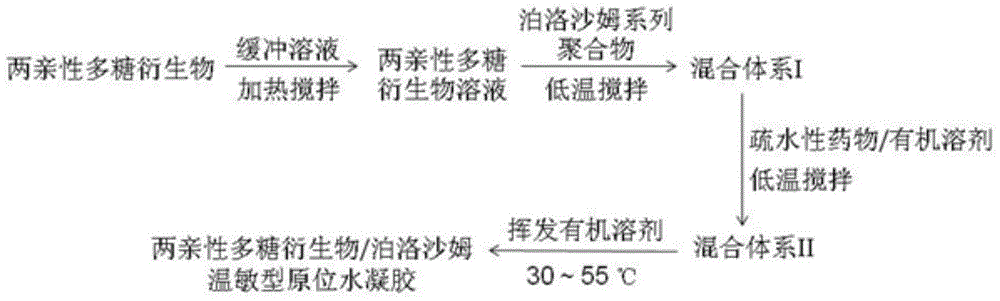
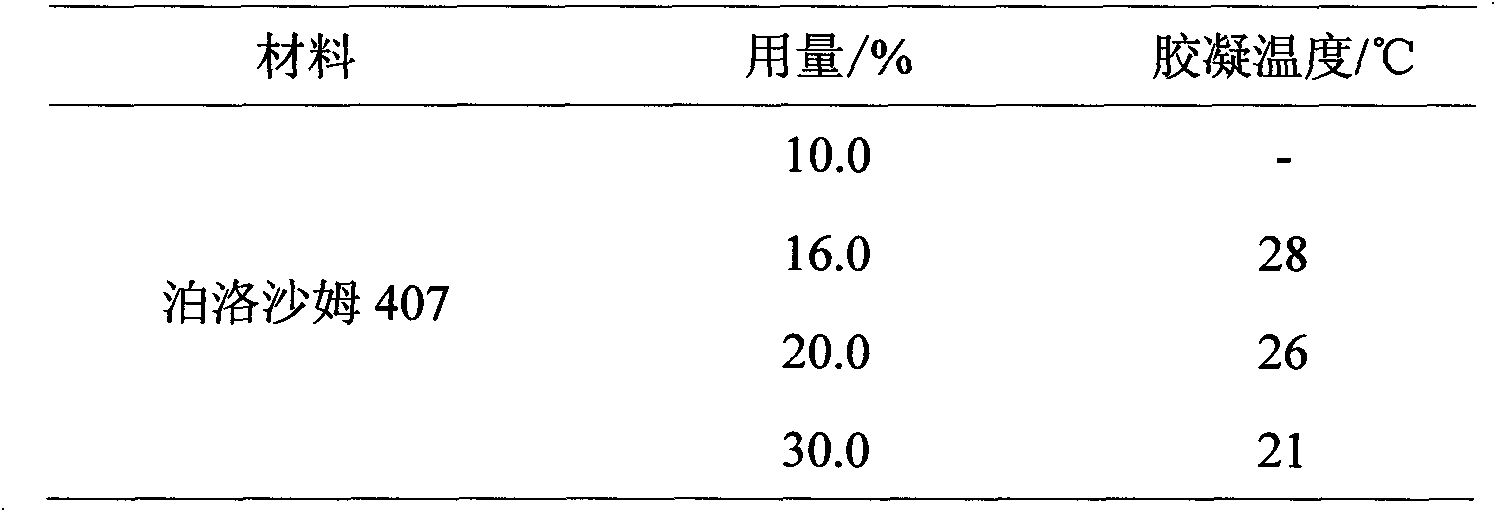
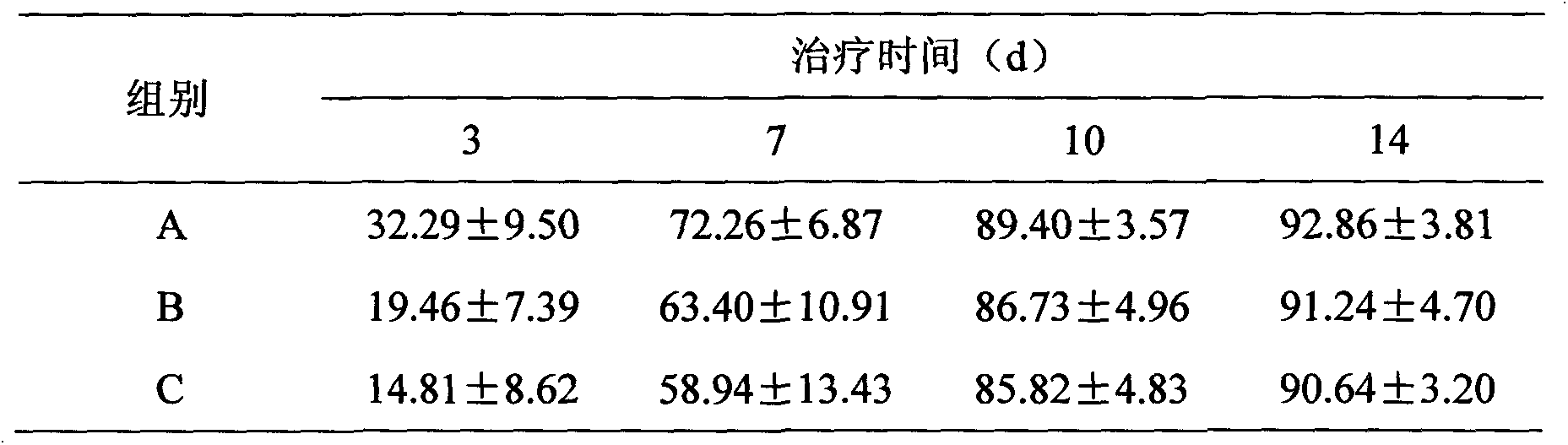
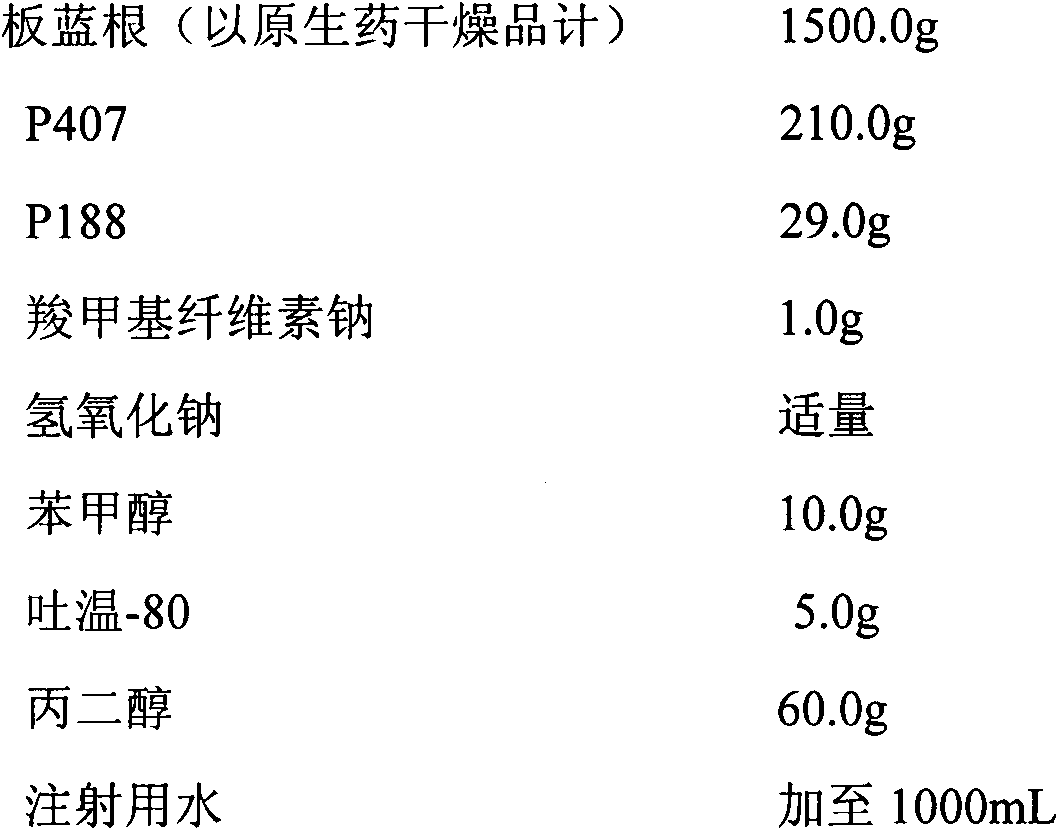
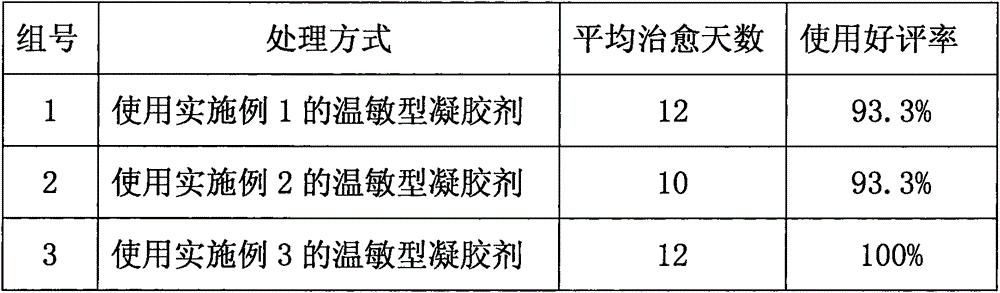
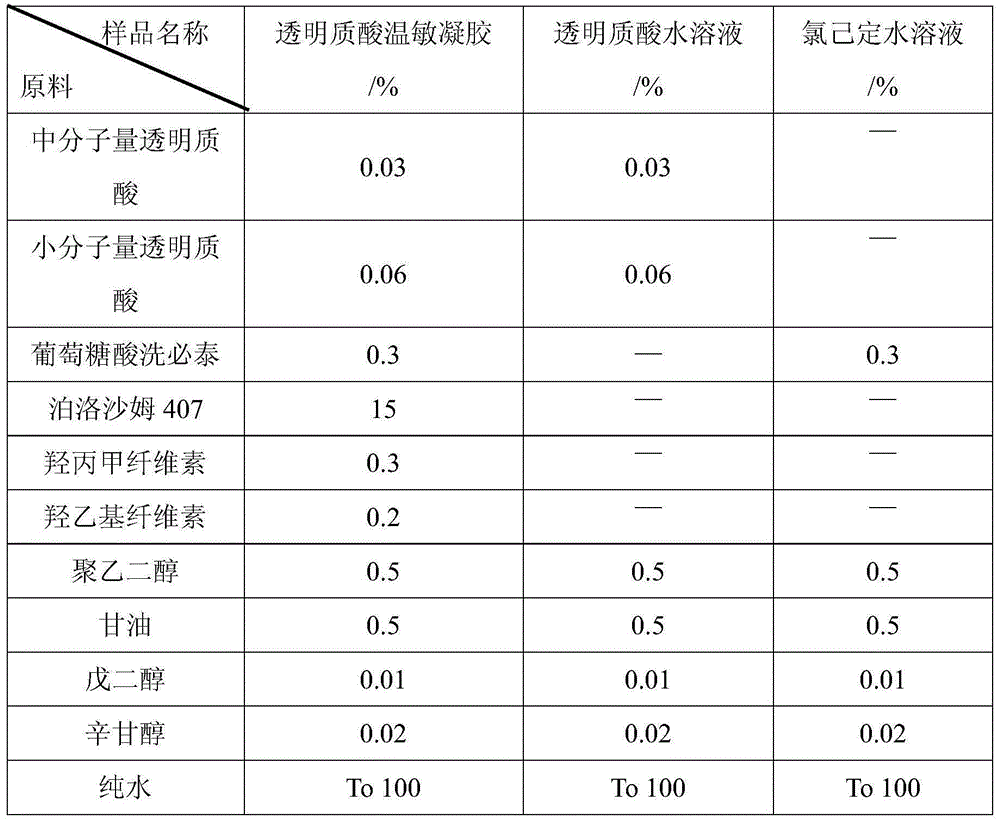
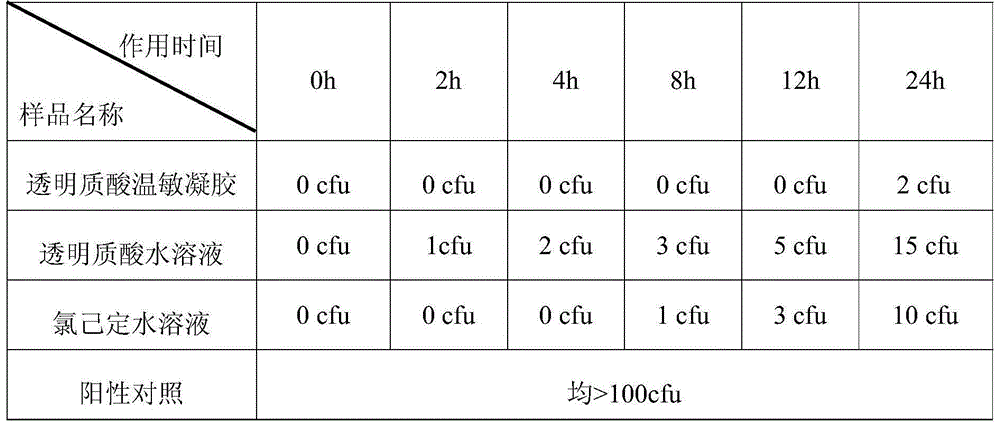
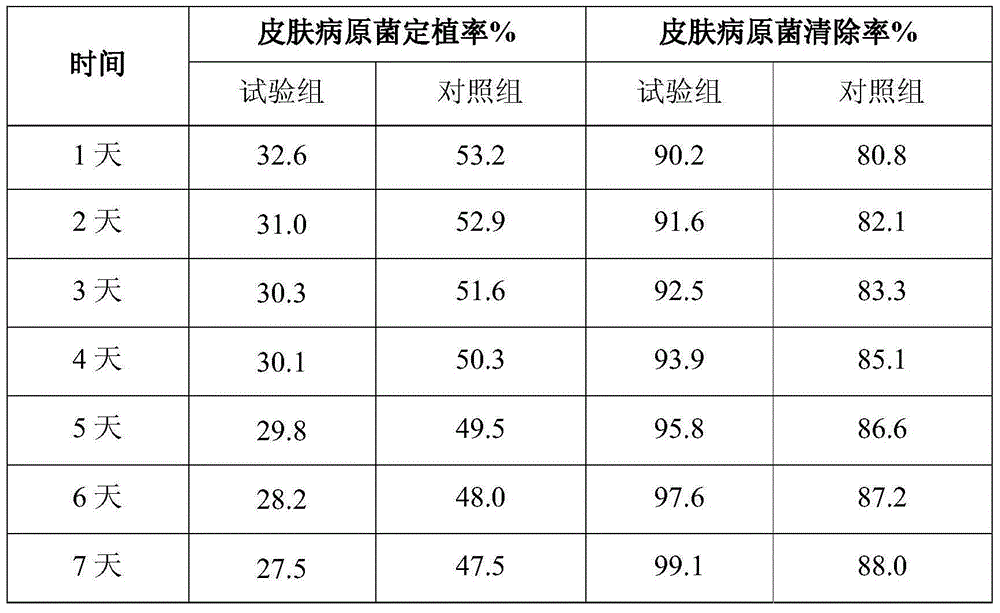
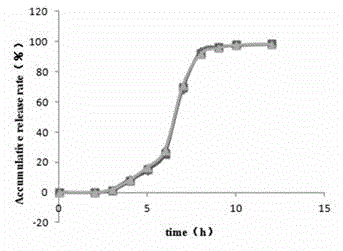
Patents
Literature
280 results about "Poloxamer 407" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Poloxamer 407 is a hydrophilic non-ionic surfactant of the more general class of copolymers known as poloxamers. Poloxamer 407 is a triblock copolymer consisting of a central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol (PEG). The approximate lengths of the two PEG blocks is 101 repeat units while the approximate length of the propylene glycol block is 56 repeat units. This particular compound is also known by the BASF trade name Pluronic F127 or by the Croda trade name Synperonic PE/F 127.
