Pharmaceutical composition
A technology of composition and water-based composition, which is applied in the direction of drug combination, drug delivery, non-active ingredients of polymer compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0196] Preferred embodiments of the present invention are exemplified below.
[0197] [1] A pharmaceutical composition comprising a compound represented by formula (1) and component (A):
[0198] Component (A): selected from nonionic surfactants, hydroxyalkylated cyclodextrins, C 1 ~C 3 Monohydric alcohol, C 2 ~C 3 Diol, C 3 ~C 6 At least one compound selected from the group consisting of triols, polyalkylene glycols, γ-lactones, polyvinylpyrrolidone, chlorogenic acids, alkyl sulfates, and salts thereof,
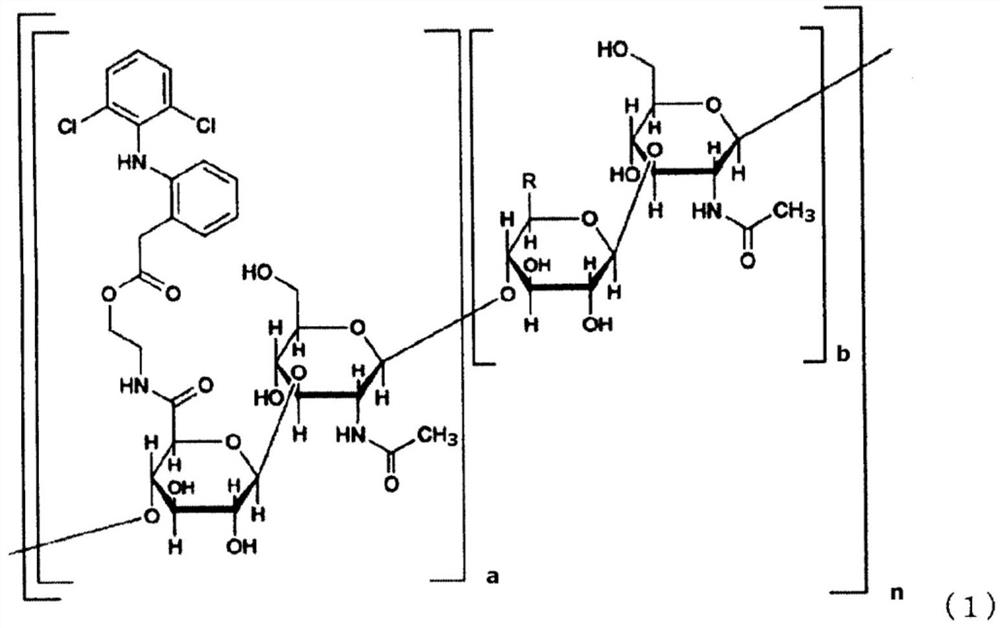
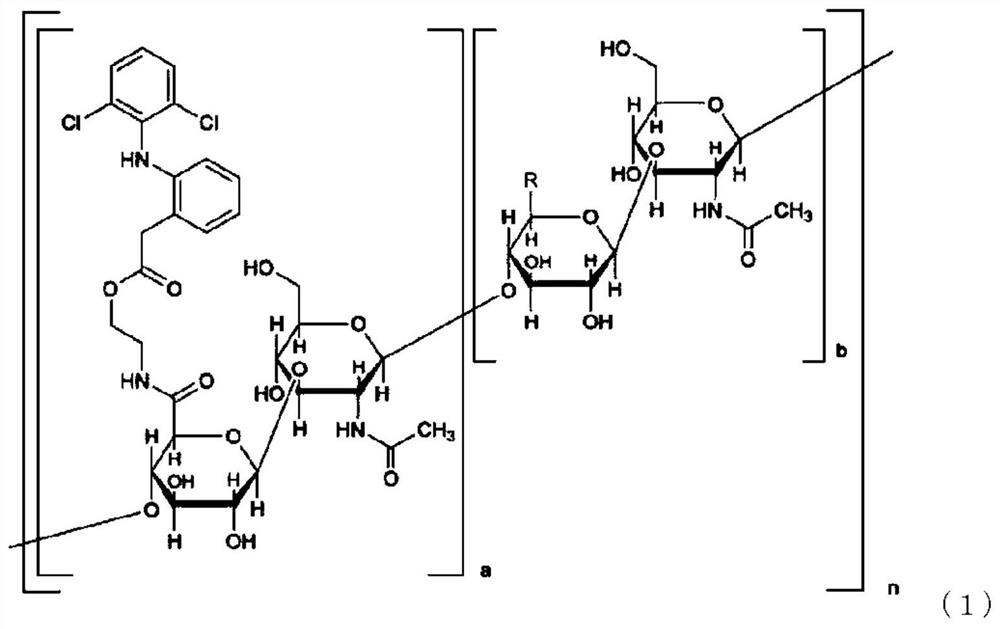
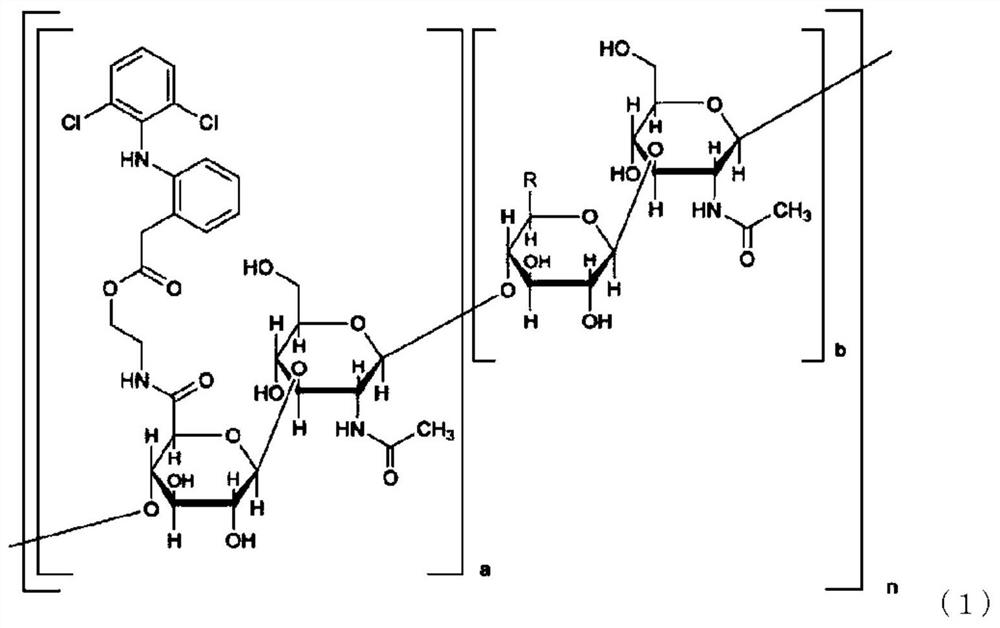
[0199]
[0200] In formula (1), a is not less than 0.01 and not more than 0.7, a+b is 1, n is an integer not less than 25 and not more than 25000, and the arrangement of each disaccharide structural unit is optionally random or block-like. R in the sugar structural unit is each independently a carboxyl group or a carboxylate group.
[0201] [2] The pharmaceutical composition according to [1] above, wherein the component (A) is selected from the group consisting of ...
Embodiment
[0279] Hereinafter, preferred embodiments of the present invention will be described in more detail using examples, but the present invention is not limited at all by the following examples.
Synthetic example
[0281]According to the method described in the examples of International Publication No. 2005 / 066214, hyaluronic acid derivatives (compounds represented by formula (1)) were synthesized (a: 0.18, n: 2 k, the mass average molecular weight of hyaluronic acid :800 000).
[0282] More specifically, it was synthesized by the following method.
[0283] Dissolve 2.155 g (10.5 mmol) of 2-bromoethylamine hydrobromide in 20 mL of dichloromethane, add 1.463 mL (10.5 mmol) of triethylamine under ice-cooling, and then add di-tert-butyl dicarbonate (Boc 2 O) 5 mL of a dichloromethane solution of 2.299 g (10.5 mmol) was stirred. After stirring at room temperature for 90 minutes, ethyl acetate was added, followed by liquid-separation washing with a 5% by weight aqueous citric acid solution, water, and saturated brine. After dehydration with sodium sulfate, the solvent was distilled off under reduced pressure to obtain Boc-aminoethyl bromide.
[0284] Ice-cool 5 mL of a dimethylformamide (D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com