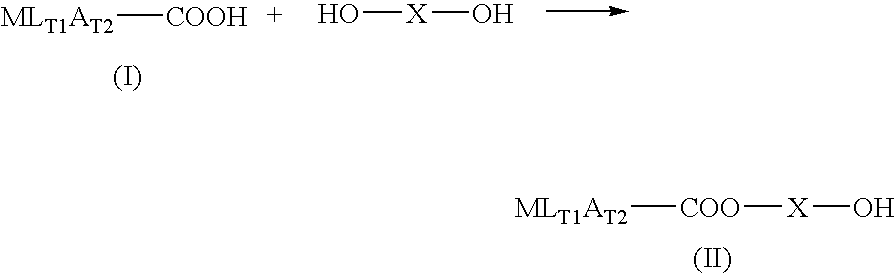Patents
Literature
73 results about "Diclofenac Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
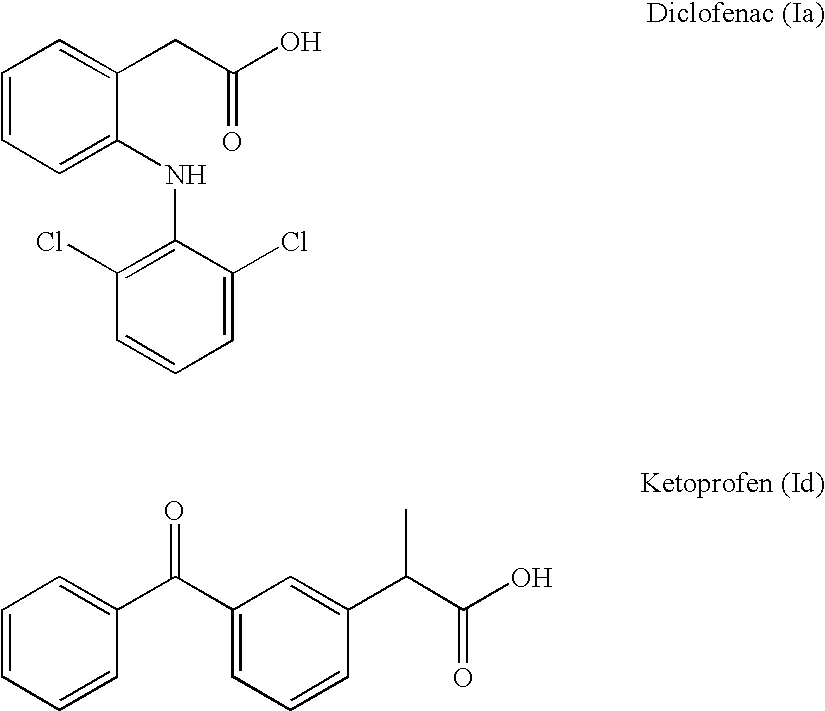
Diclofenac is a nonsteroidal benzeneacetic acid derivative with anti-inflammatory activity. As a nonsteroidal anti-inflammatory drug (NSAID), diclofenac binds and chelates both isoforms of cyclooxygenase (COX-1 and-2), thereby blocking the conversion of arachidonic acid to pro-inflammatory-proprostaglandins.
Formulations Of Low Dose Diclofenac And Beta-Cyclodextrin
InactiveUS20070232566A1Good curative effectParticular value in treating pain in a horsePowder deliveryBiocideBeta-CyclodextrinsLow dose
The present invention is directed to a pharmaceutical composition containing a unit dose of a diclofenac compound effective to induce analgesia; and a beta-cyclodextrin compound; wherein the dose of the diclofenac compound is less than 10 mg. The present invention is also directed to methods of treating a subject in need of analgesia with the pharmaceutical compositions of the invention.
Owner:JAVELIN PHARMA INC +1
Piclofenac potassium sustained release capsule and preparing technique thereof
InactiveCN101322695ASmall toxicityGood formulation stabilityOrganic active ingredientsAntipyreticAnalgesics drugsSustained release pellets
The invention provides a sustained-release capsule of diclofenac potassium, which is an anti-inflammation and analgesic drug, and a production method thereof. The diclofenac potassium sustained-release capsule is produced by preparing diclofenac potassium into sustained-release pellets and then filling the pellets into the capsule; the diclofenac potassium sustained-release pellet consists of a blank pellet core, a main drug layer coated outside of the pellet core and containing diclofenac potassium and a sustained-release coating layer covered on the main drug layer. The diclofenac potassium sustained-release capsule of the invention has fine preparation stability and prominent release effect.
Owner:海南华旗药业销售有限公司
Agentfor reducing side effects of diclofenac
InactiveUS20060122275A1Eliminate side effectsLess side effectsBiocidePeptide/protein ingredientsRenal disorderSide effect
The present invention relates to reduction of side effects of diclofenac or a salt thereof An agent for reducing side effects of diclofenac or a salt thereof which comprises ornoprostil. It is expected that a combination agent of diclofenac or a non-toxic salt thereof and ornoprostil is comparable to even superior to marketed diclofenac tablets or Arthrotec tables in effects and fast-acting property while showing little side effects (particularly digestive disorders, gastric ulcer, diarrhea / vomiting and renal disorder) and exerting excellent antipyretic, analgesic and antiinlfammatory effects. Also, by formulating the combination agent into a preparation of separation type pharmaceutical preparation, the stability of ornoprostil can be improved.
Owner:ONO PHARMA CO LTD
Anti-acne composition and methods of use thereof
The present invention provides for a topical dermatological composition that includes diclofenac and clindamycin, in a pharmaceutically acceptable medium, wherein at least one of the diclofenac and clindamycin is at least partially microencapsulated.
Owner:RAYUDU SREEDHAR RAO
Aceclofenac preparation method
ActiveCN103086907AHigh selectivityReduce breakageOrganic compound preparationAmino-carboxyl compound preparationHydrogen halideOrganic acid
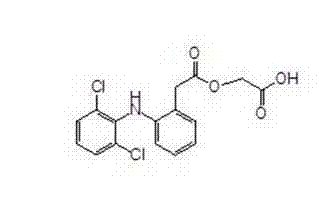
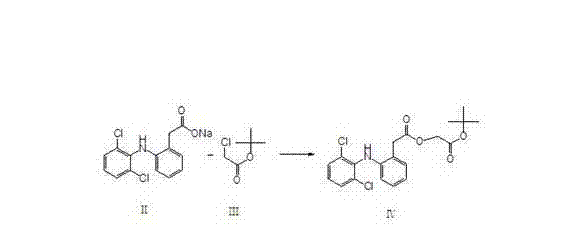
The invention relates to an aceclofenac preparation method and belongs to the chemical pharmaceutical field. The method comprises a step that acidolysis of aceclofenac tert-butyl ester is carried out in a mixed solution comprising a low-molecular-weight organic acid and hydrogen halide to obtain aceclofenac. The method which allows the acidolysis of aceclofenac tert-butyl ester to be carried out in the mixed solution comprising the low-molecular-weight organic acid and hydrogen halide has the advantages of high selectivity, reduction of the fracture of the ethoxy group in aceclofenac tert-butyl ester to a lowest limit, high acidolysis reaction conversion rate, extremely-low content of diclofenac in obtained aceclofenac, easy aceclofenac refining, and high aceclofenac purity.
Owner:HENAN DONGTAI PHARM
Immune colloidal gold method for detecting diclofenac illegally added in Chinese patent medicament
The invention relates to an immune colloidal gold method for detecting diclofenac illegally added in a Chinese patent medicament. The method comprises the following steps of: coupling the diclofenac with bovine serum albumin (BSA) by using an active ester method to synthesize an artificial immune antigen, and immunizing an animal by using the artificial immune antigen to prepare a specific diclofenac antibody; coupling the diclofenac with OVA (ovalbumin) to synthesize a detection antigen for constructing an immune detection method; and marking the separated and purified diclofenac antibody to nano colloidal gold, then immobilizing the diclofenac antibody, the diclofenac detection antigen and a goat-anti-rabbit IgG (immunoglobulin G) on a vector respectively, and performing semi-quantitative assay determination of the diclofenac according to a competitive inhibitory immuno-chromatography principle, wherein through the established detection system, the minimum detection limit of the diclofenac illegally added in a health-care product or the Chinese patent medicament is 2.0mug / kg, and quick detection can be completed within 5 minutes. By adopting the method, the nano technology and the immune technology are applied in the field of safety detection of food and medicaments, and illegal addition of the diclofenac is quickly detected in one step.
Owner:SHAOGUAN COLLEGE
Method for removing drugs in soil by utilizing magnetism molecular imprinting-electromagnetism grating combination
InactiveCN103170496AAchieve recyclingSimple structureContaminated soil reclamationDrug ContaminationClofibric acid
The invention relates to a method for removing drugs in soil by utilizing magnetism molecular imprinting-electromagnetism grating combination. The drugs are one or more of carbamazepine, clofibric acid or diclofenac. The method comprises the following specific steps of: fully mixing preprocessed soil and a magnetism molecularly imprinted polymer, absorbing the drugs in the soil on the surface of the magnetism molecular imprinting, and separating the water soil and the magnetism molecularly imprinted polymer after reaction through an electromagnetism grid; and absorbing the magnetism molecular imprinting on the grid through a magnetic field generated by the electromagnetism grid after electrification, and effectively separating the magnetism molecular imprinting and the grid. The method provided by the invention has the advantages that the operation is simple, the effect is obvious, the arbamazepine, the clofibric acid and the diclofenac and the like in the soil are removed in one time, the drugs absorbed on the magnetism molecular imprinting are recovered and separated through the electromagnetism grid; a magnetism molecular imprinting technology is utilized to restore the soil polluted by the drugs and has the advantages that the cost is low, the restoring efficiency is high and the like; and an electromagnetism grid device is utilized to recycle the resources, so that secondary pollution is not caused.
