Local application emplastrum containing heparin and diclofenac
A technology for topical application, diclofenac, applied in medical preparations containing active ingredients, organic active ingredients, nervous system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
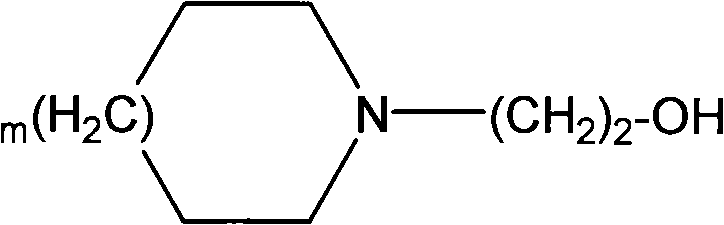
[0013] According to the plaster of the present invention, wherein diclofenac is usually used in the form of a pharmaceutically acceptable salt, and preferably the salt of a heterocyclic amine represented by the following general formula:
[0014]
[0015] where m is 0 or 1.
[0016] According to a particularly preferred embodiment, the heterocyclic amine is N-hydroxyethylpyrrolidine (pyrrolethanol).
[0017] According to the plaster of the present invention, the concentration range of the diclofenac salt is usually 0.1-5wt% of the total weight of the composition used for hydrogel matrix preparation, and the preferred concentration is 0.3-3wt%.
[0018] According to a particularly preferred embodiment, the concentration of diclofenac is 1.3% by weight of the total weight of the composition for hydrogel matrix formulation.
[0019] When the plaster of the present invention contains heparin, it is preferably one with a molecular weight of 5,000-30,000 DA.
[0020] The concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com