Novel diclofenac injection and preparation method thereof
A technology of diclofenac and diclofenac sodium, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve problems such as difficulty in ensuring effective gelation, difficulty in rapid drug absorption, and inability to detect drugs , achieve the effect of shortening the gelation time, excellent sustained release effect and good animal compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
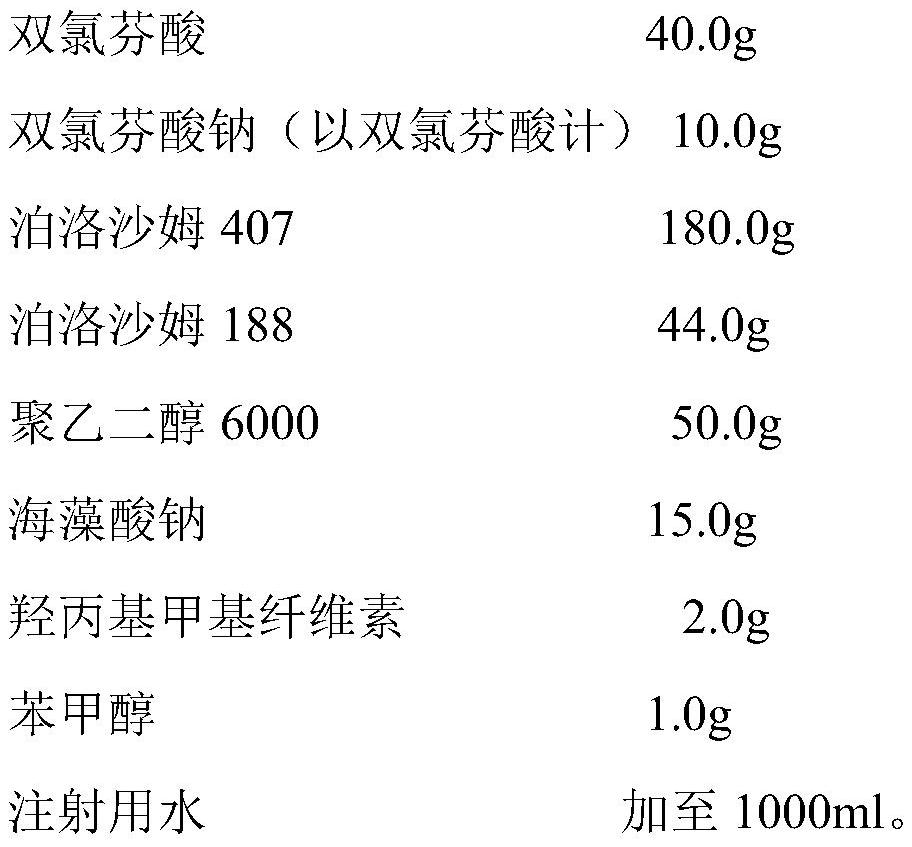
[0032] The prescription is as follows:
[0033]
[0034] Preparation:
[0035] Step 1: take each component according to the above-mentioned prescription quantity;
[0036] Step 2: Take the prescribed amount of benzyl alcohol, sodium alginate, polyethylene glycol, poloxamer 188 and diclofenac sodium, add them into water accounting for about 80% of the prescribed amount, and stir until completely dissolved to obtain a mixed solution;
[0037] Step 3: Take the prescription amount of diclofenac, poloxamer 407, and hydroxypropyl methylcellulose, mix evenly, add to the mixture in step 2, stir until a lump-free, uniformly dispersed medicinal solution is obtained, add water to volume , that is.