Owner:TONGJI UNIV
Skeleton Diclofenac Potassium Sustained-release Pellet Capsules and Production Process
ActiveCN102266292ALittle side effectsReduce the number of dosesOrganic active ingredientsAntipyreticSustained release pelletsDiclofenac Acid
The invention relates to matrix diclofenac potassium sustained-release pellet capsules and a production process thereof. The matrix diclofenac potassium sustained-release pellet capsules are prepared by filling matrix diclofenac potassium sustained-release pellets into gastric-soluble capsule shells. The matrix diclofenac potassium sustained-release pellets are prepared in one step by extrusion and rolling. The formula of the matrix diclofenac potassium sustained-release pellets comprises basic remedy diclofenac potassium 10-50%, matrix agent 1-20%, diluent 20-80%, antioxidant 0.1-5%, antisticking agent 0.1-5%, absorption promoting agent 1-10%, and any available wetting agent as balance, wherein the matrix agent is hydrophilic gel matrix agent, or hydrophilic gel matrix agent and erodiblematrix agent, or hydrophilic gel matrix agent and insoluble matrix agent. The matrix diclofenac potassium sustained-release pellet capsules provided by the invention have the advantages of high bioavailability, long in vivo holdup time, regular drug release, good in vivo (Beagle dogs) absorption and reproducibility, good sustained-release effect, simple production process, short production period, and no flying of dust during production, and are suitable for industrial production.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Deep processing method of wastewater during production of diclofenac
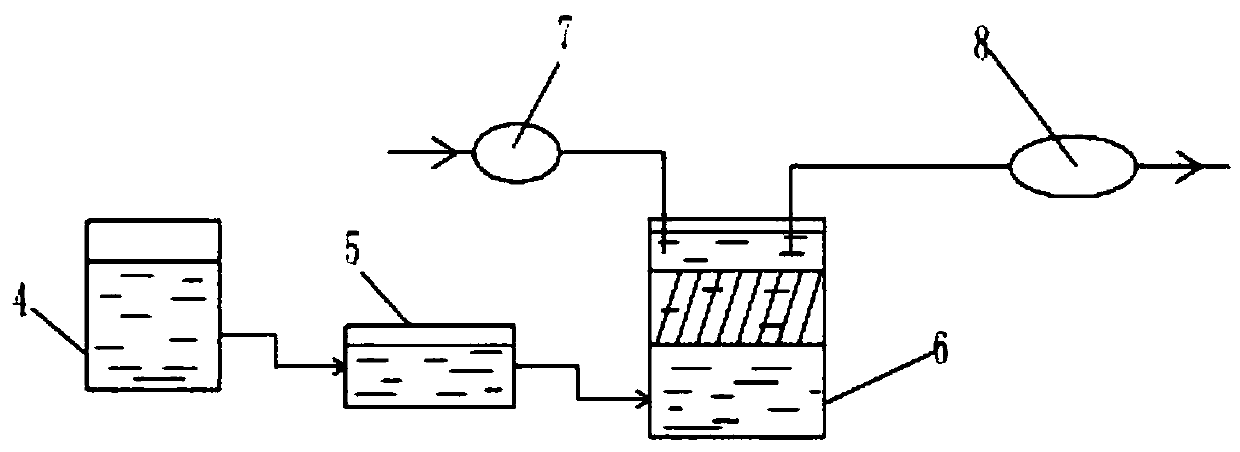
InactiveCN103193352ASimultaneous implementation of governanceSynchronize resourcesWater contaminantsMultistage water/sewage treatmentPre treatmentDiclofenac Acid
The invention discloses a deep processing method of wastewater during production of diclofenac. The deep processing method comprises the following steps of: pretreating (filtering and pH adjusting) wastewater during production of diclofenac, and forming a adsorbing gradient fluidized bed in an adsorption reaction tank by adopting an adsorption nanomaterial, so that organic matters in the wastewater during production of the diclofenac are adsorbed onto the nanomaterial; and adding a high polymer composite flocculant to carry out reinforced flocculation after the absorption is balanced, so that the nanomaterial forms a floc so as to achieve solid-liquid separation after precipitation, wherein the absorption nanomaterial is recycled after being regenerated through salt and alkali desorption. The COD (Chemical Oxygen Demand) Cr of the wastewater, during production of the diclofenac, treated by the deep processing method, can be reduced to be below 2000mg / L, the CODCr removal rate is more than 85 percent, and the vast majority of resources in the wastewater can be recycled. According to the deep processing method, wastewater processing and resource recycling can be synchronously carried out, and remarkable economical, social and environment benefits can be achieved.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Formulations of low dose diclofenac and beta-cyclodextrin
ActiveUS20110218247A1Good curative effectParticular value in treating pain in a horsePowder deliveryBiocideBeta-CyclodextrinsLow dose
Owner:JAVELIN PHARMA INC
Diclofenac epolamine jellies, preparing method and uses thereof
ActiveCN101224186ANo greasy feelingNo stimulationOrganic active ingredientsAntipyreticAdditive ingredientIrritation
The invention relates to a non-steroidal anti-inflammatory demulcent drug, which is a diclofenac epolamine gel preparation for relieving moderate pain in muscles, soft tissues and articulations. The gel preparation adopts 0.1-8.0 percent (weight percentage) of diclofenac epolamine as a main activated ingredient, 0-20 percent of grease, 0-5 percent of surfactant, 5-20 percent of cosolvent, a pH regulator, 5-20 percent of bacteriostat, 0.5-2.0 percent of gel substrate, etc. and purified water. PH value of the preparation is 6.0-8.0. The invention also provides a preparation method of the gel. The gel preparation of the invention has the advantages of good stability and good skin coupling effect, and can promote absorption of the skin to the drug and improve bioavailability and curative effect; at the same time, the substrate has stable property and no irritation to the skin, can be easily smeared and washed, has no greasiness, is beneficial to releasing of drug, especially water soluble drug and has good safety and clinical practicability.
Owner:SHANGHAI HUILUN BIOLOGICAL TECH CO LTD +1
Injectable preparations of diclofenac and its pharmaceutically acceptable salts
ActiveUS9211251B2Relieve painHigh viscosityBiocideOrganic active ingredientsInjection siteIntravenous route
Injectable formulations of water-soluble salts of diclofenac, which cause significantly less pain at the site of injection and can be administered by intradeltoid route, in addition to intragluteal and slow intravenous route.
Owner:TROIKAA PHARMA
Local application emplastrum containing heparin and diclofenac
The invention relates to a local application emplastrum with analgesic activity and simultaneously capability of enabling hematoma reabsorption, which comprises a basal layer, a pharmaceutically acceptable diclofenac salt, a hydrogel matrix form adhesion layer with heparin or heparitin, and a protective membrane capable of being removed in use.
Owner:ALTERGON
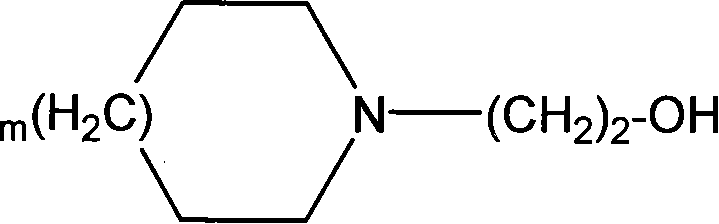
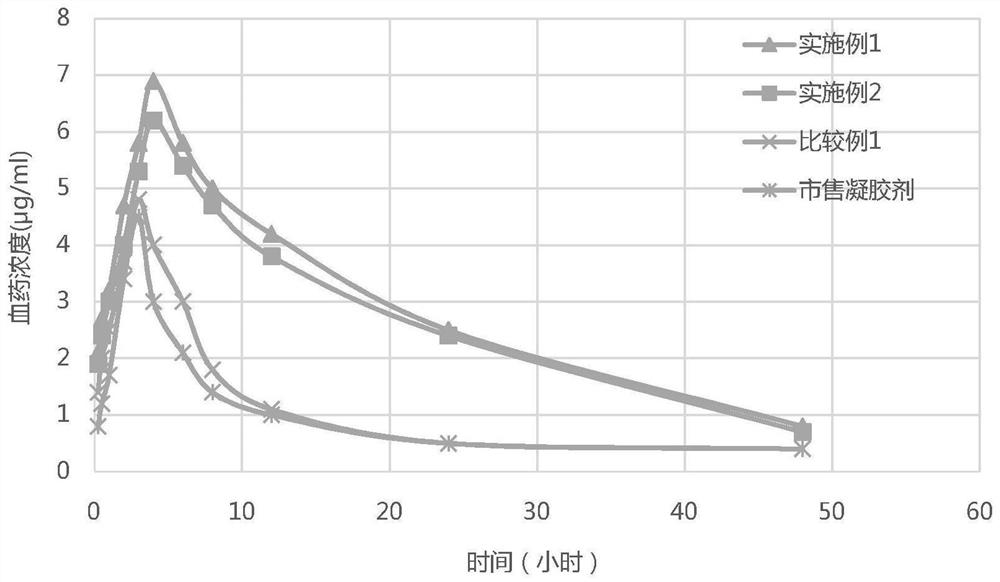
Stable diclofenac diethylamine gel plaster and preparation method thereof
PendingCN113940924AIncrease fat solubilityInhibit devitrificationOrganic active ingredientsAntipyreticDiclofenac AcidDrugs preparations
Owner:CHANGSHA JINGYI PHARM TECH CO LTD
Unique Dual-Action Therapeutics
A new family of therapeutics which provides a controlled-release delivery platform for non-steroidal anti-inflammatory agents on an ester or an ester-carbonate backbone is disclosed herein. These agents are reversible inhibitors of acetylcholinesterase and are thus useful for clinical conditions benefiting from inflammation suppression and cholinergic intervention. These compounds are of the general formula wherein n=0, 1; X═C, Si, and N+ and NSAID=ibuprofen, naproxen, indomethacin and diclofenac. Other embodiments are also disclosed.
