Long-acting diclofenac transdermal patch and preparation technology thereof
A technology of diclofenac and transdermal patch, which is applied in the field of medicine, and can solve problems such as ready-to-use, lower drug transdermal penetration rate, and pressure-sensitive adhesives that cannot be prepared in advance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
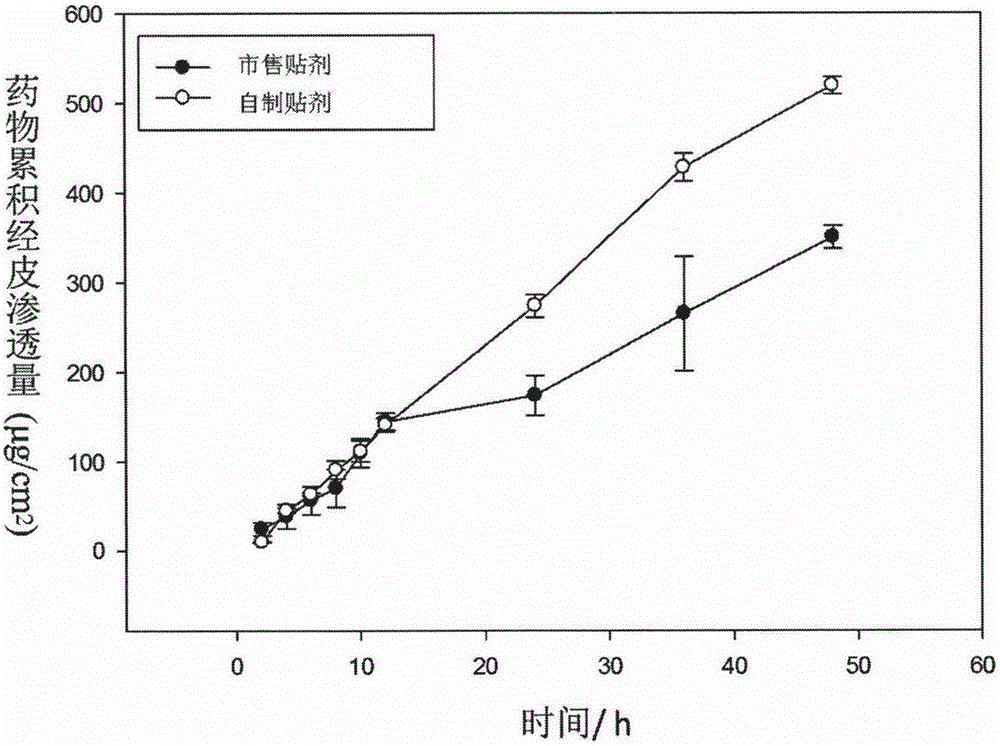
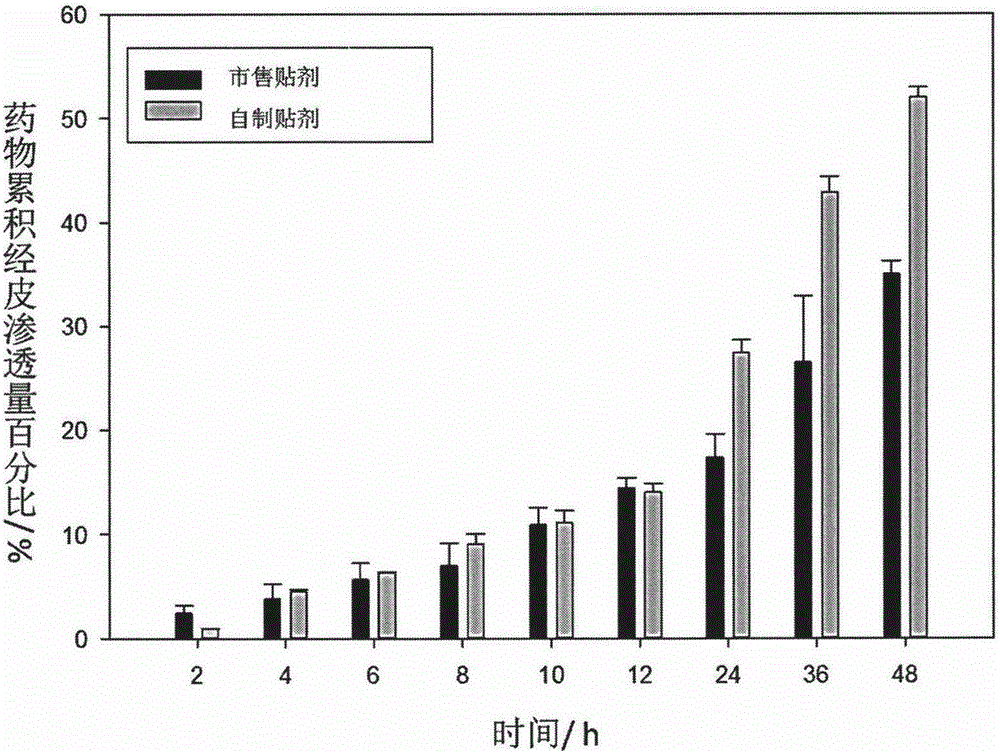
Image
Examples
Embodiment 1
[0037] Embodiment 1: Preparation of long-acting diclofenac patch, drug content per unit area: 2.00mg / cm 2
[0038]
[0039] Preparation process: Precisely weigh diclofenac and add it to a mixed solution of acetone and water to fully dissolve it, add menthol, macrogol glyceride oleate and propylene glycol, shake and mix well. Pour the above mixture into the pressure sensitive adhesive base and stir well. Mix ultrasonically for 20 min until there are no bubbles. Pour the drug glue mixture into a suitable applicator, pull it slowly at a constant speed on the side of the release film with silicone oil, so that the drug glue mixture is evenly coated on the release film, dry at 70°C for 20 minutes, take it out, and attach the backing lining.
Embodiment 2
[0040] Embodiment 2: Preparation of long-acting diclofenac sodium patch, drug content per unit area: 1.00mg / cm 2
[0041]
[0042] Preparation process: Precisely weigh diclofenac sodium and add it to a mixed solution of acetone and water to fully dissolve it, add peppermint oil, oleic acid and azone, shake and mix well. Pour the above mixture into the pressure sensitive adhesive base and stir well. Mix ultrasonically for 20 min until there are no bubbles. Pour the drug glue mixture into a suitable applicator, pull it slowly at a constant speed on the side of the release film with silicone oil, so that the drug glue mixture is evenly coated on the release film, dry at 70°C for 20 minutes, take it out, and attach the backing lining.
Embodiment 3
[0043] Embodiment 3: Preparation of long-acting diclofenac potassium patch, drug content per unit area: 0.50mg / cm 2
[0044]
[0045] Preparation process: Accurately weigh an appropriate amount of diclofenac potassium and add it to a mixed solution of ethanol and water to fully dissolve it, add menthol, caprylic acid macrogol glyceride and isopropanol, shake and mix well. Pour the above mixture into the pressure sensitive adhesive base and stir well. Mix ultrasonically for 20 min until there are no bubbles. Pour the drug glue mixture into a suitable applicator, pull it slowly at a constant speed on the side of the release film with silicone oil, so that the drug glue mixture is evenly coated on the release film, dry at 70°C for 20 minutes, take it out, and attach the backing lining.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Drug loading | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com