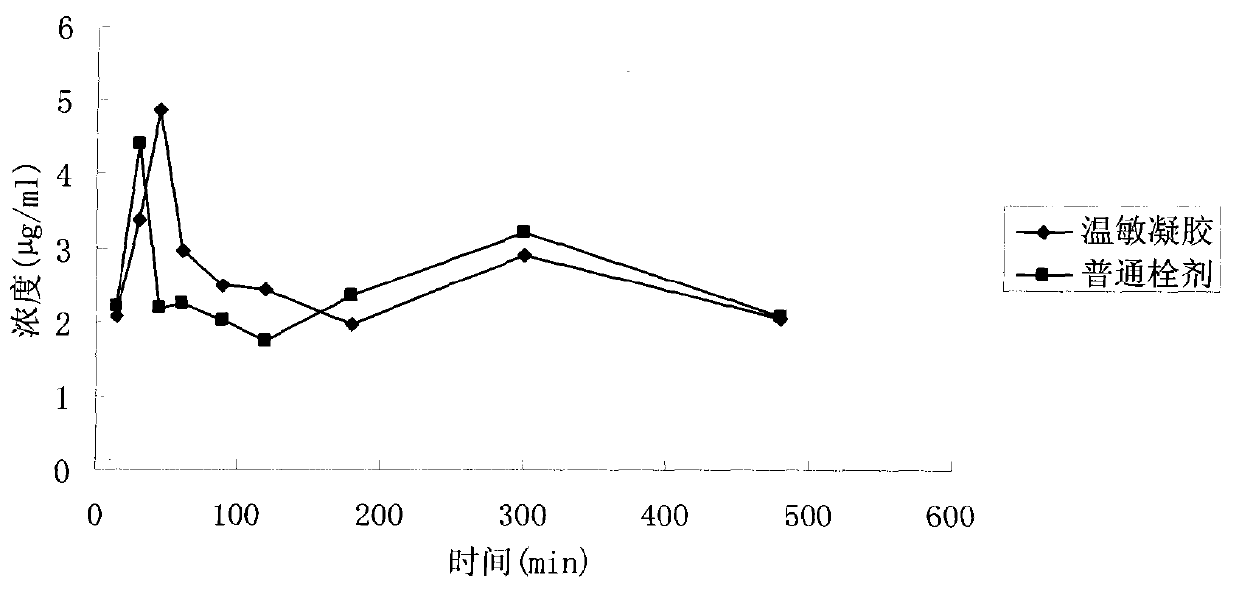
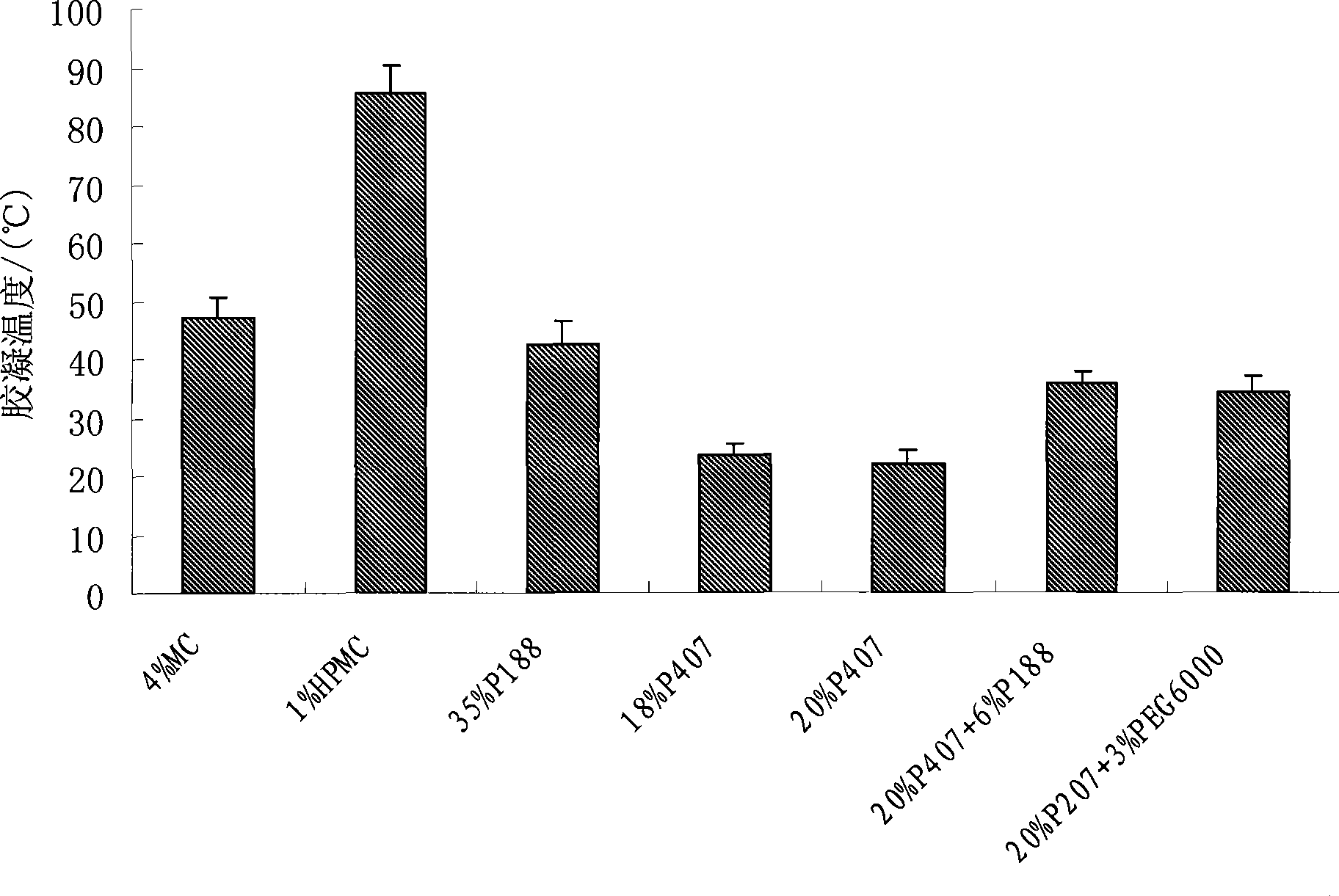
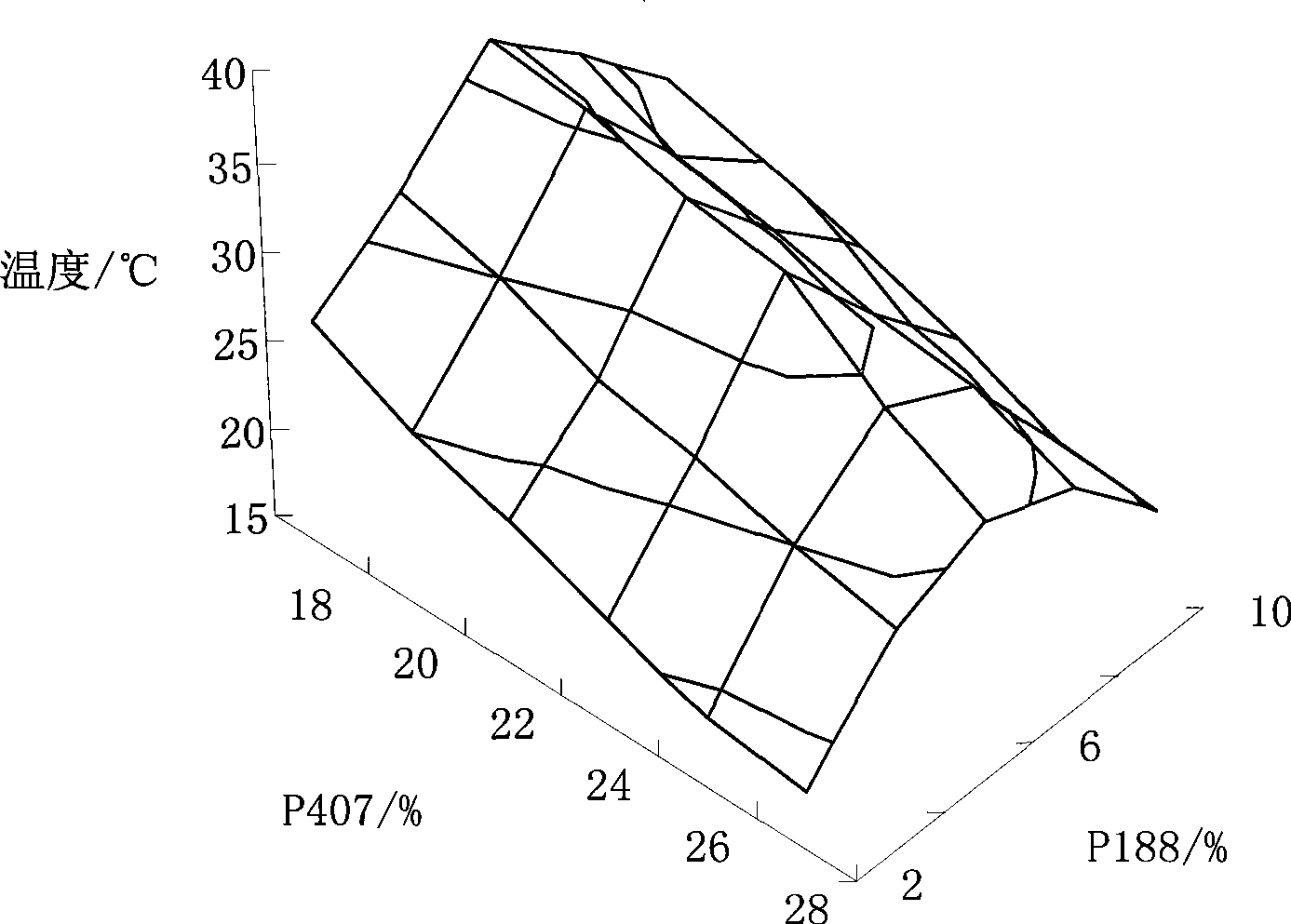
Ocular in-situ gel preparatino with proper phase conversion temperature
InactiveCN1377706AMedication convenienceReduce eliminateSenses disorderPharmaceutical delivery mechanismGel preparationTopical bioavailability
The gel preparation has the merits of both solution and gel. By means of the combination of different type poloxamers, ocular in-situ gel preparation containing medicine and water soluble polymer supplementary material is prepared. The preparation contains poloxamer 407 and poloxament 188 as well as water soluble polymer supplementary material less than 3%. The preparation has proper phase conversion temperatur and may form gel on the surface of cornea of living body after being applied in liquid state at room temperature. The present invention can delay the disappearance of medicine and raise the biological utilization in some local area and is suitable for various ocular medicines.
Owner:SHENYANG PHARMA UNIVERSITY
Baicalin thermosensitive gelatin and preparation method and use thereof
InactiveCN101732237AImprove temperature-sensitive propertiesAdjustable release rateAntibacterial agentsOrganic active ingredientsPreservativeDrug release
The invention discloses a baicalin thermosensitive gelatin and a preparation method and use thereof. The baicalin thermosensitive gelatin is prepared by the following steps: (1) taking the following components in percentage by weight: 0.6 to 15 percent of baicalin, 10 to 24 percent of poloxamer 407, 1 to 10 percent of poloxamer 188, 55.8 to 81.17 percent of solvent, 0 to 5 percent of viscosity modifier, and 0 to 0.2 percent of preservative; and (2) dissolving the baicalin and the preservative into the solvent, slowly adding the poloxamer 407 and the poloxamer 188 with stirring, mixing uniformly, adding the viscosity modifier, mixing uniformly, and placing the mixture into freely flowable semitransparent solution to prepare the baicalin thermosensitive gelatin. The baicalin thermosensitive gelatin is flowable liquid at normal temperature, which generates reversible phase change if affected by the body temperature after contacting with a mucous membrane or body fluid. Therefore, the medicament is tightly attached to the mucosal tissue, which is hard to leak. After the phase change is generated in the body fluid, the release rate of the medicament can be controlled.