Owner:RUTGERS THE STATE UNIV +1
Manufacturing process for no-donating compounds such as no-donating diclofenac
InactiveUS20060122402A1Improve filtering effectOrganic active ingredientsNervous disorderCompound (substance)Pharmaceutical formulation
Owner:NICOX SA
Novel diclofenac injection and preparation method thereof
ActiveCN112516081AImprove securityGood biocompatibilityOrganic active ingredientsAntipyreticPolythylene glycolPolymer
The invention belongs to the technical field of pharmaceutical preparations for animals, and discloses a novel diclofenac injection and a preparation method thereof. The diclofenac injection comprisesdiclofenac existing in a dispersed form, diclofenac sodium dissolved in a system, a temperature-sensitive in-situ gel matrix, polyethylene glycol, sodium alginate, a polymer retardant and water for injection, wherein the temperature-sensitive in-situ gel matrix consists of poloxamer 407 and poloxamer 188. The diclofenac injection specifically comprises the following components in percentage by mass of 2-18 percent of the diclofenac, 0-8 percent of the diclofenac sodium, 10-25 percent of the poloxamer 407, 0.1-16 percent of the poloxamer 188, 0.1-7 percent of polyethylene glycol, 0.02-5 percent of sodium alginate, 0.01-5 percent of the polymer retardant, 0.001-2 percent of a bacteriostatic agent and the balance of the water for injection. According to the diclofenac injection disclosed bythe invention, the gelling temperature is 31-35 DEG C, the gelling time is within 15 seconds, the diclofenac injection exists in a liquid state at room temperature, and a gel storage can be quickly formed at an injection part during intramuscular injection or subcutaneous injection administration.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Antimicrobial and Anti-inflammatory compositions
PendingUS20180207093A1Minimize systemic riskMinimizes GI adverse drug reactionPowder deliveryDispersion deliveryAnti-inflammatorySolvent
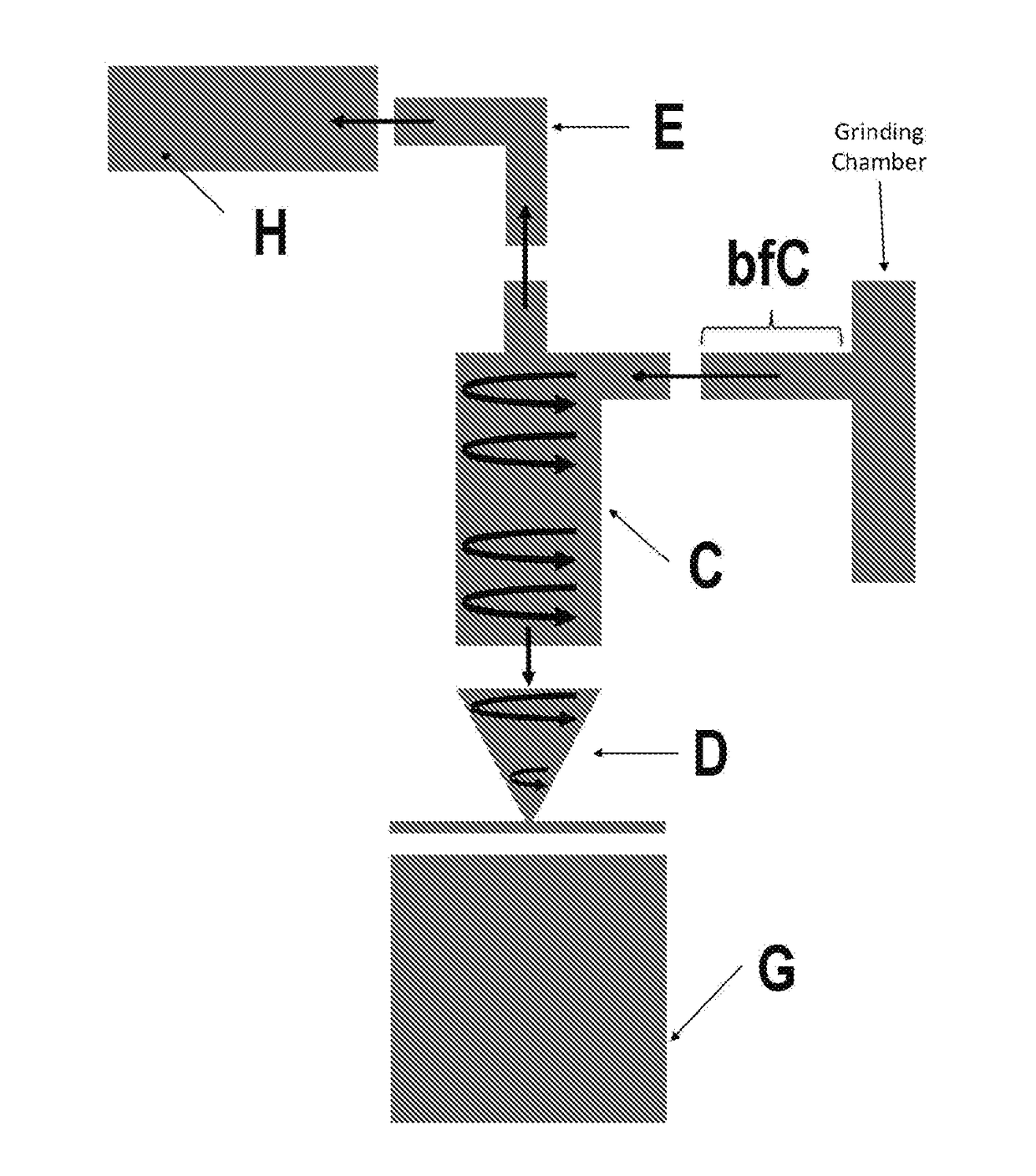
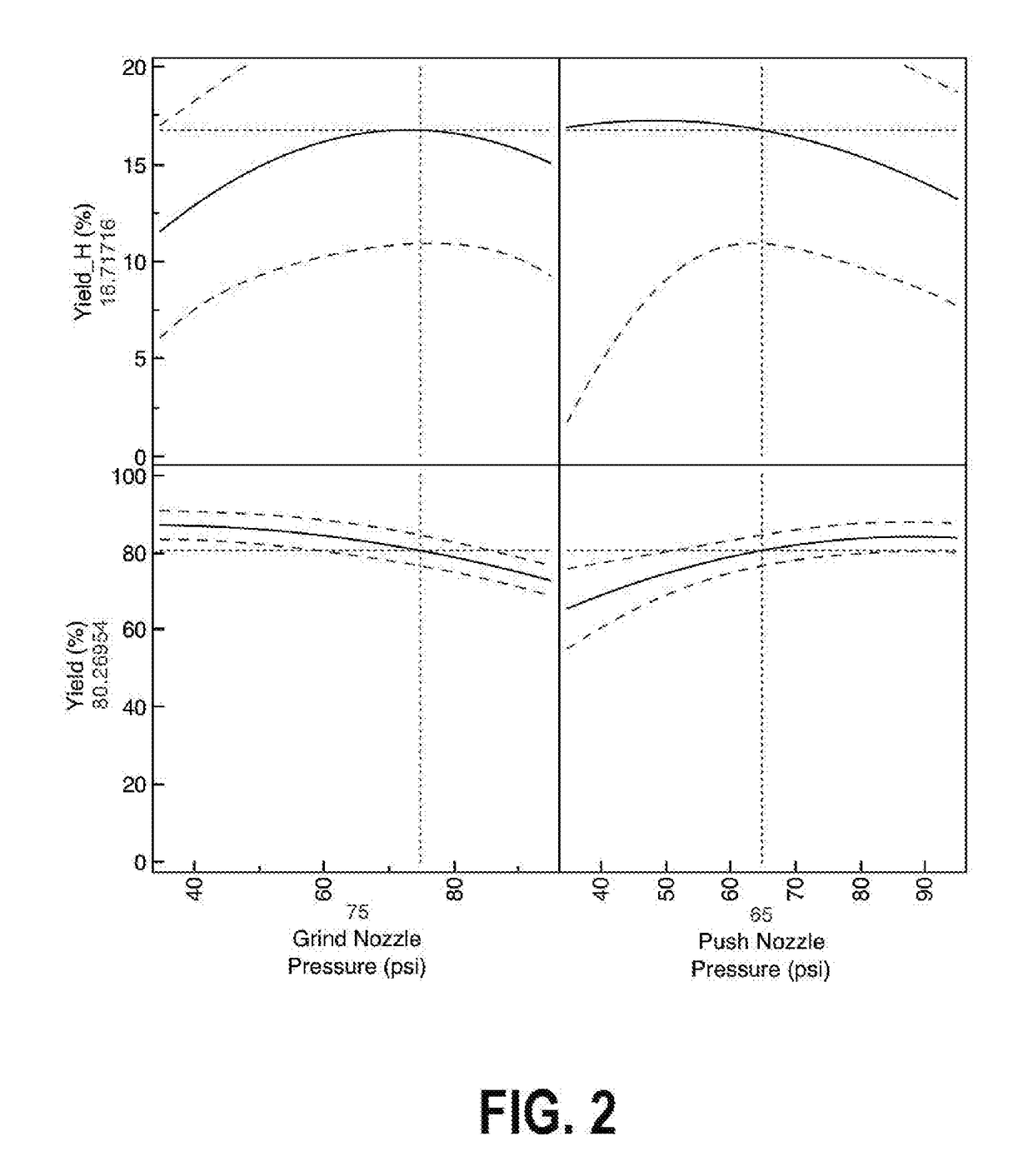
The claimed invention is directed to the use of a composition for use as an antimicrobial comprising an antimicrobial agent. In certain embodiments, the composition is used for treating or ameliorating inflammation in a subject and comprises an anti-inflammatory agent. The anti-inflammatory agent may include an NSAID, such as ibuprofen or diclofenac. The composition may further include solvents, anti-bacterial agents, anti-fungal agents, anti-parasitic agents, anti-viral agents, and buffers. The composition may include particles that have been processed via jet milling to reduce a diameter of the particles and to improve a flowability of the particles.
