Linoleic acid modified glucan and preparation method of high-molecular liposome
A technology of linoleic acid and dextran, which is applied in the field of medicine, can solve the problems of poor biocompatibility, average transdermal effect, and high cytotoxicity, and achieve good stability, good transdermal performance, and reduced cytotoxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
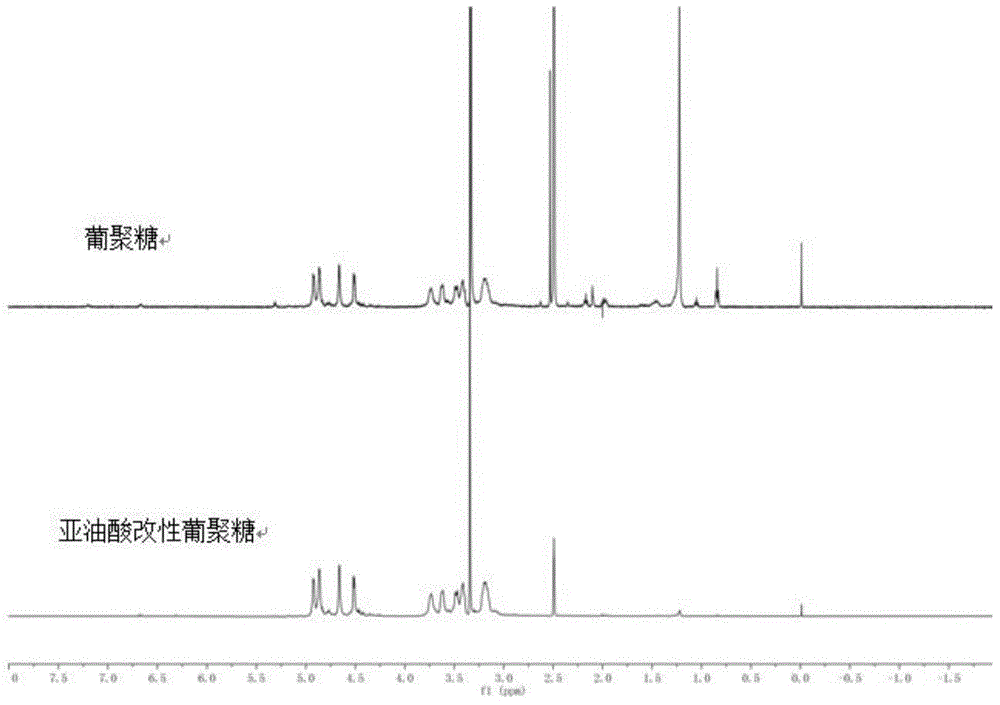
[0030] Synthesis of linoleic acid modified dextran. Take 1g of dextran with a molecular weight of 5000, 3g of linoleic acid and add them into a beaker, then add 182mL of dimethyl sulfoxide solvent, 0.6g of EDC and 0.15g of DMAP into the beaker, stir magnetically, and react at room temperature for 3 hours. After the reaction, put the product into a dialysis bag with a molecular weight of 1000 for dialysis for 3 days, and freeze-dry to obtain linoleic acid-modified dextran. Such as figure 1 As shown in the 1H-NMR analysis results, 0.8ppm and 1.2ppm are the characteristic absorption peaks of —CH3 and —CH2 in linoleic acid respectively, indicating that the linoleic acid modified dextran was successfully synthesized.
Embodiment 2
[0032] Synthesis of linoleic acid modified dextran. Take 5 g of dextran with a molecular weight of 20,000 and 1 g of linoleic acid into a beaker, then add 45 mL of dimethyl sulfoxide solvent, 1 g of EDC and 0.5 g of DMAP into the beaker, stir magnetically, and react at room temperature for 12 hours. After the reaction, put the product into a dialysis bag with a molecular weight of 5000 for dialysis for 10 days, and freeze-dry to obtain linoleic acid-modified dextran.
Embodiment 3
[0034] Synthesis of linoleic acid modified dextran. Add 1 g of dextran with a molecular weight of 10,000 and 1 g of linoleic acid into a beaker, then add 100 mL of dimethyl sulfoxide solvent, 0.5 g of EDC and 0.2 g of DMAP into the beaker, stir magnetically, and react at room temperature for 8 hours. After the reaction, put the product into a dialysis bag with a molecular weight of 3500 for dialysis for 7 days, and freeze-dry to obtain linoleic acid-modified dextran.
PUM
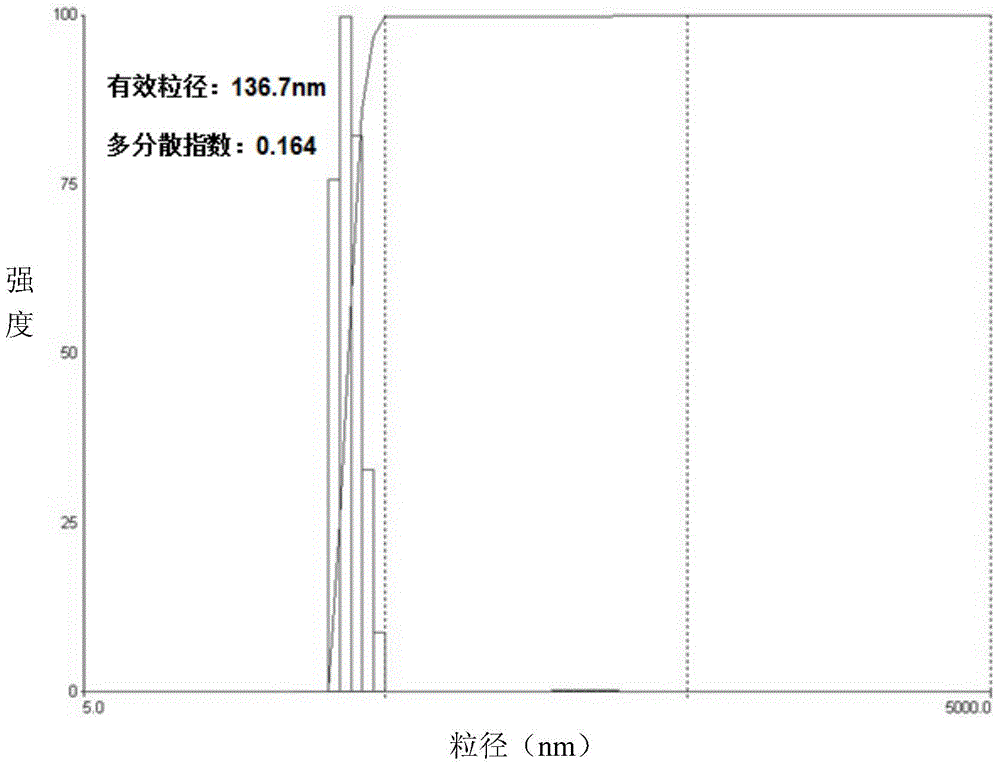
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com