Owner:LOGISTICS UNIV OF CAPF
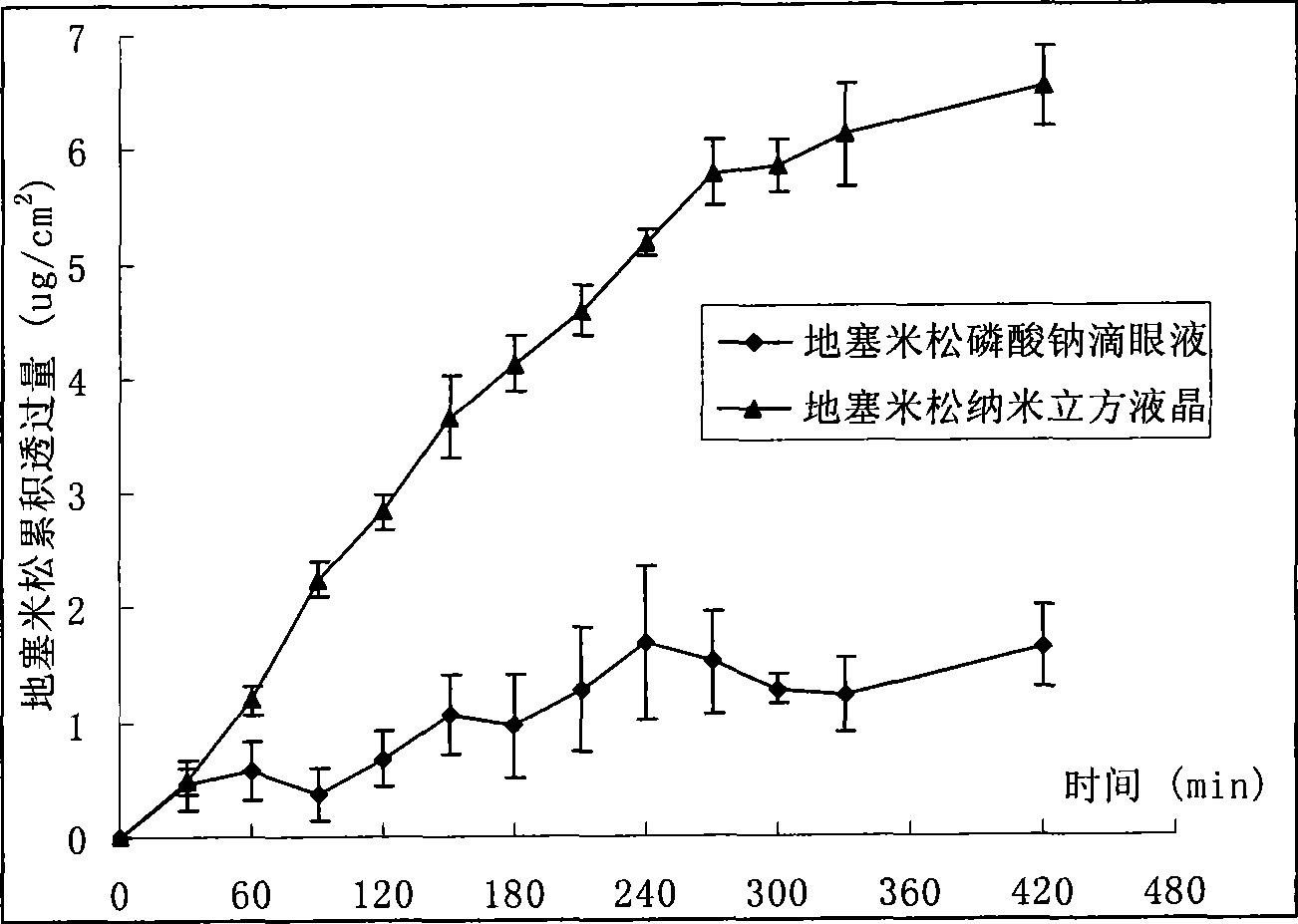
Nano cubic liquid crystal dexamethasone preparation for eye and preparation method thereof
InactiveCN101502485ASolve solubilitySolving Dispersion ProblemsOrganic active ingredientsSenses disorderLipid formationPolyvinyl alcohol
Owner:CHINA PHARM UNIV
Oil injection containing tulathromycin/poloxamer 407
The invention discloses combination of tulathromycin and poloxamer 407. The technology comprises the following steps: preparing medicine-carrying micro-particles; further dispersing the medicine-carrying micro-particles into oil medium; and preparing a long-acting oil injection containing tulathromycin and poloxamer 407 medicine-carrying micro-particles in a grinding manner. The injection is simple in preparation technology, good in slow-release effect, good in biocompatibility, and free of an irreversible damage to the tissue of the injection part.
Owner:王玉万
Conception control gel composition for vagina as well as preparation method and application thereof
InactiveCN101559036AOptimize content ratioSensitive to temperaturePharmaceutical delivery mechanismMacromolecular non-active ingredientsDiseaseBuffering agent
The invention discloses a gel composition for vagina, containing buffering agent, bioadhesive polymer, water, phase transition temperature regulator and one or more poloxamer of poloxamer 237, poloxamer338 and poloxamer407. The weight percentage of the poloxamer is 15-30 percent, the weight percentage of the phase transition temperature regulator is 1.5-6.75 percent; the weight ratio of the poloxamer and the phase transition temperature regulator is 20:1-20:9; and the pH of the composition is 3.0-5.5. The invention also discloses a preparation method of the gel composition and the application of the gel composition in preparing conception control preparation, vagina lubricant, preparation or medicament for preventing sexually transmitted diseases or medicaments for preventing gynecological diseases. The gel composition for vagina is liquid under room temperature and is semi-solid in vagina, is convenient to prepare and use, has the pH value similar to the vagina environment and has better acidic buffering capability and viscosity without damaging the vagina.
Owner:SHANGHAI INST OF PHARMA IND
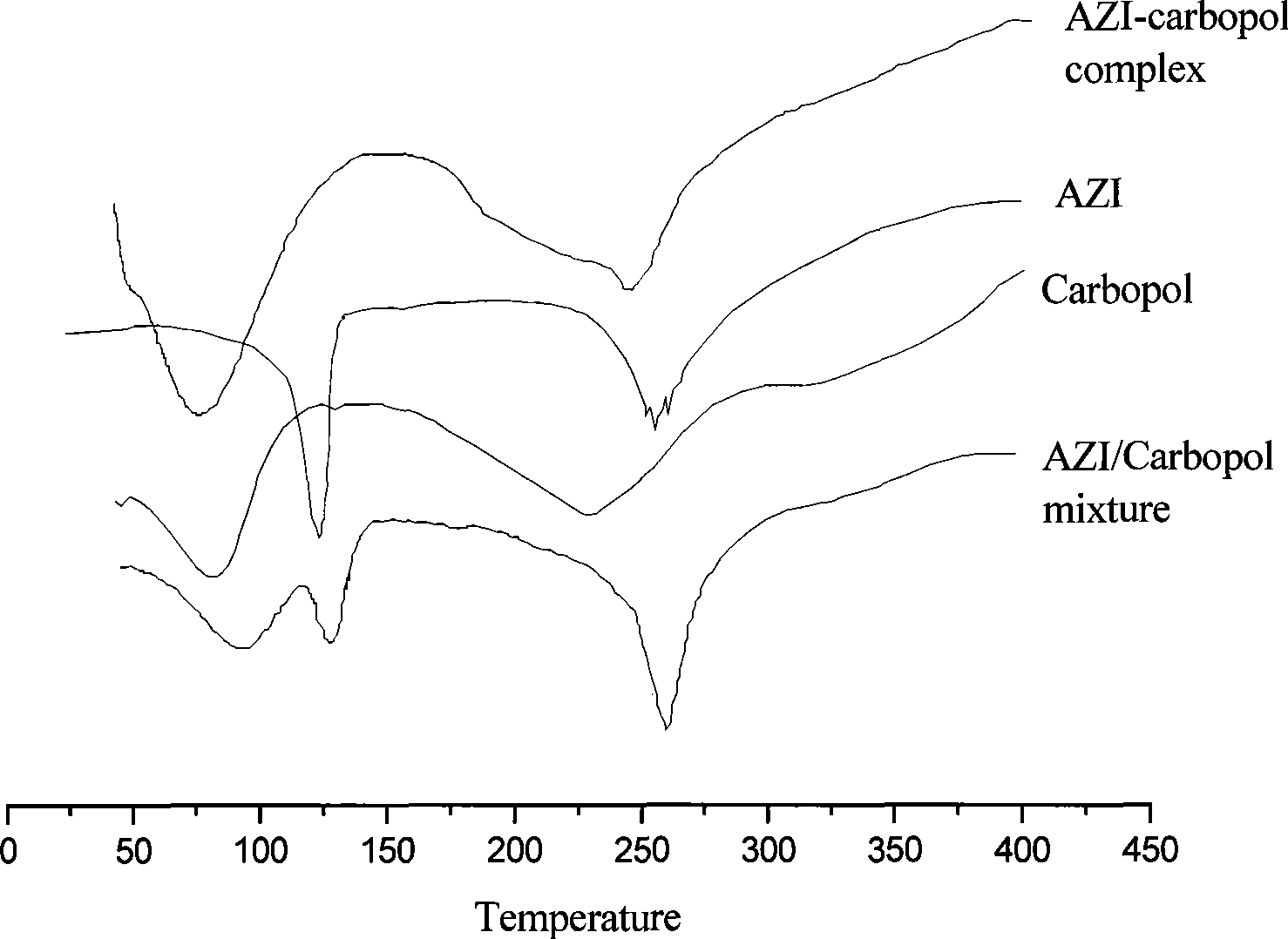
Azithromycin ophthalmic instant molding gel and preparation method thereof
InactiveCN101444477AProlong the action timeImprove complianceAntibacterial agentsOrganic active ingredientsAzithromycinBiocompatibility Testing
The invention relates to the field of pharmaceutical preparation, in particular relates to an azithromycin ophthalmic instant molding gel. The azithromycin ophthalmic instant molding gel is characterized in that the azithromycin ophthalmic instant molding gel comprises azithromycin, a temperature sensing substrate, carbomer, a permeation pressure conditioning agent and water for injection, wherein the temperature sensing substrate comprises poloxamer 407 and poloxamer 188. The invention further discloses a preparation method thereof. The invention uses the poloxamer 407 and the poloxamer188 as the temperature sensing substrate with good biocompatibility. The preparation is flowable fluid preparation under the condition of invitro storage; the preparation forms gel when the external environment of dropping in ocular region is changed, so as not to flow in ocular region; therefore, the pharmic action time is remarkably prolonged, time administer drug is reduced and the adaptability of the patient is improved.
Owner:CHINA PHARM UNIV
Preparation method of in-situ gel sustained-release preparation for treating Bovine mastitis
InactiveCN101664381AAdhesiveIncrease lethalityOrganic active ingredientsPharmaceutical delivery mechanismMass ratioMastitis
The invention relates to a preparation method of in-situ gel sustained-release preparation for treating Bovine mastitis, which is characterized in that: the preparation steps are as follows: weighingciprofloxacin hydrochloride and film formation high molecular material chitosan according to a weight ratio of 0.1-0.5:1; dissolving the mixture of the two in water to prepare an aqueous solution containing 1 percent of chitosan, spraying and drying the aqueous solution to prepare a chitosan microsphere containing ciprofloxacin hydrochloride; distributing the obtained microsphere containing drug into the mixture aqueous solution formed by poloxamer 188 and poloxamer 407 according to the mass ratio of 0.1-5:1, thus obtaining the temperature-sensitive in-situ gel sustained-release preparation for treating Bovine mastitis. The sustained-release microcapsule containing inflammation diminishing medicine is distributed in the solution of high polymer material capable of forming gel at the body temperature. The in-situ gel containing inflammation diminishing medicine can treat Bovine mastitis by perfusion in breast; and the preparation method is simple, the product is safe in use, and the curative effect is accurate.
Owner:TIANJIN SHENGJI GRP CO LTD
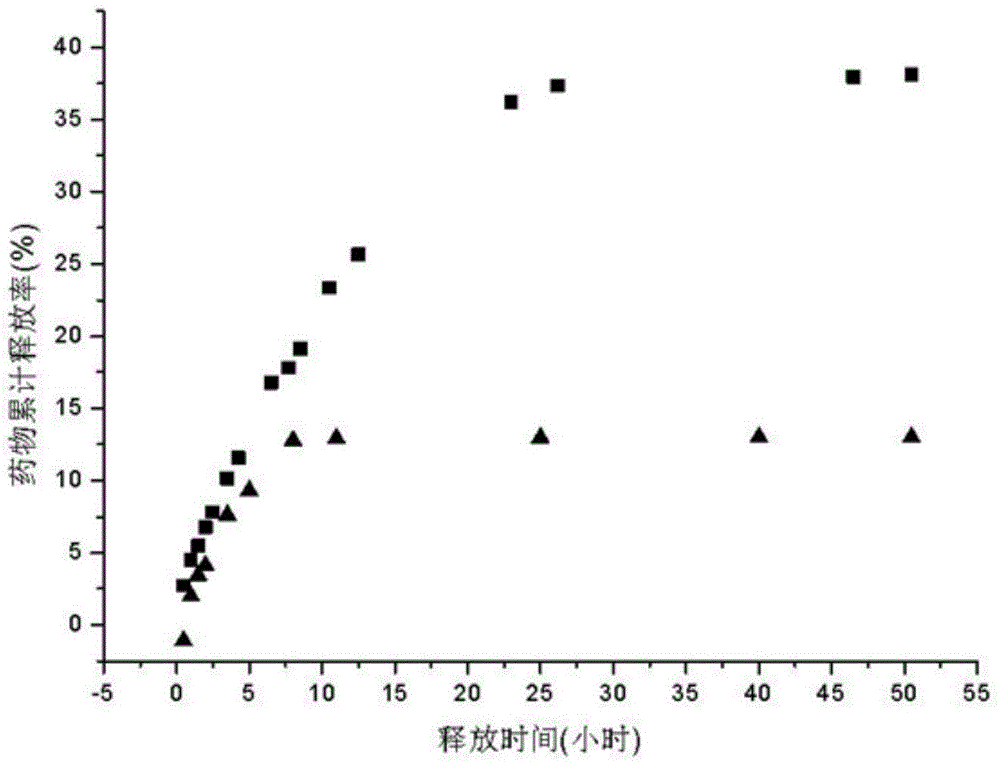
Amphipathic polysaccharide derivative/poloxamer thermo-sensitive type in-situ hydrogel and preparation method thereof
InactiveCN104888224ATo achieve the purpose of slow-release drugsGelation time is shortPharmaceutical non-active ingredientsPerylene derivativesBioavailability
The invention discloses amphipathic polysaccharide derivative / poloxamer thermo-sensitive type in-situ hydrogel and a preparation method thereof. The thermo-sensitive type in-situ hydrogel is prepared in the mode that amphipathic polysaccharide series derivatives, poloxamer polymer and hydrophobic drugs interact to form stable in-situ hydrogel, the gelatinization temperature of the thermo-sensitive type in-situ hydrogel ranges from 34 DEG C to 37 DEG C, and the gelatinization time for forming the in-situ hydrogel at the gelatinization temperature ranges from one to three minutes. The poloxamer polymer is the mixture of poloxamer 407 and any one kind of poloxamer of other types. Compared with common amphipathic / poloxamer in-situ hydrogel, the in-situ hydrogel is higher in stability and longer in drug sustained release time, parenteral drug delivery and improving of drug bioavailability are made possible, and the in-situ hydrogel can be applied to mucous membrane drug delivery, transdermal drug delivery and parenteral drug delivery systems; the preparation method of the mphipathic polysaccharide derivative / poloxamer thermo-sensitive type in-situ hydrogel is convenient to operate, simple in process and cheap in needed equipment and raw material.