Owner:TEXAS A&M UNIVERSITY +1
A conjugate containing diclofenac, a preparing method thereof and uses of the conjugate
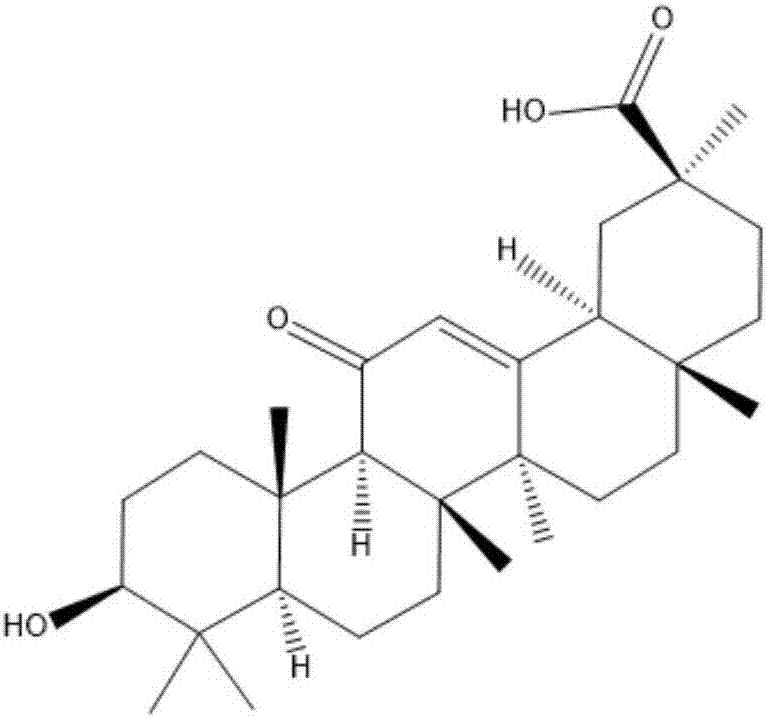
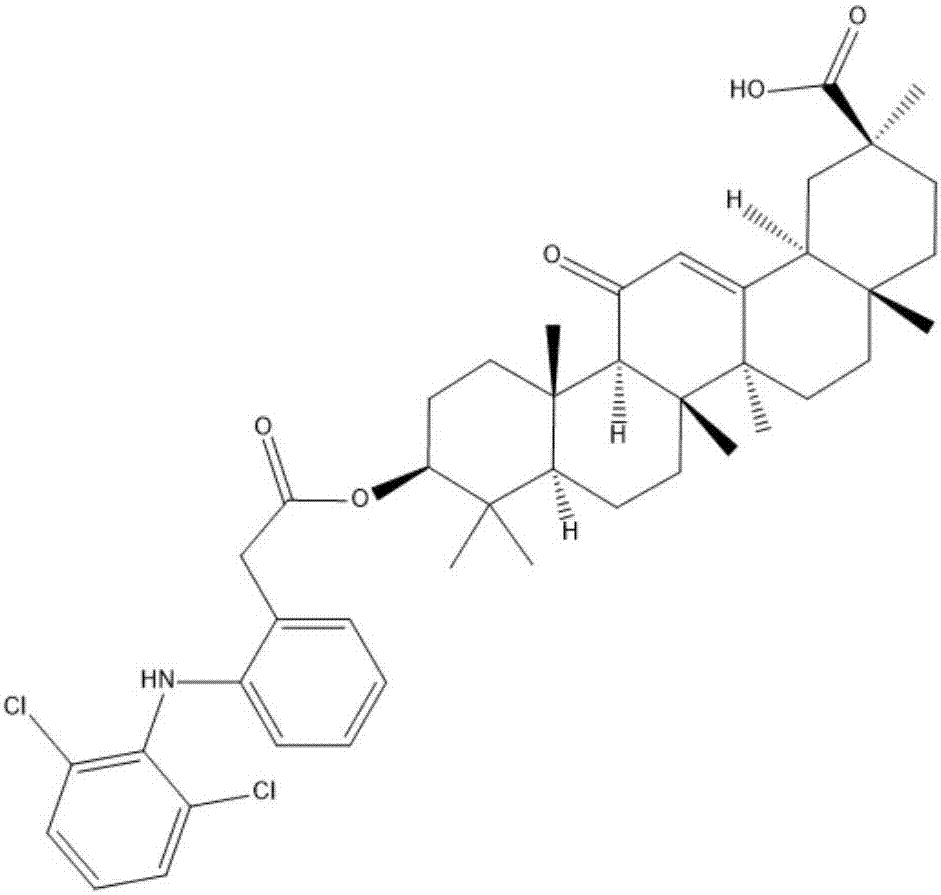
InactiveCN107474098AImprove solubilityHigh activityOrganic active ingredientsCosmetic preparationsCancer cellApoptosis
The invention belongs to the fields of natural medicines and medicine therapy, and discloses a conjugate containing diclofenac, a preparing method thereof and uses of the conjugate. The conjugate is formed by coupling glycyrrhetinic acid and the diclofenac and has a structure shown as a formula (I) as follows. The method includes reacting the glycyrrhetinic acid and the diclofenac in anhydrous dichloromethane at room temperature overnight under catalysis of pyridine and 4-dimethylaminopyridine, performing filtration, allowing a filtration product to pass through a silica gel chromatographic column, performing rotary evaporation, and performing drying treatment to obtain a product. In-vitro MTT detection experiments found that the conjugate has good inhibiting effects on tumor cell strains (MCF-7, BC-3 and HepG2 cells), the IC50 value of the conjugate is 3-4 times lower than that of the glycyrrhetinic acid, and the conjugate has low toxicity for normal liver cells L-O2. The compound can treat cancer in a manner that two medicine molecules are coupled to form a target prodrug, overcomes a problem that the glycyrrhetinic acid has low solubility, combines the diclofenac that is an anti-inflammatory drug to enhance anticancer activity, and achieves effects of removing cancer cells through a route of enhancing cell apoptosis.
Owner:佛山市第五人民医院 +1
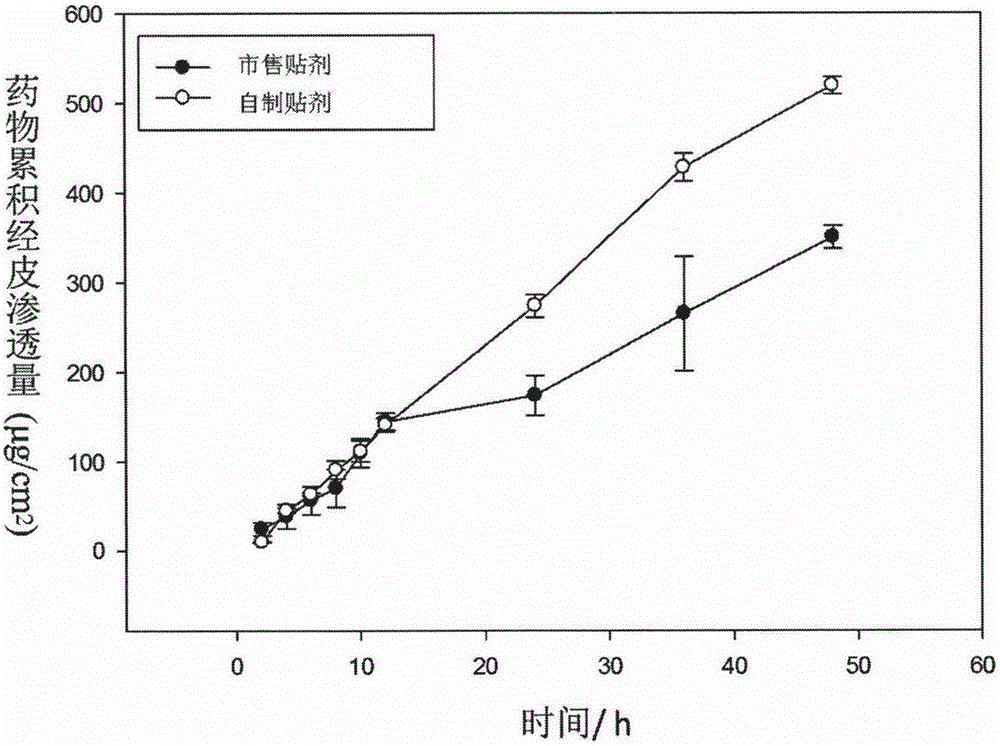
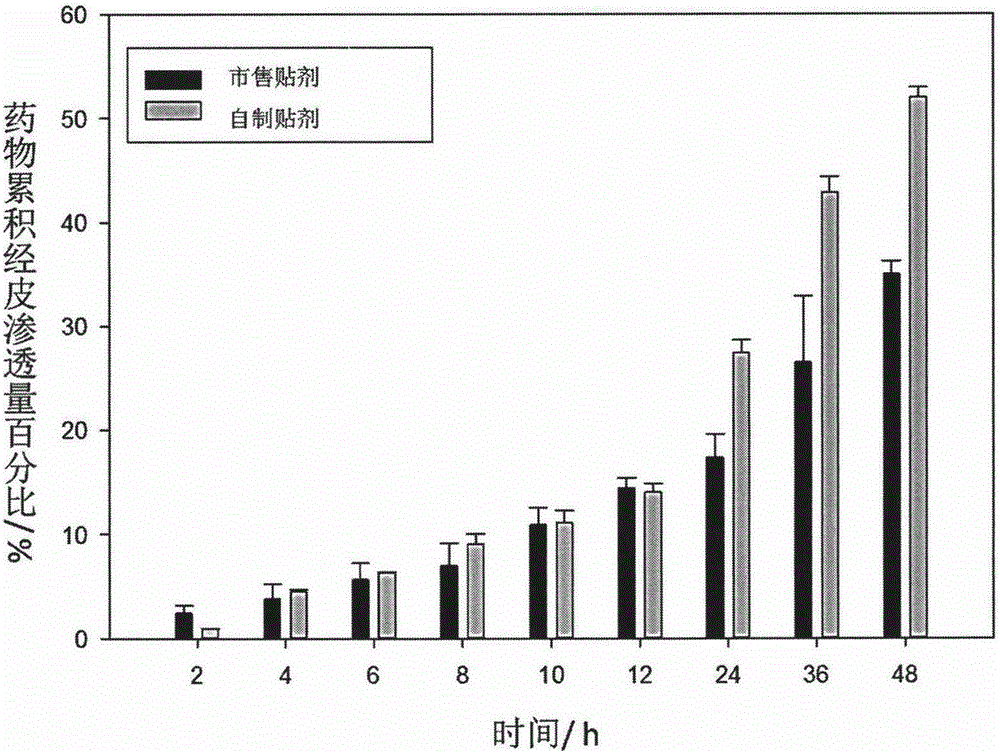
Long-acting diclofenac transdermal patch and preparation technology thereof
InactiveCN105708823AReasonable drug loadingImprove skin penetrationOrganic active ingredientsAntipyreticDiclofenac transdermal patchPercutaneous absorption
The invention discloses a long-acting diclofenac transdermal patch and a preparation technology thereof and belongs to the technical field of medicine. The preparation is mainly prepared from a diclofenac bulk pharmaceutical chemical, a penetration enhancer, a dispersing solvent and a polyacrylate pressure-sensitive adhesive. The long-acting diclofenac transdermal patch and the preparation technology thereof have the following advantages: (1) the drug can be released from a matrix continuously for 12-48 h, the drug remaining amount is low, the percutaneous absorption is excellent, and the drug delivery times and dosage can be reduced; (2) a product has no skin irritation and has good adhesion and flexibility; (3) the main drug and the additives are stable in a viscose matrix; (4) the preparation technology is simple, convenient and pollution-free.
