes, G 3 Filtration with a vertically fused glass funnel and a 0.22um microporous membrane; the filtered medicinal solution is divided into 2ml / vials, and freeze-dried by a freeze dryer to make a freeze-dried powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com