Owner:SUN YAT SEN UNIV
Heat-sensitive gel containing matrine alkaloid and its preparing method
InactiveCN1850076AGood thermal propertiesEasy and effective applicationOrganic active ingredientsPharmaceutical delivery mechanismGel preparationPhosphate
The present invention relates to a heat-sensitive gel preparation containing matrine alkaloid and its preparation method. Said preparation is composed of matrine alkaloid, hydroxypropyl methylcellulose, poloxamer 407, phosphate buffer solution whose pH value is 5.0-7.0, glycerin and preservative. It is flowable fluid at normal temperature, and when the temperature is 28-33 deg.C, it can produce phase change, and can be formed into gel, so that it can be conveniently applied to vagina, mucous membrane of external genitalia, skin and eye.
Owner:SHANGHAI HUATUO MEDICAL SCI CO LTD
Moldable paste composition
The invention concerns a novel moldable and / or flowable composition for application to a bone defect site to promote new bone growth at the site. The composition includes a therapeutic material and a carrier comprising means for achieving reverse phase characteristics. In one embodiment, the therapeutic material can be a resorbable alloplastic material and the carrier can be a poloxamer. In a specific embodiment, the resorbable alloplastic material is a biphasic material composed of hydroxyapatite and tricalcium phosphate (HA-TCP), and the carrier is poloxamer 407 (Pluronic® F127). The invention further includes methods for using the novel composition.
Owner:BOURGEOIS NICOLAS +3
Preparation method of ceftiofur acid long-acting injection
InactiveCN103230364ALong injection half-lifeImprove solubilityAntibacterial agentsOrganic active ingredientsHalf-lifeDissolution
The invention belongs to the technical field of preparation of medicines and in particular relates to a preparation method of ceftiofur acid long-acting injection. The preparation method comprises the followings steps of: adding ceftiofur acid and 2-hydroxypropyl-beta-cyclodextrin in a molar ratio of 1:(1-2) to a ball mill for uniformly mixing; sufficiently grinding under the room temperature, and sufficiently uniformly and sieving to obtain a ceftiofur acid clathrate compound; dissolving sodium alginate in sterile water and adding poloxamer 407 and poloxamer 188, storing for 12-24 hours under the temperature condition of 4 DEG C, so that the poloxamer 407 and the poloxamer 188 are completely dissolved and sterilized under the temperature of 121 DEG C, carrying out ice-bath cooling to obtain a transparent solution; magnetically stirring under the temperature condition of 4 DEG C and the rotation speed condition of 150r / min; adding the ceftiofur acid clathrate compound in a weight ratio of the sodium alginate to the ceftiofur acid clathrate compound of (0.1-0.3):(5-10), so that the ceftiofur acid clathrate compound is sufficiently dispersed uniformly to obtain the ceftiofur acid long-acting injection. According to the preparation method of the ceftiofur acid long-acting injection, the preparation process is simple, the product intramuscular injection half-life period is long, the dissolution degree of the ceftiofur acid is high, the production and treatment cost is low, the cure rate is high and the environment is friendly.
Owner:QINGDAO AGRI UNIV
Medical antiblocking intelligent gel rubber material and preparation thereof
The invention relates to a medical temperature response intellectual gelatin material for preventing postoperative adhesions and a manufacturing method thereof. When the temperature is less than 20 DEG C, the material is colorless and clear liquid with good fluidity; when the temperature is heated to 20-40 DEG C, the material is transferred to be a steady gelatin state, and the transformation from a liquid state to be a gelatin state can be realized at body temperature; poloxamer 407 is taken as host material, after sodium hyaluronate, glycerin and propanediol with certain proportion are added, dissolution at low temperature, vacuum degassing, millipore filtration and autoclaving are adopted, thus forming the material. The gelatin material is filled in blocking bottles, Schering bottles and pre-filled injectors with fixed amount and is used in the clinical postoperative adhesions, the curative effect is good, and use is convenient.
Owner:HANGZHOU SINGCLEAN MEDICAL PROD
Oily injection containing valnemulin hydrochloride/poloxamer 407
ActiveCN103705454AReduce dosageGood sustained release effectAntibacterial agentsSolution deliveryValnemulin HydrochlorideBiocompatibility Testing
The invention discloses an oily long-acting injection containing valnemulin hydrochloride / poloxamer 407 drug-loading particles, which is prepared by combining the valnemulin hydrochloride with poloxamer 407 into drug-loading particles and further dispersing the drug-loading particles into an oily medium and grinding. Hydroxypropyl methyl cellulose or high-substituted hydroxypropyl cellulose also can be added into the drug-loading particles, and the sustained-release effect of the preparation is obviously enhanced. The preparation process of the injection is simple, the drug release is uniform, the biocompatibility is good, irreversible damage to the tissue of the injection part is avoided, and the adopted sustained-release carrier can be degraded and excreted.
Owner:河北威远药业有限公司
Recombinant human-derived collagen vagina gel for vaginal dryness and preparation method thereof
ActiveCN104548071AIncrease elasticityImprove drynessOrganic active ingredientsPeptide/protein ingredientsGlycerolSodium methylparaben
The invention relates to an ordinary treatment medicine for vagina dryness, and in particular relates to recombinant human-derived collagen vagina gel for vaginal dryness. The recombinant human-derived collagen vagina gel comprises the following components in percentage by mass: 0.05-1% of recombinant human-derived collagen, 10-30% of poloxamer 407, 0.5-6% of poloxamer 188, 0.1-2% of sodium hyaluronate, 1-4% of glycerinum, an antibacterial preservative which comprises 0.1-1% of phenoxyethanol, 0.05-3% of sodium methylparaben, 0.01-2% of propylparaben, and the balance of water. By adopting the recombinant human-derived collagen vagina gel, the vagina elasticity can be improved, vaginal atrophy can be alleviated, dyspareunia can be relieved, and the sex satisfaction can be improved; by increasing secreta, improving the vagina wetness and alleviating the dryness of vaginas, pruritus, hotness and chapped skin caused by dryness can be remedied, and the symptoms of dryness and stabbing pain of vulvovagina can be alleviated. In addition, the product cannot affect the pH value of vaginas when used in the vaginas, is free of adverse reaction, and is worthy of clinical popularization and application.
Owner:SHANXI JINBO BIO PHARMA CO LTD
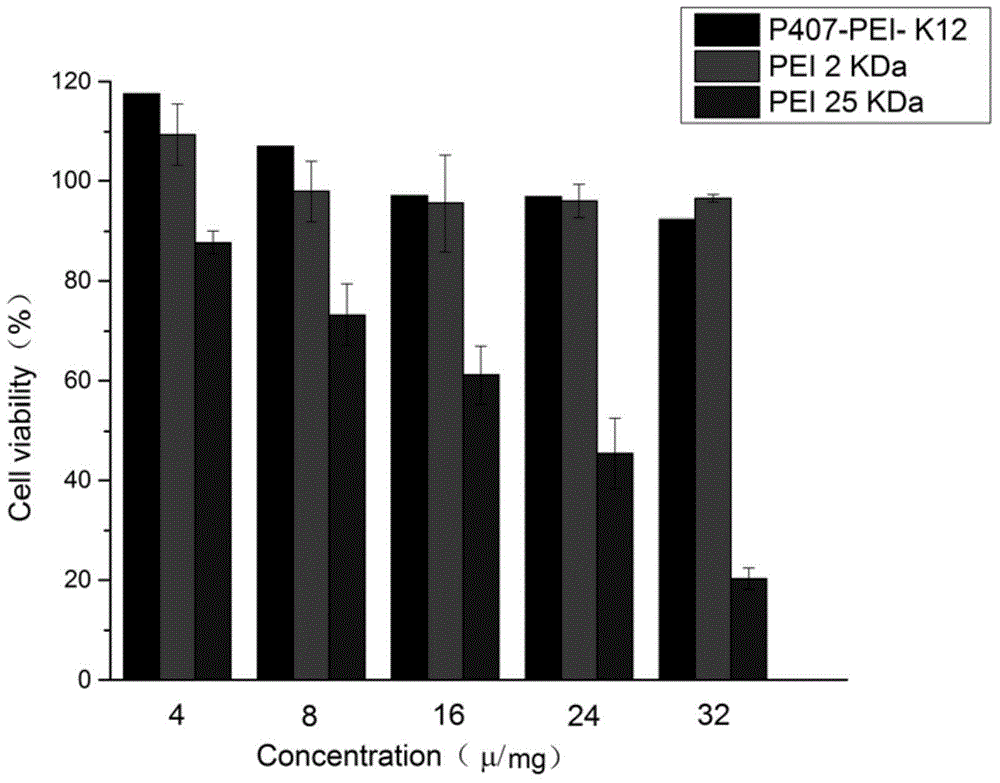
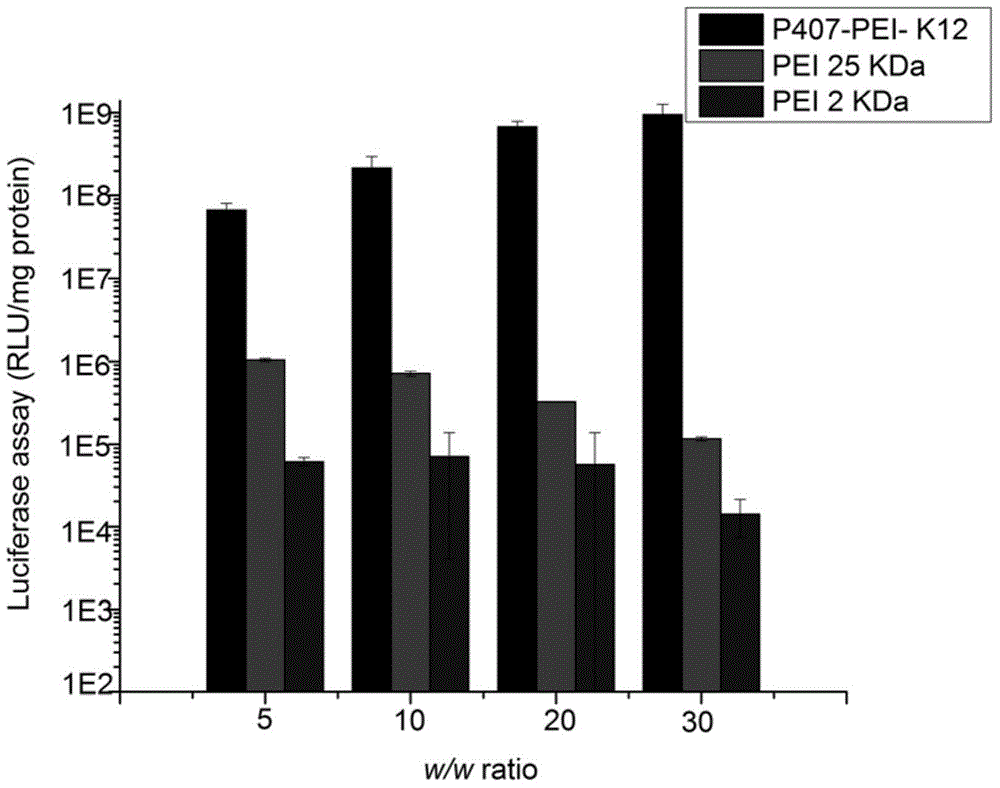
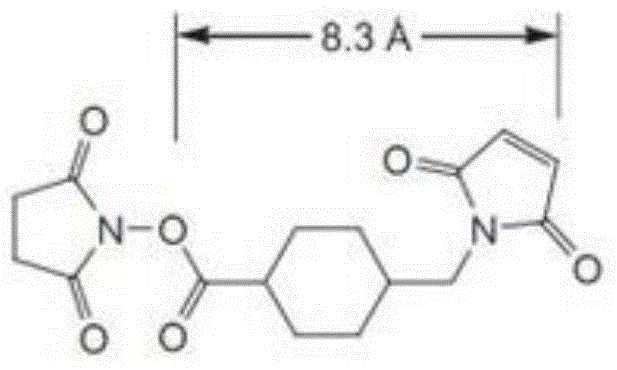
Polypeptide K12-based transgenic vector and application thereof
InactiveCN104830903ALow toxicityExcellent transfection effectPeptide/protein ingredientsGenetic material ingredientsCytotoxicityHigh molecular mass
The invention relates to a polypeptide K12 and a K12-modified gene vector. The sequence of the polypeptide K12 is Lys-Lys-Lys-Arg-Lys-Cys-Gly-Asn-Lys-Arg-Thr-Arg; and the gene vector is prepared by the following steps: firstly, selecting poloxamer 407 (P407) and connecting to PEI (polyether-imide) to obtain a high molecular weight PEI derivative with a multi-branch form or a network structure; selecting a tLyP-1 oligopeptide, connecting with a nuclear localization signal peptide (NLS), and synthesizing the polypeptide K12 which targets NRP and is capable of improving the nuclear delivery ability; coupling the K12 to the EPI derivative by employing a crosslinking technique, and building a novel non-viral gene vector P407-PEI-K12. The cytotoxicity and transfection experiment results prove that the novel non-viral gene vector system P407-PEI-K12 provided by the invention is low in toxicity and has relatively high targeting property; and the cell and in-vivo transfection effects of the gene vector are superior to those of a control group.