Owner:CHINA PHARM UNIV
Tryptamine derivatives, their preparation and their use in gastropathy
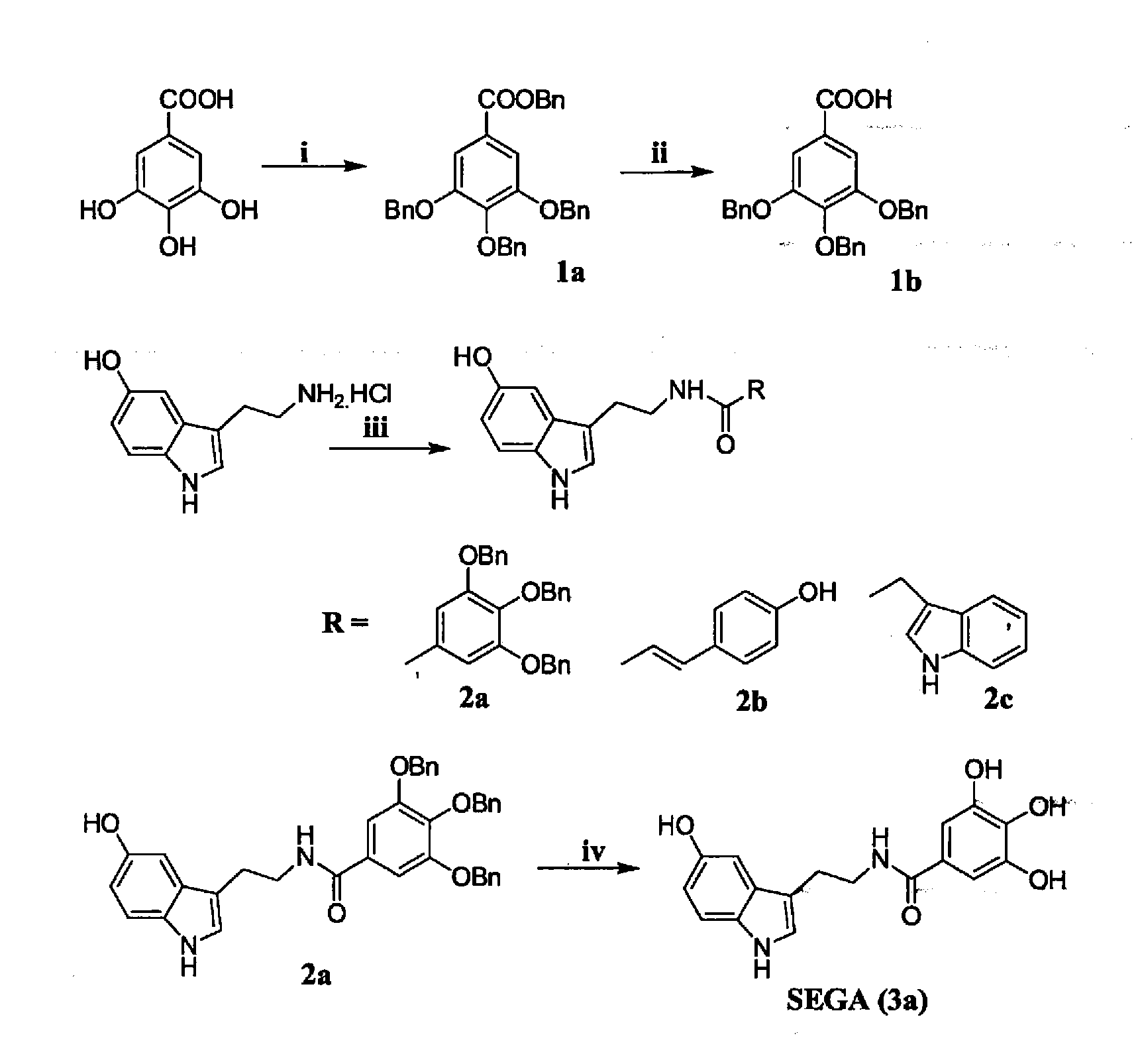
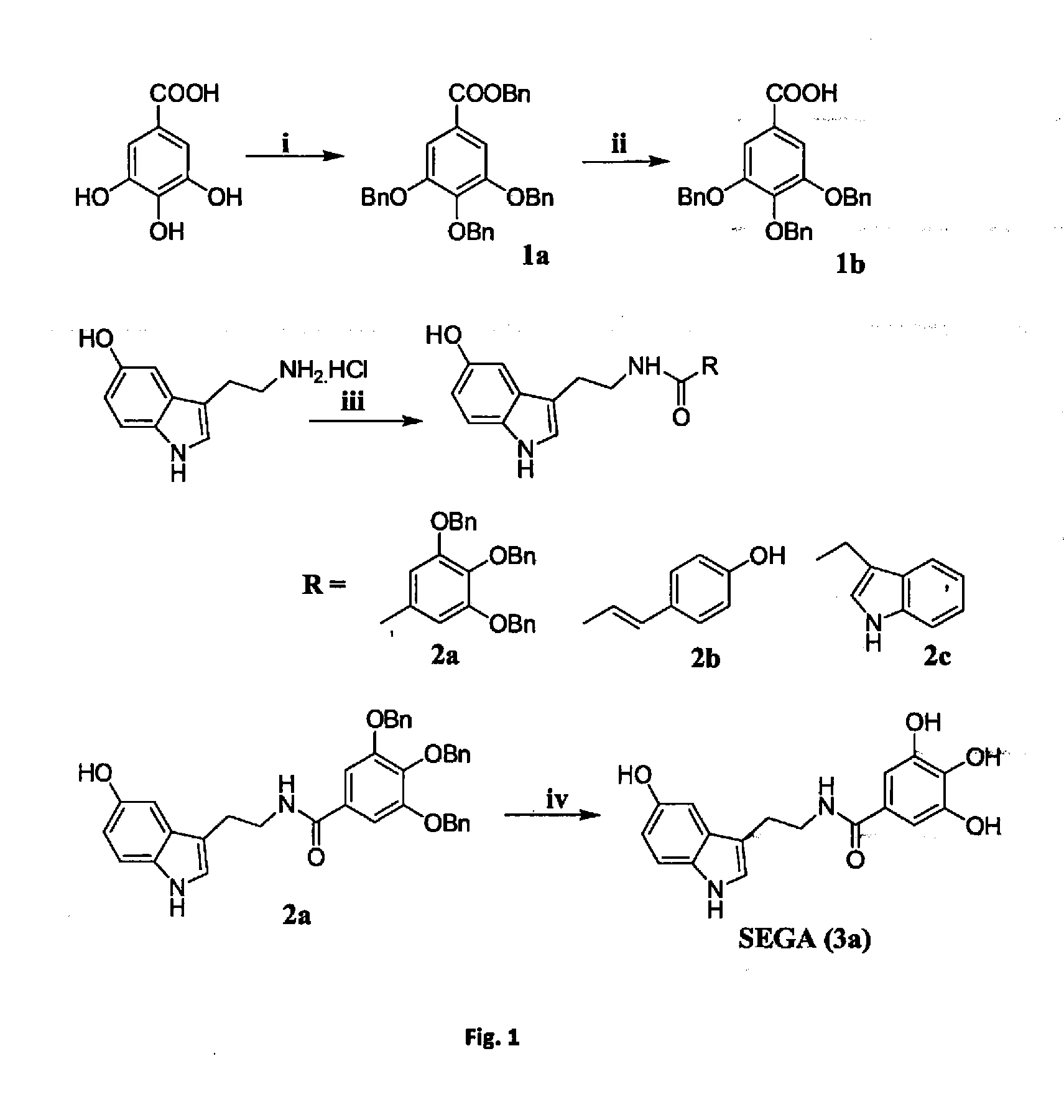
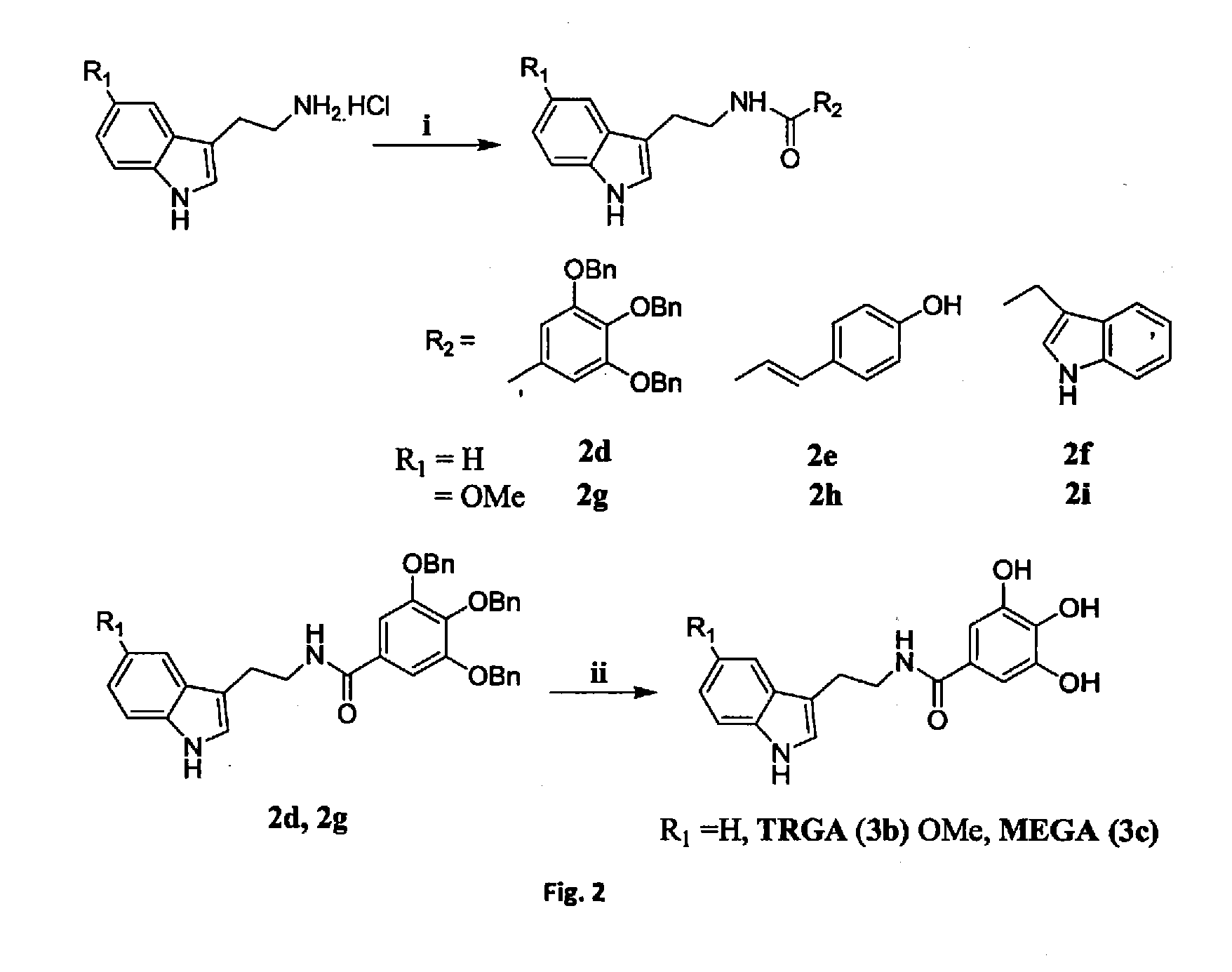
ActiveUS20130197052A1Increase absorbanceImprove suppression propertiesBiocideOrganic active ingredientsScavengerIMP dehydrogenase activity
The synthesis and evaluation of gastroprotective effect of different tryptamine derivatives. Tryptamine derivatives have been synthesized by formation of amide or ester with some known anti oxidant molecules. These derivatives show excellent antioxidant property in vitro. Among all the derivatives the compound SEGA (3a), that was prepared by the combination of serotonin with gallic acid shows the greater antioxidant property than the other synthesized compounds both in vivo and in vitro. SEGA(3a) shows the gastroprotective effect against NSAIDs (indomethacin or diclofenac)-induced gastropathy in dose dependent manner and also accelerates the healing from injury. It prevents the NSAIDs-induced mitochondrial oxidative stress in vivo. This derivative prevents NSAID-induced mitochondrial oxidative stress-mediated apoptosis in vivo by preventing the activation of caspase 9 and caspase-3 and restores NSAIDs-mediated collapse of mitochondroial transmembrane potential and dehydrogenase activity. SEGA (3a) plays an important role as an iron chelator as well as intra mitochondrial ROS scavenger. Thus, SEGA (3a) is a potent antioxidant antiapototic molecule, which efficiently prevents NSAID-induced gastropathy and stress or alcohol-mediated gastric damage.
Owner:COUNCIL OF SCI & IND RES +1
Freeze dry preparation containing diclofenac salt and lidocaine and its preparation method
InactiveCN1279897CReduce volumeSolve solubilityPowder deliveryOrganic active ingredientsDiclofenac AcidFreeze-drying