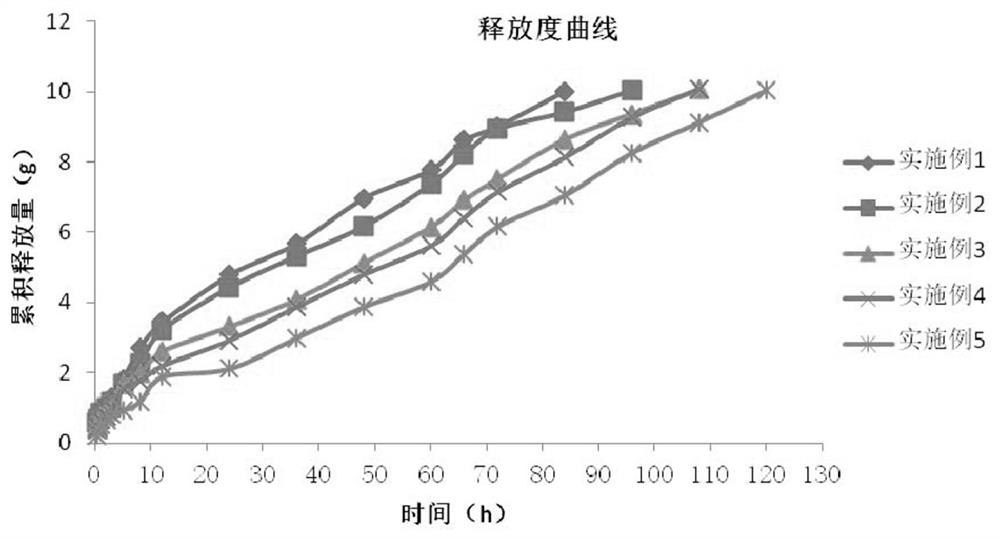
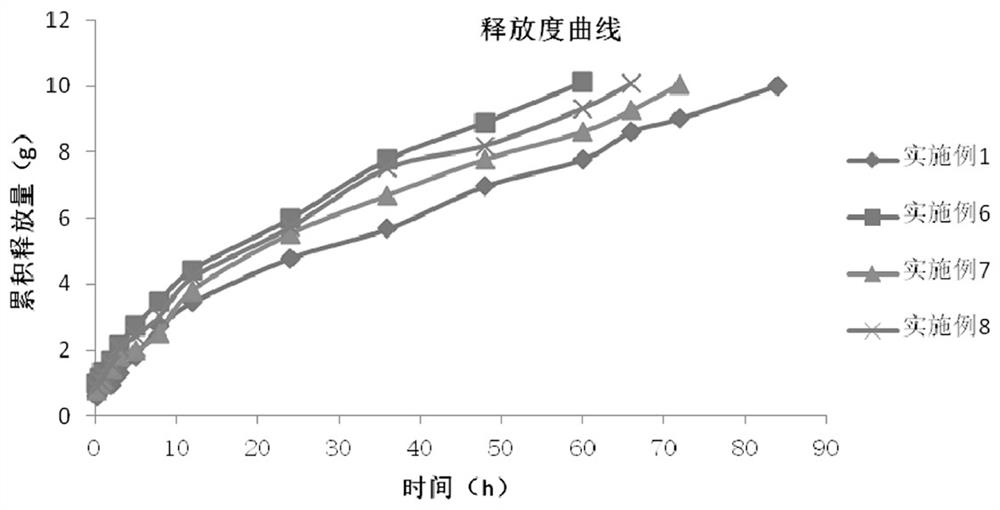
[0038] The in vitro performance evaluation of Diclofenac Sodium Injection was carried out, including the determination of properties, needle passability, gelation temperature, gelation time, thermal reversibility, and release rate. The in vitro release profile is as figure 1 shown. Th...
Embodiment 2
[0045] The prescription is as follows:
[0046]
[0047] Preparation:
[0048] Step 1: take each component according to the above-mentioned prescription quantity;
[0049] Step 2: Add the prescribed amount of ethyl p-hydroxybenzoate, sodium alginate, polyethylene glycol, poloxamer 188 and diclofenac sodium into water accounting for about 80% of the prescribed amount, and stir until completely dissolved to obtain a mixed solution;
[0050] Step 3: Take the prescribed amount of diclofenac, poloxamer 407, and povidone K30, mix evenly, add to (2), stir until a lump-free, uniformly dispersed medicinal solution is obtained, add water to volume, and the product is ready.
[0051] The in vitro performance evaluation was carried out, including the determination of properties, penetrability, gelation temperature, gelation time, thermal reversibility, and release rate. The in vitro performance evaluation method is the same as in the examples. The in vitro release profile is as fig...
Embodiment 3
[0053] The prescription is as follows:
[0054]
[0055] Preparation:
[0056] Step 1: take each component according to the above-mentioned prescription quantity;
[0057] Step 2: Take the prescribed amount of benzoic acid, sodium alginate, polyethylene glycol, poloxamer 188 and diclofenac sodium, add them into water accounting for about 80% of the prescribed amount, and stir until completely dissolved to obtain a mixed solution;
[0058] Step 3: Take the prescribed amount of diclofenac, poloxamer 407, and sodium carboxymethylcellulose, mix evenly, add to the mixed solution in step 2, stir until a lump-free and evenly dispersed medicinal solution is obtained, add water to volume, Instantly.
[0059] The in vitro performance evaluation was carried out, including the determination of properties, penetrability, gelation temperature, gelation time, thermal reversibility, and release rate. The in vitro performance evaluation method is the same as that in Example 1. The in vi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com