Owner:SHANGHAI OCEAN UNIV
Temperature sensitive biogel preparation and application thereof
ActiveCN109820816AEasy to storeConvenient for clinical operationAerosol deliveryOintment deliveryDosing FrequencyMesenchymal stem cell
The invention relates to a temperature sensitive biogel preparation comprising, by mass, 20-30 parts of mesenchymal stem cell exosome, 20-28 parts poloxamer 407, 5-10 parts of poloxamer 188, 0.9-2.4 parts of polymer stabilizer for adjusting gelation temperature and drug release characteristics and 32-54 parts of water. The biocompatible degradable polymer is used as a drug-loading matrix of the mesenchymal stem cell exosome, and a polymer stabilizer is added to adjust the gelation temperature and the drug release characteristics, so that the loaded MSCs exosome can be released continuously toreduce the dosing frequency, prevent leakage of liquid medicine, improve the bioavailability and provide the safe effective preparation convenient to apply clinically for treatment of IUA.
Owner:江苏拓弘生物科技有限公司
Nimesulide temperature sensitive hydrogel and preparation method thereof
InactiveCN102068404AUnique phase transition propertiesReduce onset timeAntipyreticAnalgesicsPreservativeSuppository
The invention provides Nimesulide temperature sensitive gel and a preparation method thereof. The Nimesulide temperature sensitive gel comprises Nimesulide, a temperature sensitive gel material, a pH regulator, a preservative and the like and is characterized in that: the temperature sensitive gel material is poloxamer, and the weight ratio of the poloxamer to the Nimesulide is 9:1; and the optimized temperature sensitive gel material is poloxamer 407. The temperature sensitive gel is sensitive to temperature changes, and rabbit rectum experimental results show that the temperature sensitive gel has higher medicament release rate and higher bioavailability which are obviously better than those of the conventional Nimesulide common suppositories. The Nimesulide temperature sensitive gel reserves the advantages of the conventional suppositories, overcomes the defects of the conventional suppositories to a certain extent and has a wide clinical application prospect. The Nimesulide temperature sensitive gel uses common safe pharmaceutical excipients, has a simple production process, stable and easily-controlled quality and high process reproducibility, and is suitable for industrial production.
Owner:LOGISTICS UNIV OF CAPF
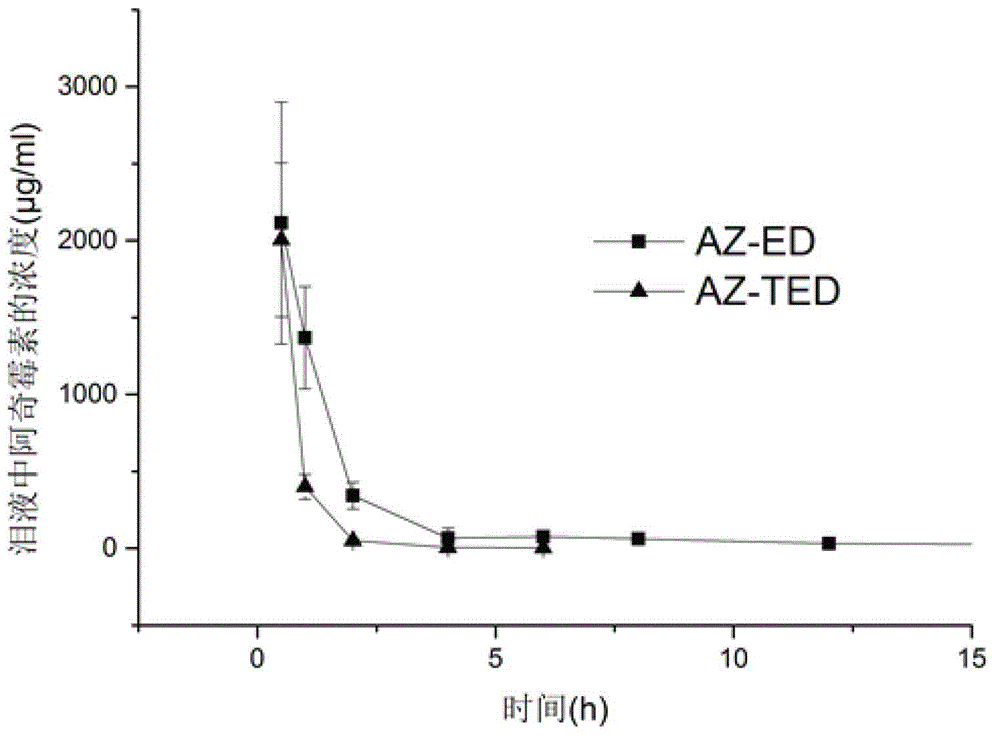
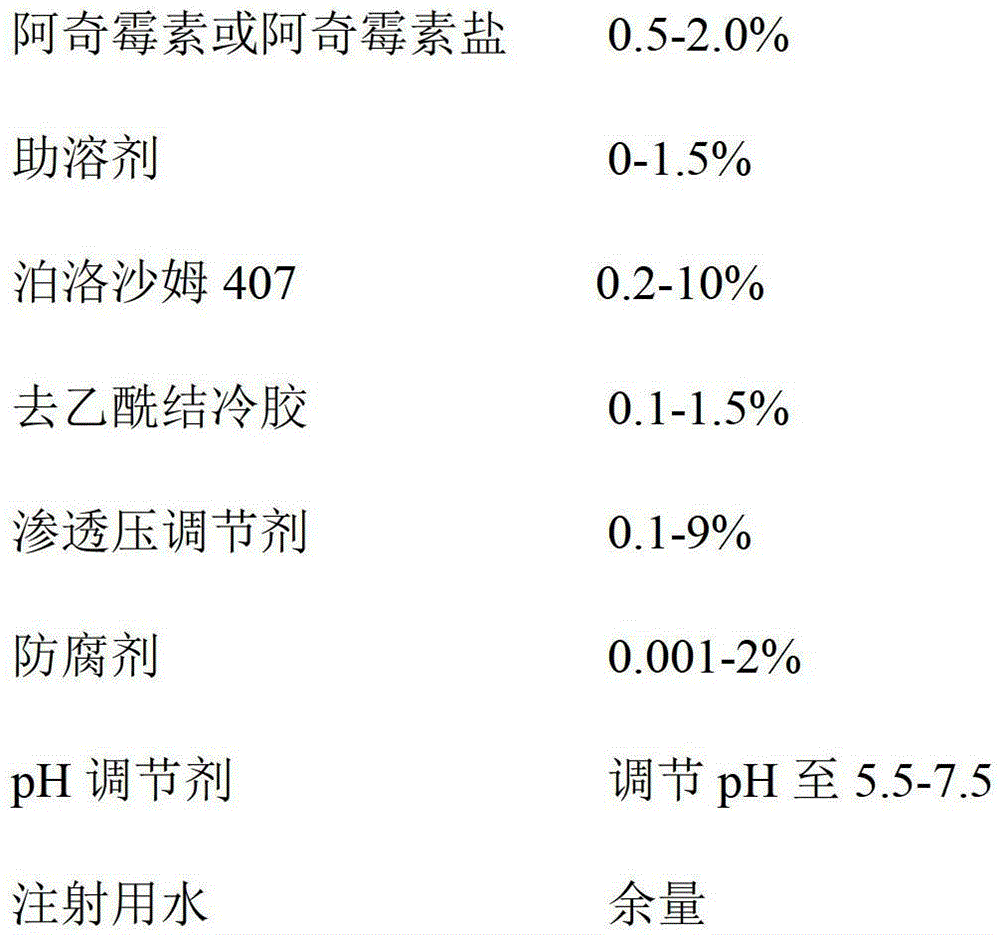
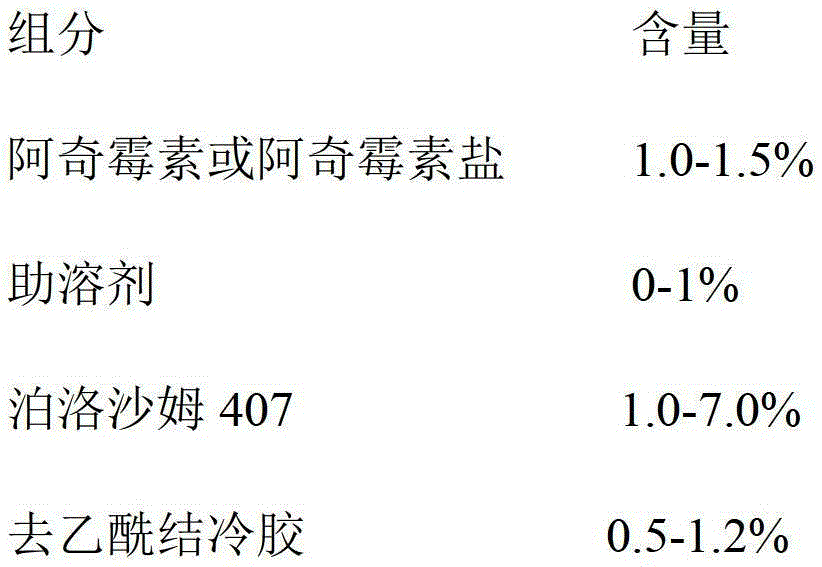
Azithromycin eye drops and preparation method thereof
InactiveCN104055729AImprove complianceImprove toleranceAntibacterial agentsOrganic active ingredientsSide effectSurvivability
The invention discloses azithromycin eye drops and a preparation method thereof, the azithromycin eye drops comprise the following components by weight: 0.5-2.0% of azithromycin or azithromycin salt, 0-1.5% of cosolvent, 0.2-10% of poloxamer 407, 0.1-1.5% of deacetylated gellan gum, 0.1-9% of osmotic pressure regulator, 0.001-2% of antiseptic; pH value is adjusted to 5.5-7.5 by a pH conditioning agent, and injection water is the balance. The azithromycin eye drops contain ion sensitive and temperature sensitive gel matrix, and has dual gelling characteristic, compared with usage of a single matrix, the azithromycin eye drops have better characteristic, preparation technology is reasonable, stability of the azithromycin eye drops can be guaranteed, metering is easily and accurately controlled, little loss can be avoided, the therapeutic time of the medicine can be maintained for a longer time, the side effect is less, the azithromycin eye drops have good biocompatibility without influence of eyesight, and the survivability of the patients is good.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
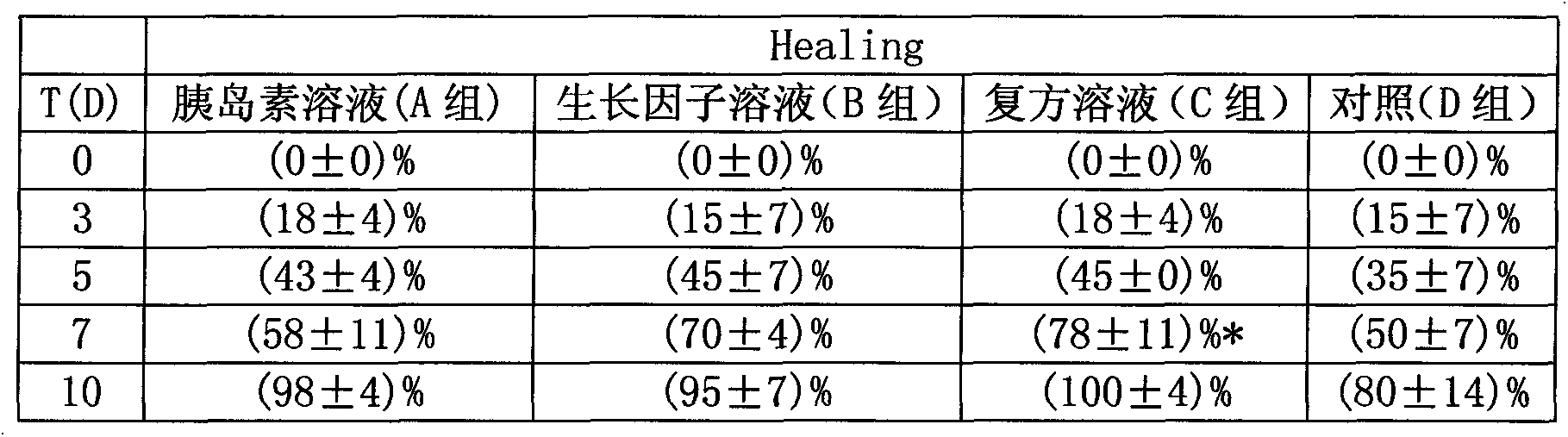
Compound temperature-sensitive gel for treating skin ulcer and preparation method thereof
InactiveCN103055304AGood temperature sensitivityImprove stabilityPeptide/protein ingredientsAerosol deliveryCuticleOperability
The invention relates to a compound temperature-sensitive gel for treating skin ulcer and a preparation method thereof. The compound temperature-sensitive gel is composed of insulin and recombinant human epidermal factor by taking one or more of poloxamer 407, poloxamer 188 and chitosan as a substrate; and the compound temperature-sensitive gel is prepared through preparation of a gel substrate, preparation of a mixed solution of the insulin and the recombinant human epidermal factor, preparation of the compound temperature-sensitive gel and observation of a sample in turn. The compound temperature-sensitive gel disclosed by the invention is in the liquid state in preparation and storage processes and easy to coat or spray at a wound, so that the compound temperature-sensitive gel is conveniently used clinically; gel can be rapidly formed under the body temperature condition; the gel is difficult to run away, effective for long-term retention and high in bioavailability; furthermore, due to the aqueous environment of the gel, biological activity of two protein medicaments can be ensured more easily; release of the two protein medicaments is more easy; and the preparation method of the compound temperature-sensitive gel disclosed by the invention is simple and practical and strong in operability.