The invention relates to a freeze-dried powder preparation containing diclofenac and lidocaine, which consists of Tween 80 containing solubilization, a pharmaceutically acceptable pH regulator for adjusting the pH value of the solution, a therapeutically effective amount of diclofenac and The solution of lidocaine, the pH value of the solution is greater than 7.0, is obtained after freeze-drying. The solution may also contain other pharmaceutically acceptable auxiliary materials. The preparation of the invention has stable performance, convenient transportation, long storage period, and no adverse reactions caused by organic solvents such as ethanol during use.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Device and method for treating diclofenac wastewater in low-temperature environment
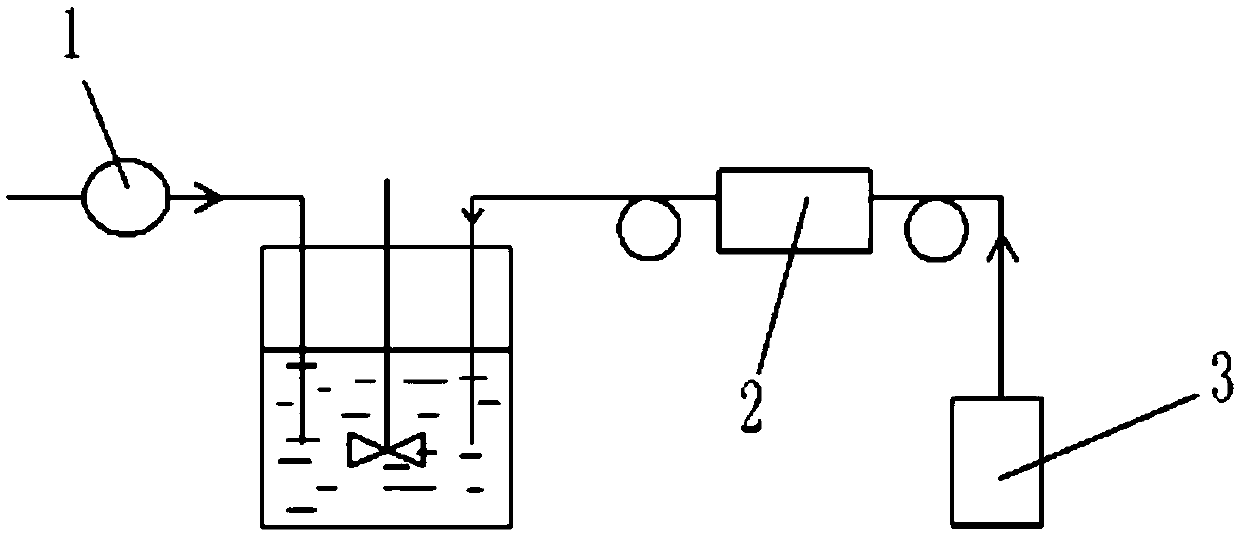
InactiveCN105565602AEasy to handleSolve the problem of heat loss and energy lossWater contaminantsWater/sewage treatment by electrochemical methodsElectrical resistance and conductanceDiclofenac Acid
The invention belongs to the technical field of wastewater treatment and relates to a device and method for treating diclofenac wastewater in a low-temperature environment. A cathode bar (13) of the device is connected with a direct-current power supply (1) through a cathode wire (2), an anode plate (14) is connected with the upper end of a spiral coil (11) of an electromagnetic field generating device, the lower end of the spiral coil (11) of the electromagnetic field generating device is connected with the direct-current power supply (1) through an anode wire (3) and a slide resistor (22). The device is simple in structure and compact in arrangement and achieves the removal purpose through physical, chemical and biological treatment conducted on the diclofenac wastewater under the low-temperature condition.