Owner:卓阳
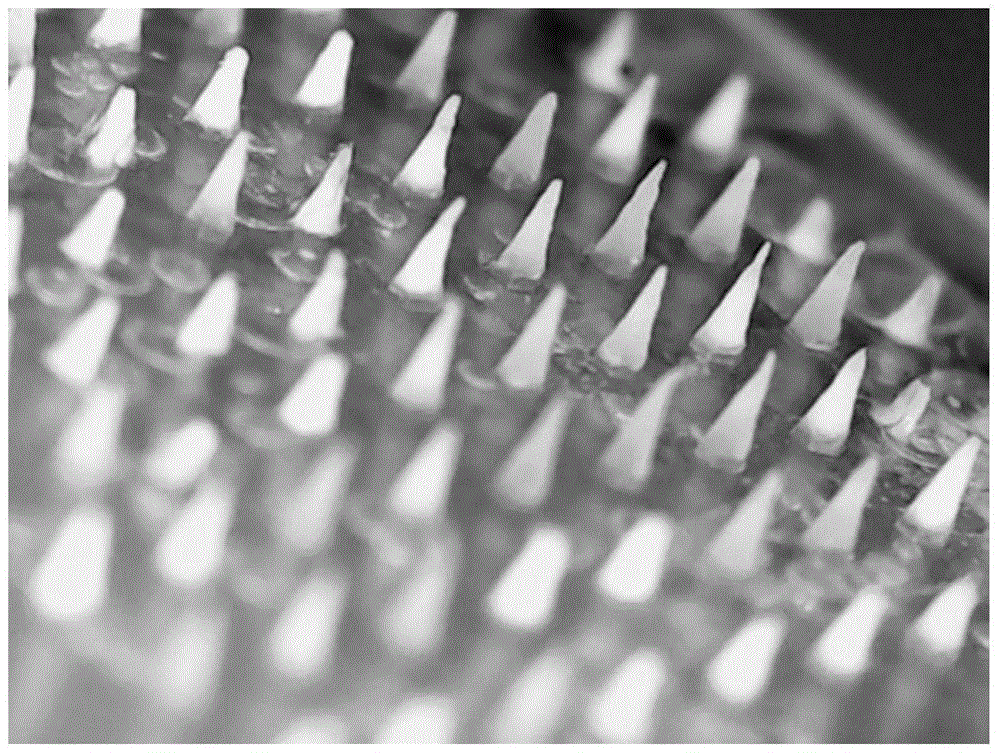
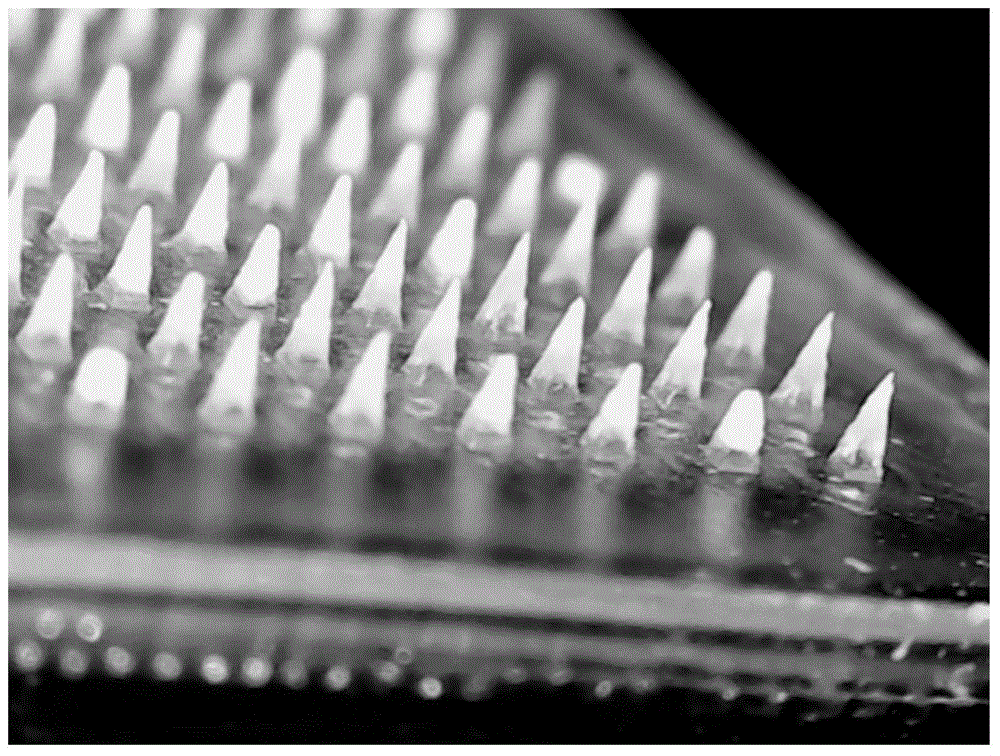
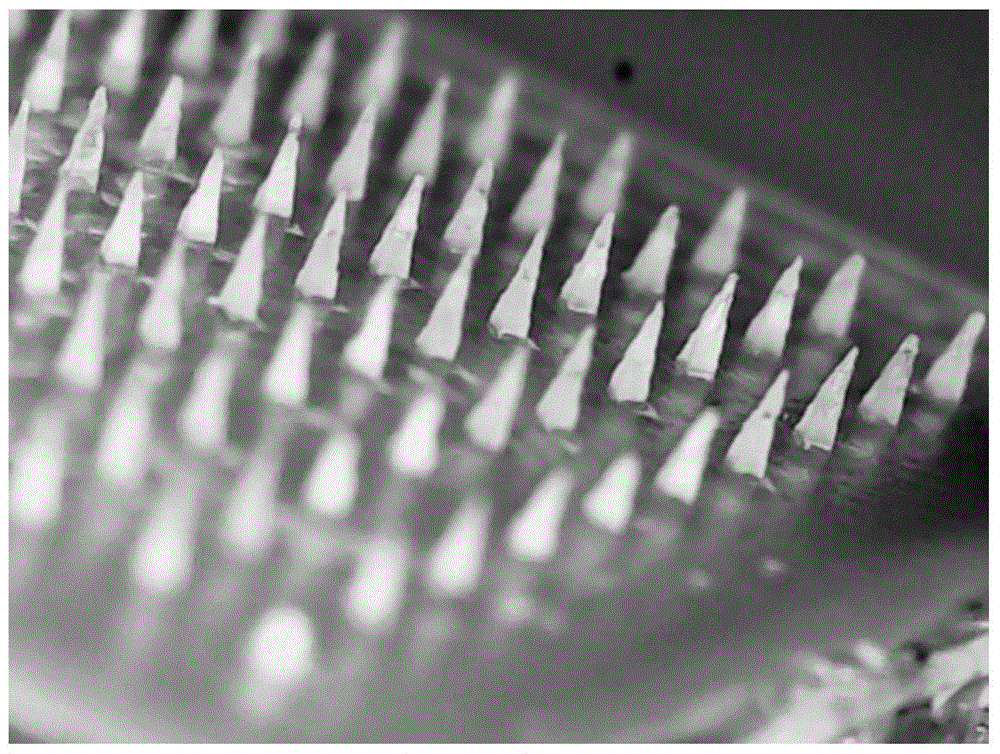
Temperature-sensitive soluble microneedle and preparation method thereof
ActiveCN105726458AFast dissolutionGood formabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIrritationPolyvinyl alcohol
The invention relates to a temperature-sensitive soluble microneedle and a preparation method thereof.The microneedle comprises a needle tip and a base layer, the needle tip is prepared from a temperature-sensitive material and a macromolecular shaping material, and the base layer is prepared from a macromolecular material; the temperature-sensitive material is composed of a material A and a material B, wherein the material A is selected from one or more of chitosan, polyvinyl alcohol and methylcellulose or hydroxypropyl methyl cellulose, the material B is selected from one or more of beta-sodium glycerophosphate, elastic protein polypeptides, poloxamer 184, poloxamer 188 and poloxamer 407, and the mass ratio of the material A to the material B is 1:(1-20); the mass ratio of the temperature-sensitive material to the macromolecular shaping material is 1:(1-10).The microneedle can be quickly dissolved in the skin, therefore, irritation of the microneedle to the skin is reduced, and medicine can be more safely and effectively released.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD +1
Pharmaceutical composition for treating periodontitis, and preparation method and application thereof
ActiveCN102240277ASpecial physical propertiesSensitive to temperatureOrganic active ingredientsAerosol deliveryControlled releaseLiquid state
The invention relates to a pharmaceutical composition for treating periodontitis and a preparation method thereof, wherein the pharmaceutical composition comprises chlorhexidine or derivatives thereof, poloxamer 407, a release speed adjusting agent, a stabilizer and other additives. The pharmaceutical composition has particular physical characteristics; when the temperature reaches the gel point, the composition can undergo phase transition from liquid to semisolid gel; the composition is liquid at the room temperature; after being used, the composition changes into semisolid gel quickly under the human body temperature condition and then is adhered on the surface of periodontal mucosa to release the medicine, thus a therapeutic effect is obtained; the composition has specific controlled-release property, and can achieve a controlled-release effect of 4 to 5 days of sustained medicine release by adjusting types and dosages of the release speed adjusting agents, thereby reducing the medicine using times, and improving the medication compliance of patients; and the composition is good in stability, and convenient to produce, transport and store.
Owner:SHENZHEN SOUTH CHINA PHARMA
Nasal administration gel preparation for treating brain diseases
ActiveCN102283801AImprove adhesionExtended stayOrganic active ingredientsNervous disorderDiseaseNasal cavity
The invention discloses a nasal drug delivery gel preparation for curing cerebral diseases. Water is used as solvent in the preparation. The preparation includes geniposide or total iridoid glycoside of cape jasmine fruit, poloxamer 407 and poloxamer 188. The phase transformation temperature of the preparation is 25-35 DEG C. Based on the weight of the preparation, the geniposide or total iridoid glycoside of cape jasmine fruit is 1-5%; the poloxamer 407 is 16-18%; the poloxamer 188 is 0-5%; preferably, based on the weight of the preparation, the preparation is composed of 2% of the geniposide, 16% of the poloxamer 407, 2.5% of the poloxamer 188, 0.2% of ethylparaben, 0.9% of sodium chloride and the balance of water; and the phase transformation temperature of the preparation is 25-33 DEG C. According to the preparation disclosed by the invention, the gel preparation has proper phase transformation temperature by adjusting kinds and proportions of gelling matrixes in the preparation; after the gel preparation is sprayed in a nasal cavity in the form of solution so that gel is rapidly formed under a body temperature condition, therefore, the contact time of the geniposide or total iridoid glycoside of cape jasmine fruit and nasal mucosa is increased; and the distribution of drugs in brain tissue is increased.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Injectable Isatis Root in-situ gel, and preparation method thereof
InactiveCN103520227ASimple preparation processGood physiological compatibilityAerosol deliveryOintment deliveryGel preparationAnti virus
The invention belongs to the field of medicinal preparations for animals, and relates to an injectable Isatis Root in-situ gel preparation, and a preparation method thereof. The injectable Isatis Root in-situ gel preparation comprises, by weight, 30.0-75.0 parts of an Isatis Root extract liquid, 14.5-31.5 parts of poloxamer 407, 0.7-9.5 parts of poloxamer 188, 0.02-10.0 parts of a high-molecular retarding agent, 0.01-10.0 parts of a solubilizing stabilizer, a proper amount of a pH adjusting agent and 0.01-3.0 parts of an antiseptic, and each milliliter of the obtained preparation equals to 1.5-5.0g of a dried raw medicine. The preparation is a free-flowing liquid at room temperature, forms a semisolid gel after intramuscular or hypodermic injection, is slowly released, realizes only one-time administration for one treatment course, and has the advantages of simple preparation, convenient administration and definite therapeutic effect. The preparation has anti-virus, anti-bacterium, anti-endotoxin and immunoloregulation effects, and is suitable for controlling diseases of animals comprising pigs, cattle, sheep, dogs, cats and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Temperature-sensitive gel for skin injury and preparation method of temperature-sensitive gel
InactiveCN106619489AEasy to administerNo stimulationOrganic active ingredientsPeptide/protein ingredientsBiocompatibility TestingSkin repair
The invention discloses a temperature-sensitive gel for a skin injury and a preparation method of the temperature-sensitive gel and relates to the technical field of skin repair administration. The temperature-sensitive gel is prepared from the following raw materials in parts by weight: 5-20 parts of poloxamer 407, 0.01-0.1 part of a bacteriostatic agent, 0.001-0.05 part of a human epidermal growth factor, 0.2-1.0 part of a humectant, 2-10 parts of sodium hyaluronate and the balance of water for injection water. The temperature-sensitive gel is prepared from the poloxamer 407 as a main raw material, and exists in a solution form at a low temperature; administration is convenient; the temperature-sensitive gel is converted into a gel state at a high temperature and is smooth and soft in surface, free of thrill to the administration part, free of foreign body sensation and good in biocompatibility; the sodium hyaluronate is very high in biocompatibility, beneficial to long-term moisture retention of the skin and beneficial to tissue cell regeneration and is capable of compacting the skin and preventing dehydration; and the human epidermal growth factor has an acceleration effect on mucosa repairing of the skin injury, so that the wound healing cycle is shortened to meet the structure design and clinical requirements.
Owner:XIAN HUIPU BIOTECH CO LTD
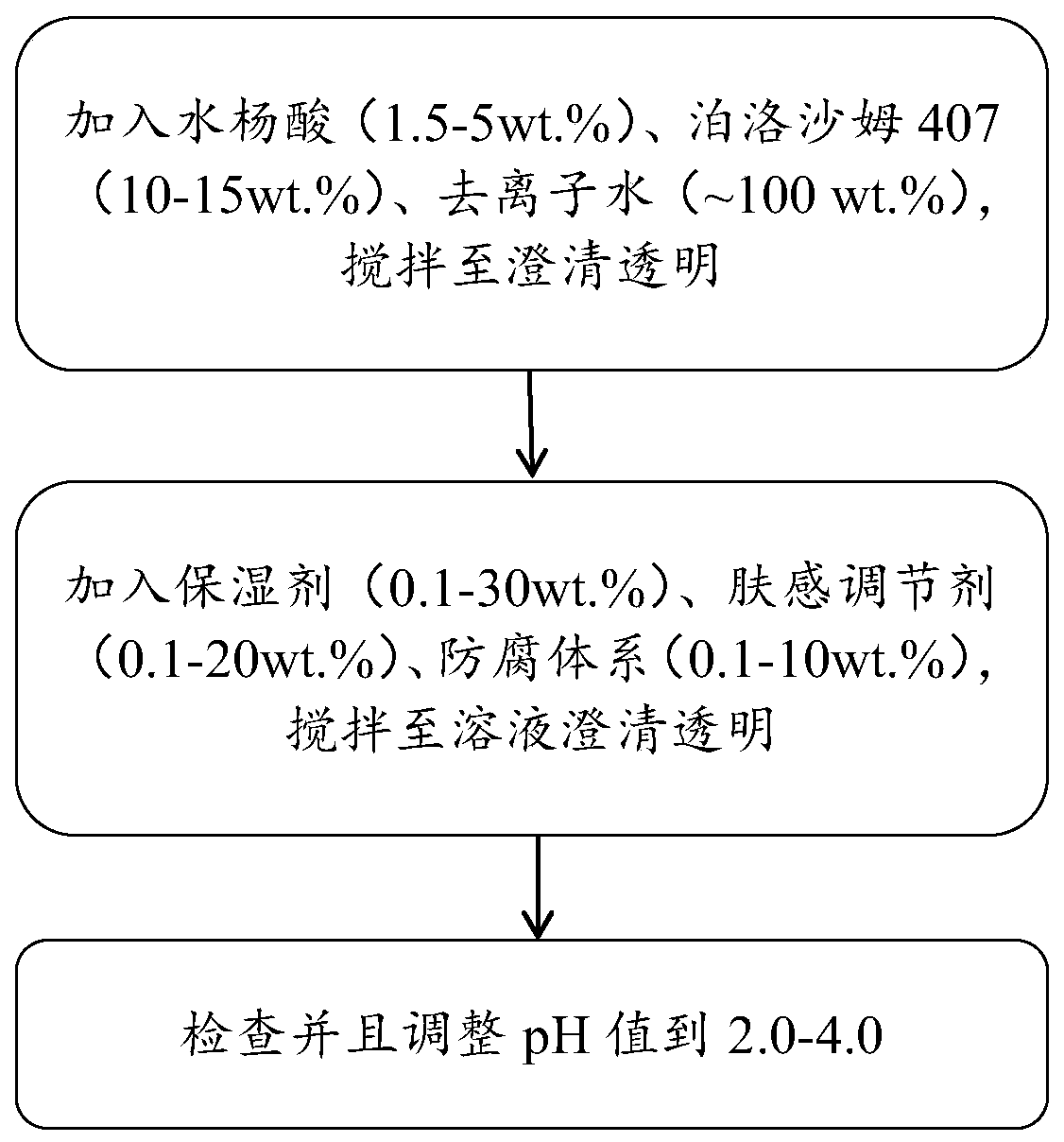
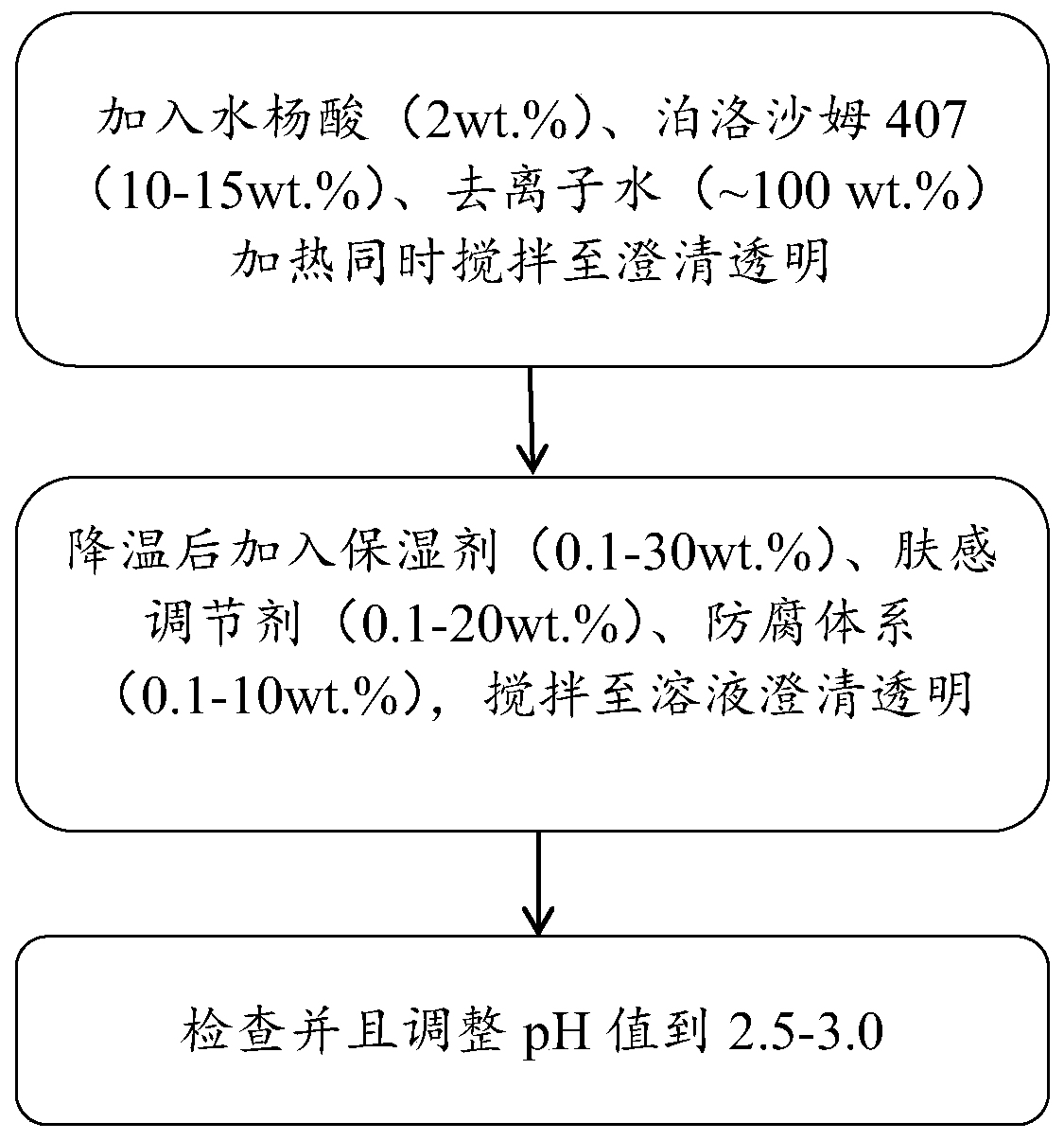
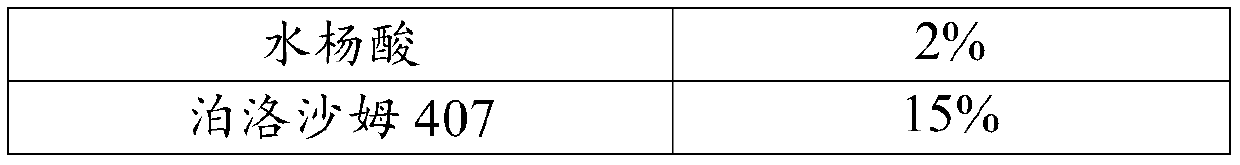
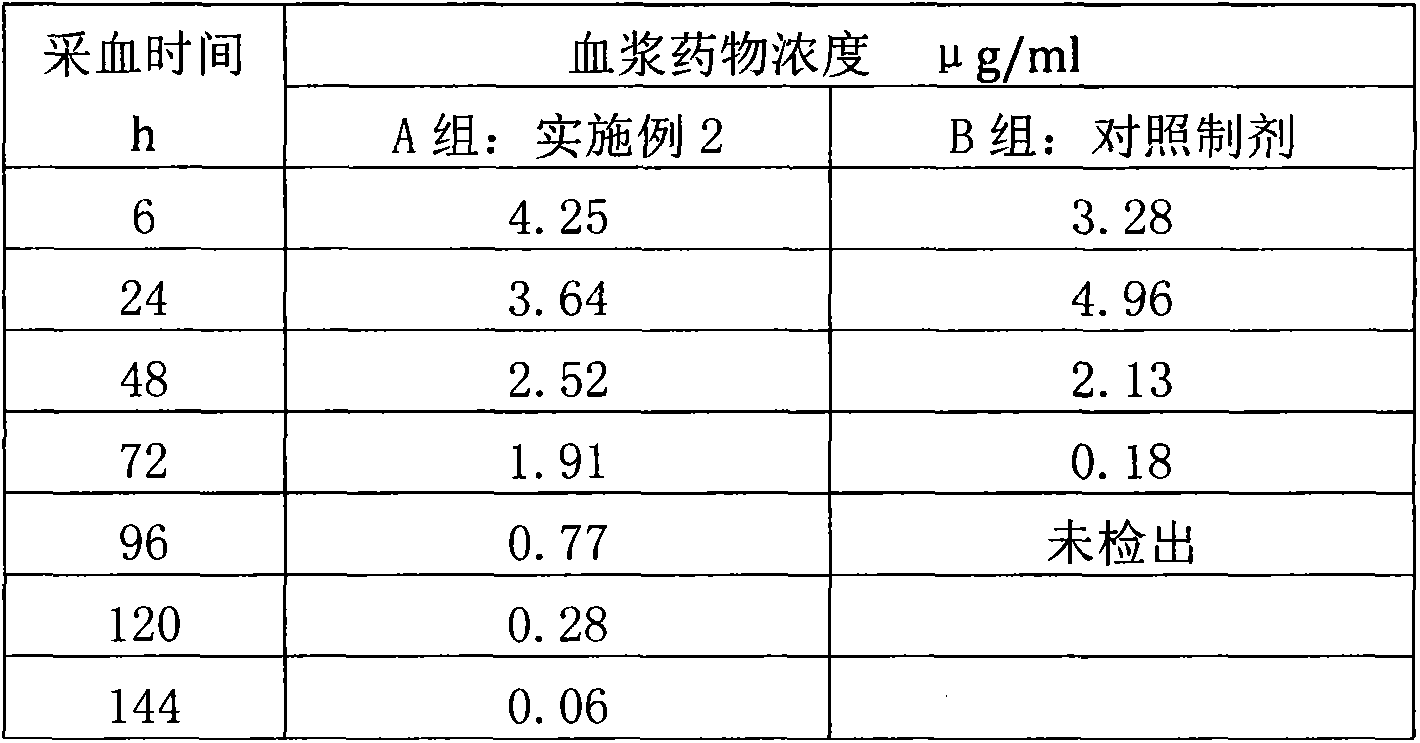
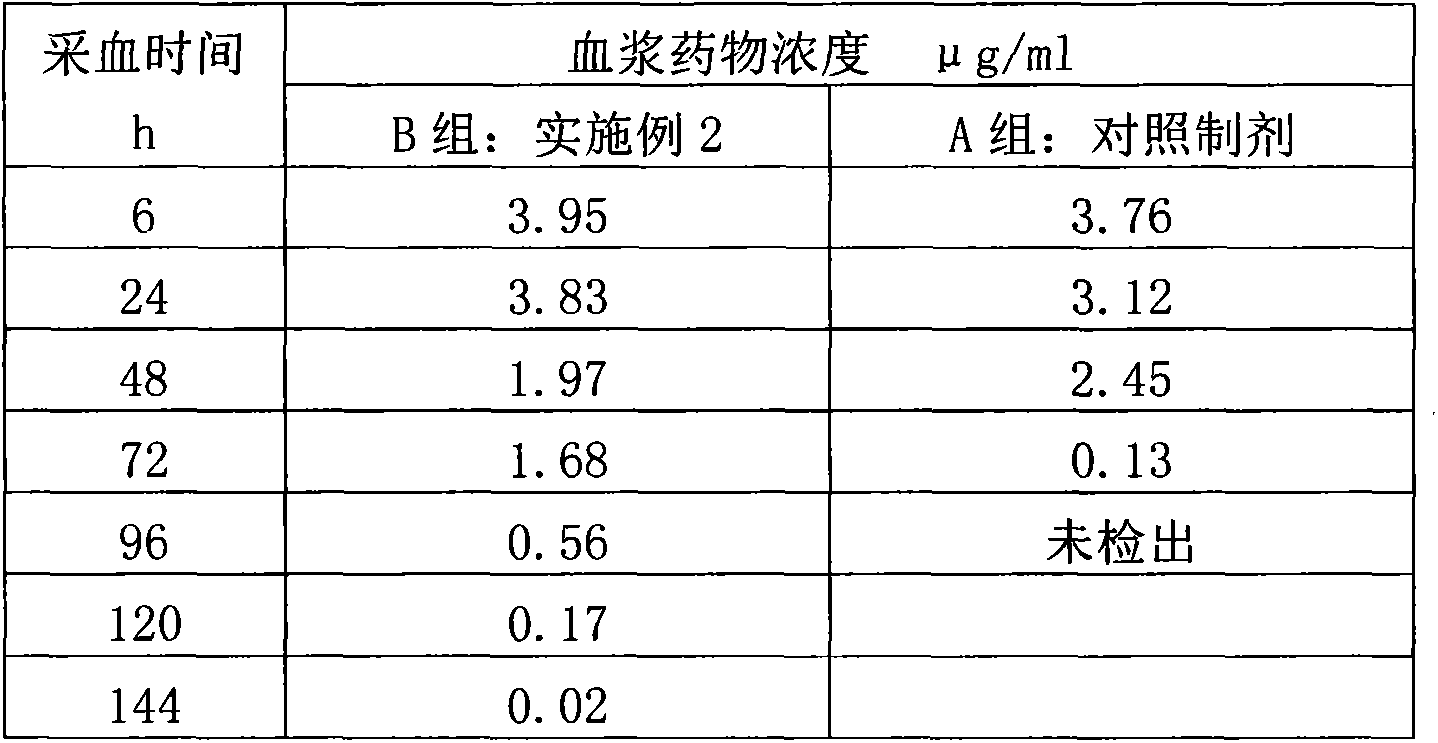
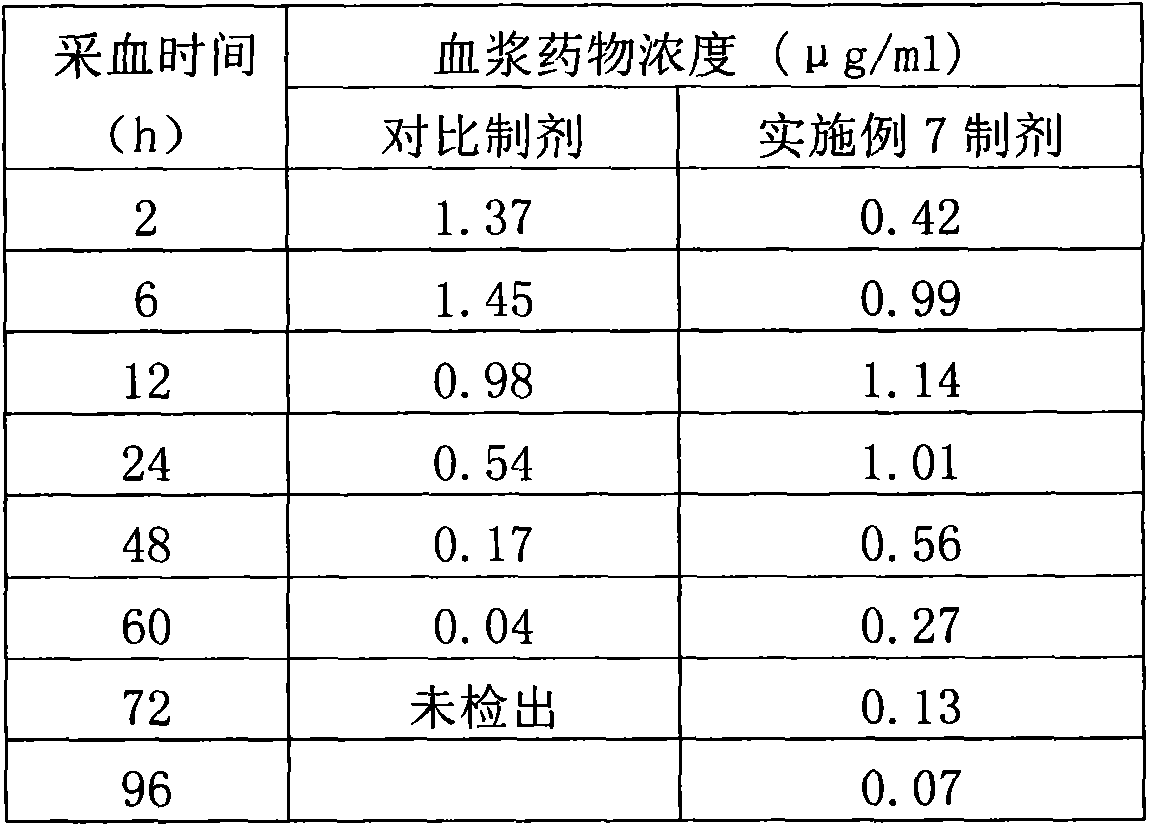
Salicylic acid solubilizing slow-release composition and preparation method and application thereof
InactiveCN109771333AImprove solubilityImprove stabilityCosmetic preparationsToilet preparationsSolubilityIrritation
The invention provides a salicylic acid solubilizing slow-release composition. The salicylic acid solubilizing slow-release composition is prepared from a specific proportion of active ingredient salicylic acid, solubilizing slow-release agent poloxamer 407, a humectant, a skin feeling conditioning agent, a preservative system and deionized water. The salicylic acid solubilizing slow-release composition has the advantages that under the conditions without alcohol and at a low pH value, the salicylic acid is prepared into a stable, easy-to-use and low-irritant water-soluble liquid, gel, or an intermediate state between the water-soluble liquid and the gel; on one hand, the solubility of the salicylic acid in an aqueous phase can be significantly improved, thereby increasing the biological utilization rate of the salicylic acid, on the other hand, the irritation of the salicylic acid to the skin can be significantly reduced, thereby solving the technical problem of the high-concentrationsalicylic acid intolerance of wide crowds. In addition, the invention further relates to a preparation method and application of the salicylic acid solubilizing slow-release composition.
Owner:HARMEN LEIMOGAOSHI ESSENCE
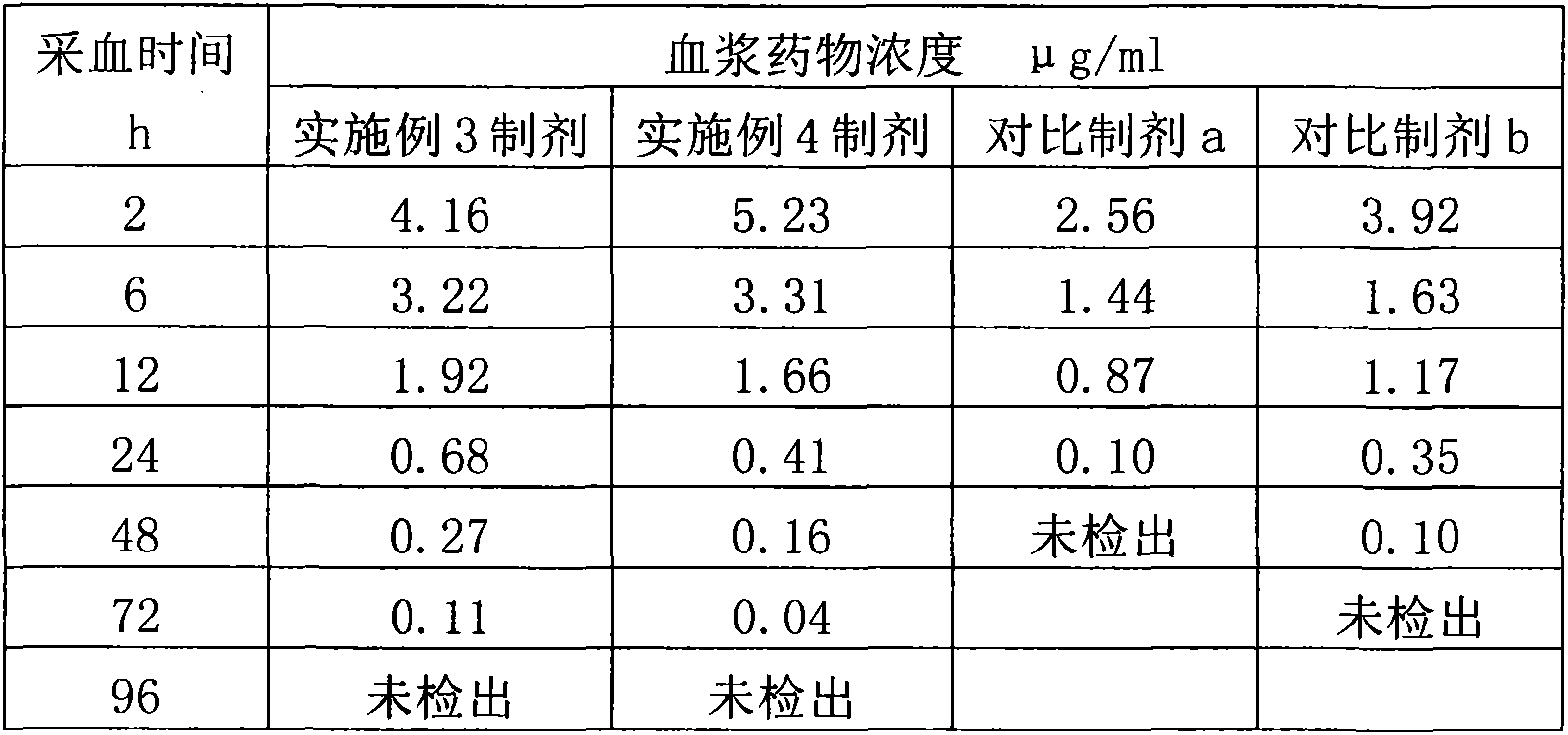
Antibacterial drug injection containing poloxamer 407 and oily medium
ActiveCN103705452AReduce dosageGood sustained release effectAntibacterial agentsSolution deliveryInjection siteMedicine
The invention discloses an oily long-acting injection containing antibacterial drug / poloxamer 407 drug-loading particles, which is prepared by combining the antibacterial drug with poloxamer 407 into drug-loading particles and further dispersing the drug-loading particles into an oily medium and grinding. Hydroxypropyl methyl cellulose or high-substituted hydroxypropyl cellulose also can be added into the drug-loading particles, and the sustained-release effect of the preparation is obviously enhanced. The preparation process of the injection is simple, the drug release is uniform, the biocompatibility is good, irreversible damage to the tissue of the injection part is avoided, and the adopted sustained-release carrier can be degraded and excreted.
Owner:荷本(北京)大药厂有限公司
Hyaluronic temperature-sensitive gel and preparation method and application thereof
InactiveCN105168238AImprove the bactericidal effectImprove repair effectAntibacterial agentsOrganic active ingredientsPolyethylene glycolGlycerol
The invention discloses hyaluronic temperature-sensitive gel and a preparation method and application thereof. The hyaluronic temperature-sensitive gel comprises, by mass, 0.01-0.3% of medium-molecular-weight hyaluronic acid, 0.01-0.5% of small-molecular-weight hyaluronic acid, 0.05-2% of chlorhexidine, 8-25% of poloxamer 407, 0.1-1% of hydroxypropyl methylcellulose, 0.05-0.5% of hydroxyethyl cellulose, 0.1-1% of polyethylene glycol, 0.1-1.0% of glycerol, 0.01-0.2% of pentanediol, 0.01-0.1% of octanediol and the balance of water. The hyaluronic temperature-sensitive gel has efficacy of moisturizing, repairing and sterilizing, can form a protecting film on the surface of skin or damaged skin and is suitable for body surface de-planting and sterilization nursing and sterile maintaining of barrier damaged skin of skin trauma and postoperative wound surface of inpatients.