Owner:UNIV OF JINAN
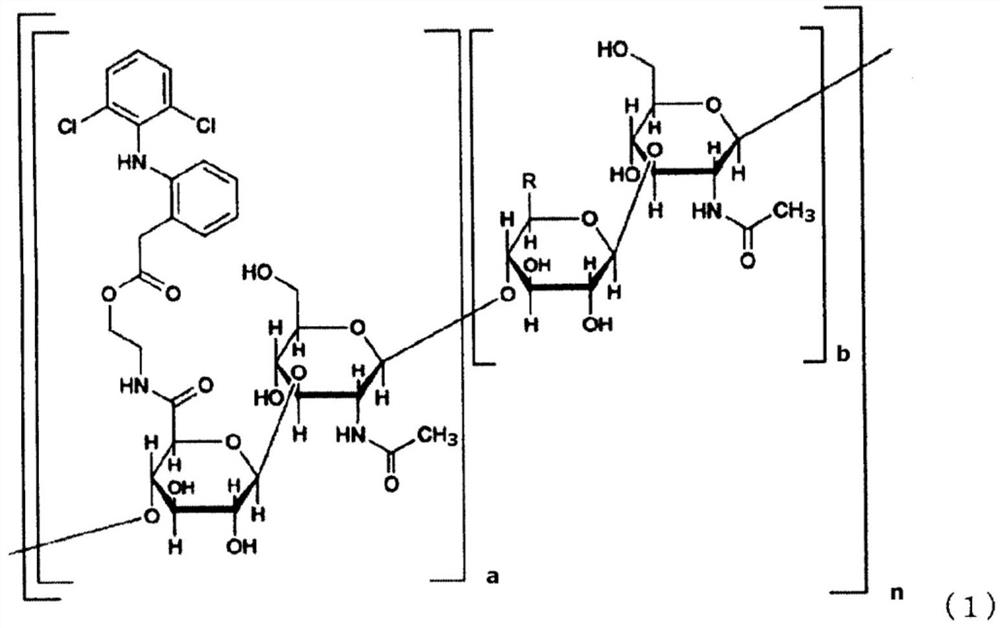
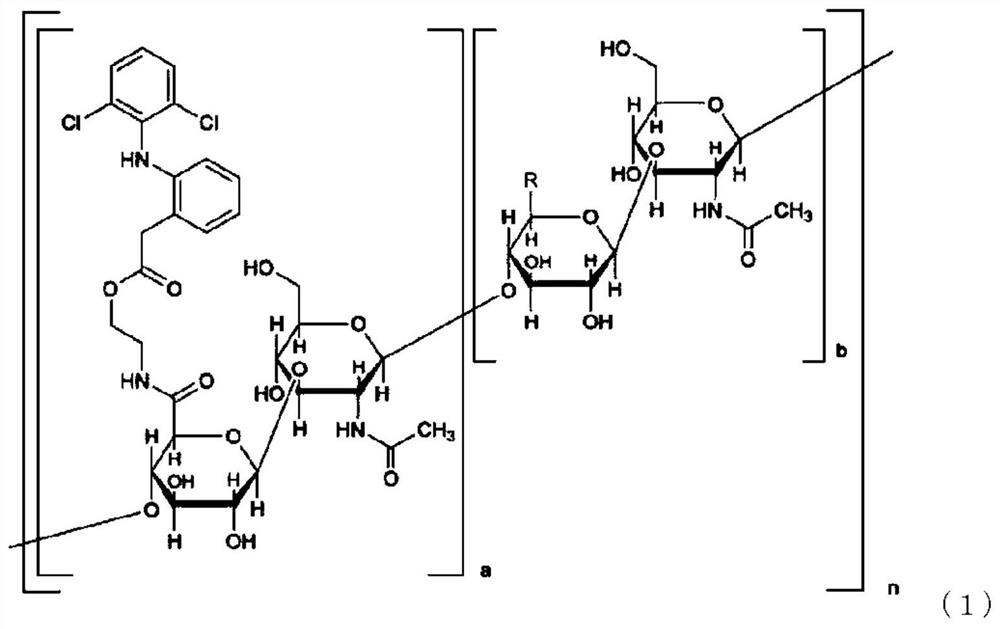
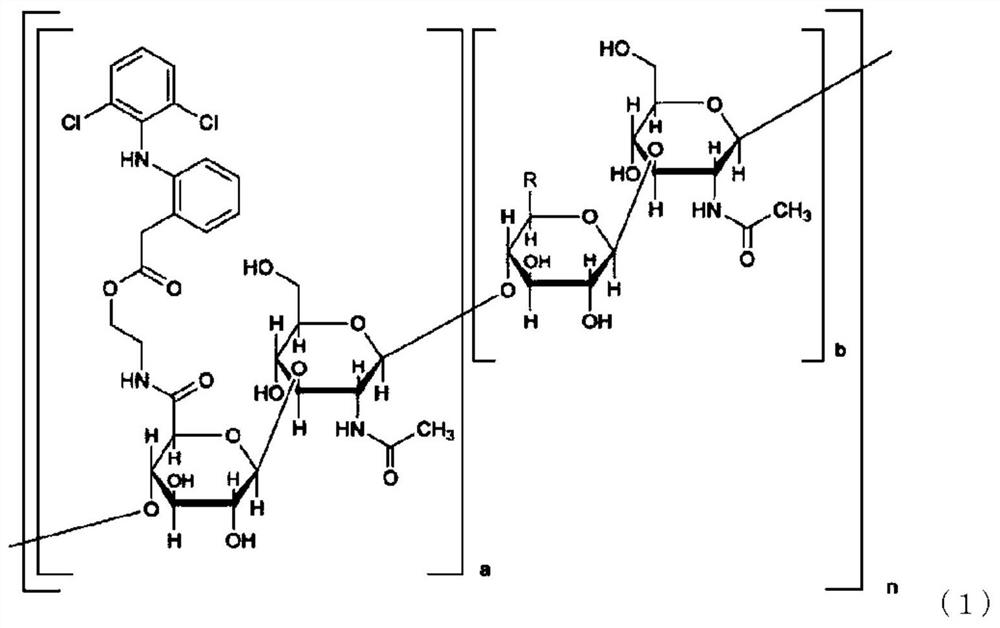
Pharmaceutical composition
The purpose of the present invention is to provide a pharmaceutical composition that comprises a hyaluronic acid derivative represented by formula (1) in the description or a pharmaceutically acceptable salt thereof, a method for producing the same, and a method for inhibiting the formation of diclofenac lactam from a compound represented by formula (1). Provided is a method for inhibiting the formation of diclofenac lactam from a compound represented by formula (1) in the description, said method comprising allowing the compound of formula (1) to coexist with component (A) that is at least one kind of compound selected from the group consisting of a nonionic surfactant, a hydroxyalkylated cyclodextrin, a C1 to C3 monoalcohol, a C2 to C3 dialcohol, a C3 to C6 trialcohol, a polyalkylene glycol, gamma-lactone, polyvinylpyrrolidone, a chlorogenic acid, an alkyl sulfate and salts thereof. Also provided are a method for producing a pharmaceutical composition that comprises a compound represented by formula (1) and component (A), and a pharmaceutical composition produced thereby.
Owner:SEIKAGAKU KOGYO CO LTD
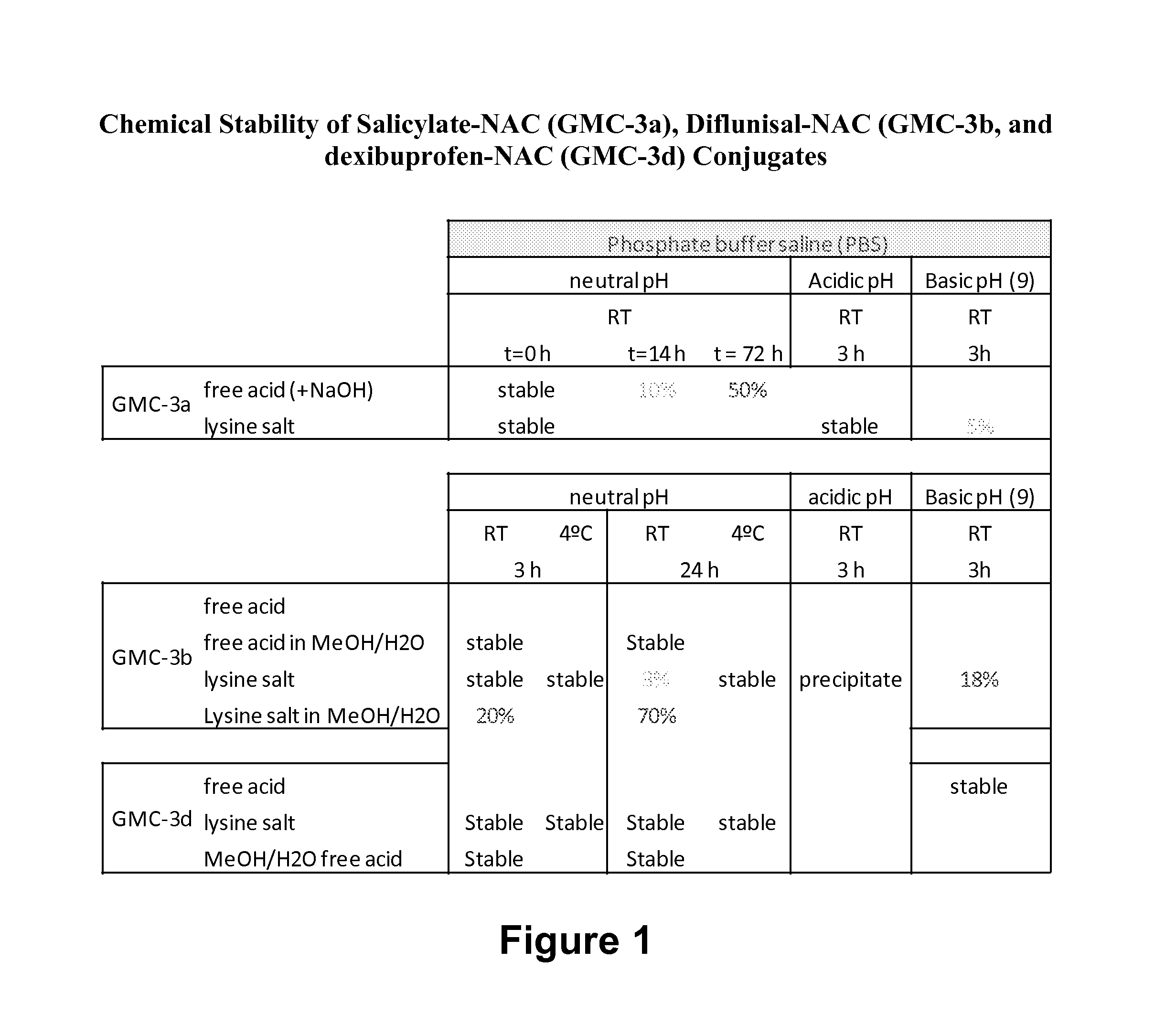
Anti-Inflammatory And Antioxidant Conjugates Useful For Treating Metabolic Disorders
InactiveUS20130281520A1Eliminate side effectsReduce riskBiocideOrganic chemistryDiclofenac AcidAntioxidant
The present invention is directed to methods for treating metabolic disorders with compounds that are conjugates. The conjugates of the present invention are comprised of salicylic acid, triflusal, diflusinal, salsalate, IMD-0354, ibuprofen, diclofenac, licofelone, or HTB, and one or more antioxidants.