Owner:NANJING TZONE BIOLOGICAL SCI & TECH
Temperature-sensitive nasal spray gel for treating rhinitis
InactiveCN105031615AReduce the risk of infectionPeptide/protein ingredientsAerosol deliveryNasal cavityAdhesive
The invention belongs to the field of medicine, and relates to temperature-sensitive nasal spray gel for rhinitis treatment and daily protection of the nasal cavity. The temperature-sensitive nasal spray gel contains poloxamer 407, a PLGA-PEG-PLGA (poly(lactic-co-glycolic acid-polyethylene glycol-poly(lactic-co-glycolic acid) three-block polymer, gulcomannan, an epidermal growth factor and water, wherein poloxamer 407 is taken as a temperature-sensitive material, and the using amount of poloxamer 407 is 15 to 25 percent; the PLGA-PEG-PLGA three-block polymer is taken as a phase change temperature regulator, and the using amount of the PLGA-PEG-PLGA three-block polymer is less than 2 percent; the PLGA-PEG-PLGA three-block polymer is the phase change temperature regulator; gulcomannan with a molecular weight of 1 to 3 millions is taken as a mucous adhesive, and the using amount of gulcomannan is 0.2 to 1 percent; the epidermal growth factor is taken as a mucous healing agent, and the using amount of the epidermal growth factor is less than 0.1 percent.
Owner:SUZHOU JIOU BIOTECH CO LTD
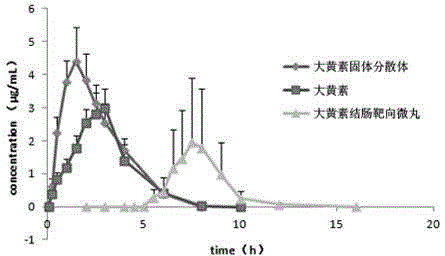
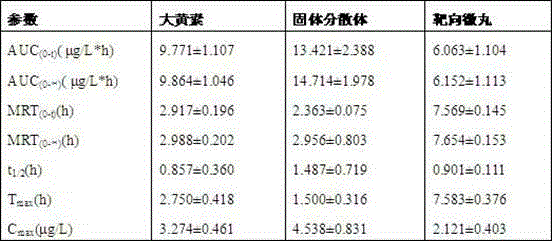
Emodin solid dispersion, drug-containing pellet core, colonic targeted micropill, and applications of three
InactiveCN103550158AImprove solubilityImprove bioavailabilityOrganic active ingredientsPowder deliveryPharmaceutical SubstancesOrganic chemistry
The invention relates to emodin solid dispersion, a drug-containing pellet core, a colonic targeted micropill, and applications of the three. The emodin solid dispersion is prepared from emodin and a carrier material, wherein the carrier material is one or several selected from poloxamer 188, poloxamer 407, Kollidon 12 PF, Kollidon VA 64, Kollicoat IR or Soluplus, and the weight ratio of emodin to the carrier material is 1:2-1:15. The drug-containing pellet core and the colonic targeted micropill both contain the emodin solid dispersion. The emodin solid dispersion is substantially improved in solubility of indissolvable drug emodin; and the targeted micropill helps to realize colonic positioning release in vivo / vitro of emodin, and has substantial protective effect on intestinal barrier of a rat severe acute pancreatitis pancreatitis model.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV +1
Method for topical treatment of carpal tunnel syndrome
InactiveUS6979441B2Easy to useInhibiting PKC activityBiocideKetone active ingredientsApigeninCTS - Carpal tunnel syndrome
This invention relates to the topical treatment of the Carpal Tunnel Syndrome by the use of a selected protein kinase C inhibitor and an effective penetrating agent selected from lecithin organogel or poloxamer 407 lecithin organogel. The protein kinase C inhibitors may be selected from sphingosine, sphinganine, phytosphingosine, curcumin, tetrahydrocurcumin, curcuminoids or apigenin.
Owner:CRANDALL WILSON T
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com