Owner:GENMEDICA THERAPEUTICS SL
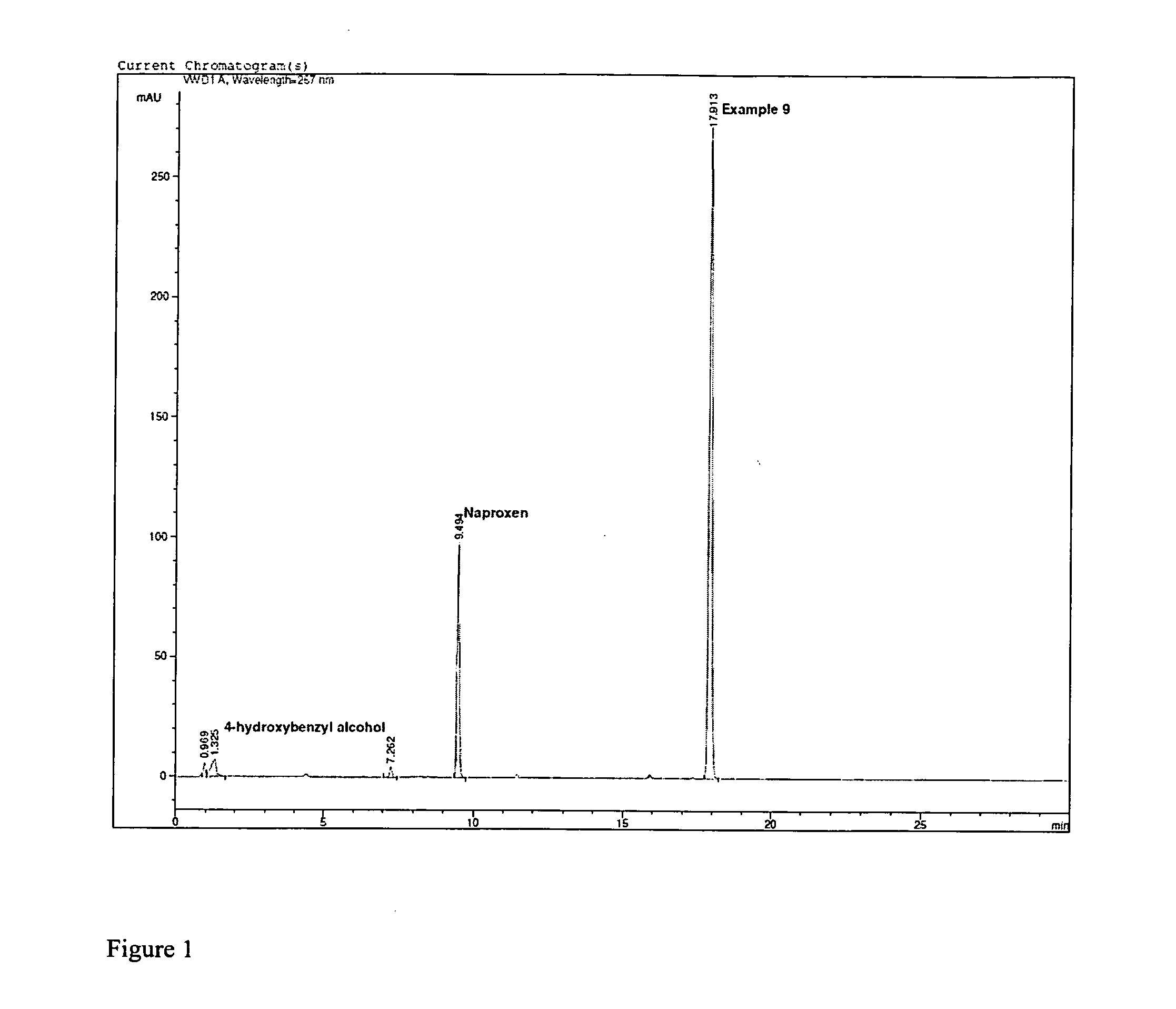
Diclofenac-glycine-resveratrol conjugate, preparation method and application
ActiveCN109847067AMild reaction conditionsFew stepsHydroxy compound active ingredientsAntipyreticGlycineSolubility
The invention discloses a diclofenac-glycine-resveratrol conjugate, a preparation method and an application. The conjugate is formed by coupling glycine serving as a linking arm with diclofenac and resveratrol respectively; diclofenac and glycine are linked by an amido bond; a diclofenac-glycine conjugate is linked with resveratrol by multiple sites of ester bonds. Due to introduction of glycine,the solubility of the conjugate is increased, due to introduction of diclofenac and resveratrol, the anti-arthritis effect of the conjugate is significantly enhanced, adverse reactions are reduced, and safety is higher. The synthesis method of the conjugate is simple and suitable for industrial production.
Owner:药大制药有限公司
Non-aqueous topical solution of diclofenac and process for preparing the same
A non-aqueous topical solution composition of pharmaceutically acceptable salt of diclofenac is disclosed. The non-aqueous topical solution composition comprises therapeutically effective amount of pharmaceutically acceptable salt of diclofenac, solublizer, penetration enhancer and solvent, and optionally a humectant, counter irritant, additional penetration enhancer and anti-oxidants and a process for preparing the same.
Owner:TROIKAA PHARMA
Topical formulation
InactiveUS20100029769A1Improve throughputOrganic active ingredientsBiocideSodium acetateSODIUM LAURYL SULFOACETATE
There is described a topical formulation. The topical formulation comprises: (i) diclofenac or a pharmaceutically acceptable salt thereof, (ii) a first compound, and (iii) a second compound. The first compound and second compound are different, and each is selected from the group consisting essentially of N-lauroyl sarcosine, sodium octyl sulfate, methyl laurate, isopropyl myristate, oleic acid, glyceryl oleate and sodium lauryl sulfoacetate. It has been discovered that certain combination of compounds are excellent penetration enhancers and, as such, can be incorporated in a topical formulation to facilitate administration of diclofenac or a pharmaceutically acceptable salt thereof. The increased penetration enhancement can also lead to a reduction in the total concentration of skin irritants in the formulation.
Owner:CRESCITA THERAPEUTICS INC
Diclofenac and hyaluronic acid combination treatment for oral leukpoplakia
An oral gel composition suitable for the treatment of oral leukoplakia comprising a combination of diclofenac with hyaluronic acid and methods of administering the oral gel that may include a device that prolongs contact time of the oral gel with the areas of the mucosa that are affected by oral leukoplakia.
Owner:GI PHARM INC AKS E2BIO CONSULTANTS
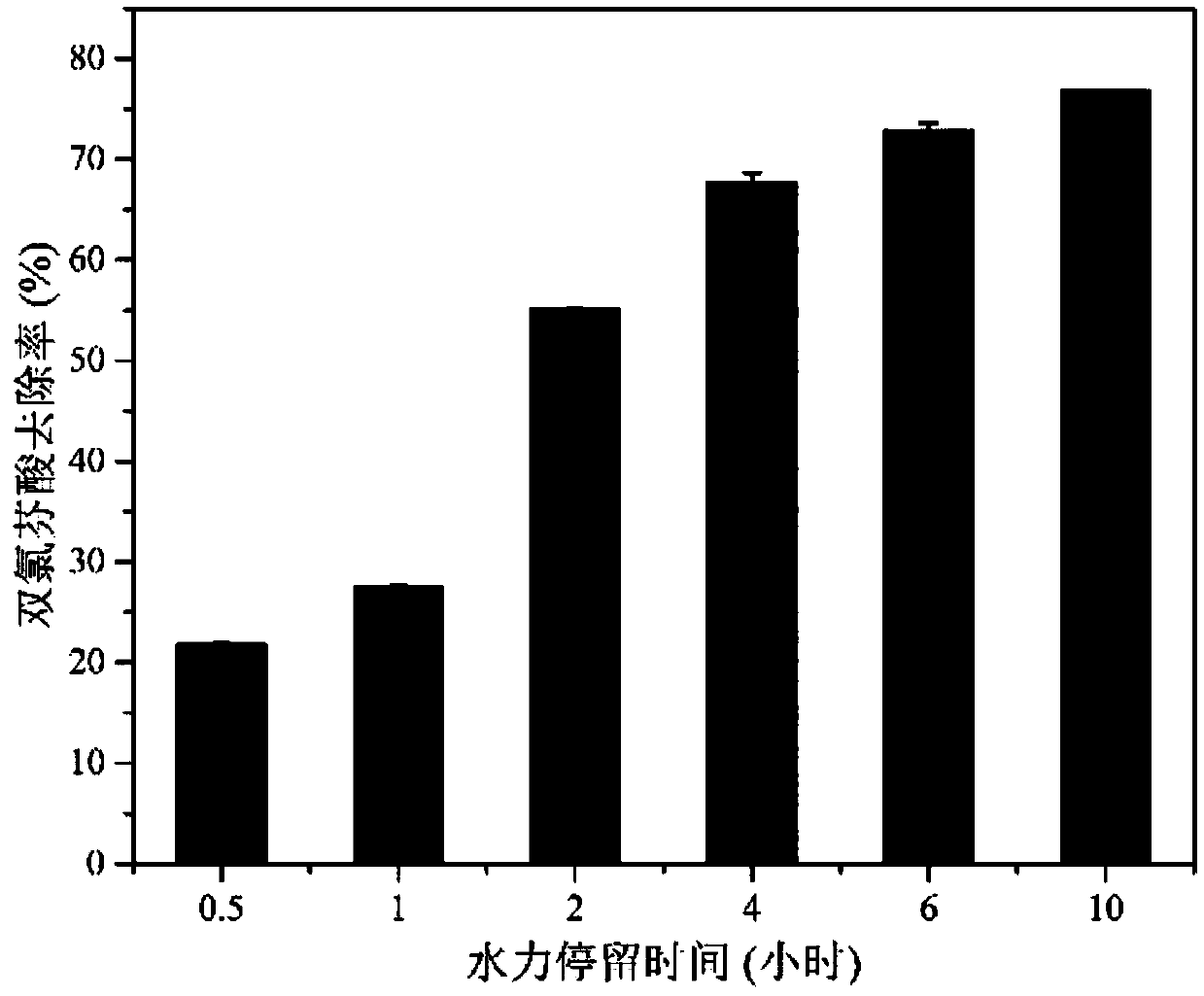
Method for removing diclofenac in sewage in strengthened manner on basis of enriched nitrifying bacteria
ActiveCN109574399ASimple and fast operationLow costWater treatment parameter controlSpecific water treatment objectivesTreatment resultsWater source
The invention discloses a method for removing diclofenac in sewage in a strengthened manner on the basis of enriched nitrifying bacteria. The method includes steps of 1), domesticating and enriching the nitrifying bacteria to obtain enriched and domesticated nitrifying sludge; 2), allowing the sewage to automatically flow into secondary sedimentation tanks and carrying out solid-liquid separation;3), carrying out sewage treatment on the enriched and domesticated nitrifying sludge in MBR (membrane bioreactor) devices; 4), analyzing treatment results for effluent. The method has the advantagesof simple equipment, simplicity and convenience in operation, low cost and the like. Besides, the method is free of pollution and high in stability; the diclofenac in the sewage can be effectively removed by the aid of the method, accordingly, the requirements on sewage discharge can be met, environmental pollution can be prevented, and economic treatment effects can be realized; the shortcomingsof existing purification processes can be overcome by the aid of the method, the shortcomings of poor diclofenac removal effects and instable operation in the prior art can be overcome by the aid of the method, and gap of domestic and overseas related technologies for removing diclofenac in water sources can be filled.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com