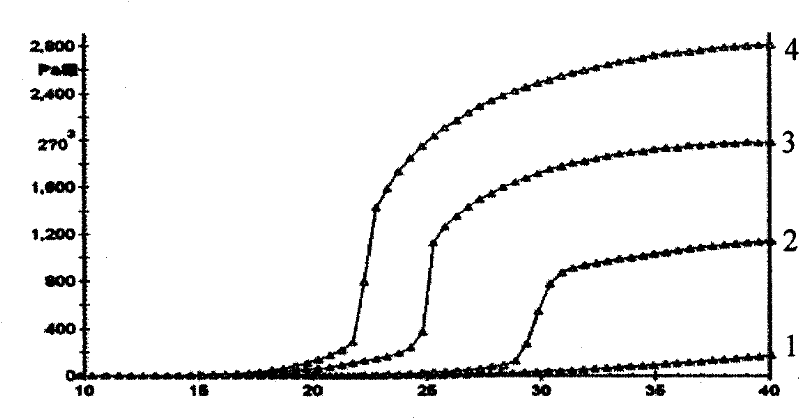
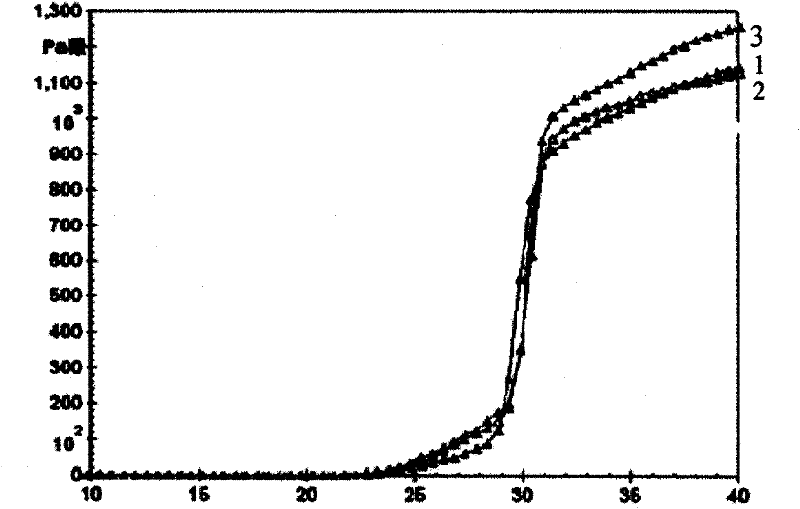
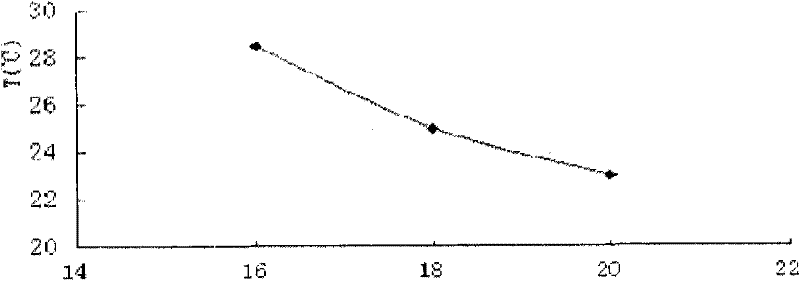
Nasal administration gel preparation for treating brain diseases
A technology of gel preparation and nasal administration, which is applied in the direction of blood diseases, antiviral agents, and extracellular fluid diseases, etc., to achieve the effects of improving compliance, obvious slow-release effect, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Embodiment 1 geniposide nasal administration gel preparation
[0245] 1) Weigh 5 g of geniposide, 16 g of poloxamer 407, 0.9 g of sodium chloride, and 0.2 g of ethylparaben;
[0246] 2) Dissolve geniposide, sodium chloride, and ethylparaben in water, add poloxamer 407 while stirring to make the dispersion uniform, and place it at 4°C for 24 hours to make the gel fully swell and disperse evenly to obtain The clear solution is filled into a nasal cavity inhalation device and made into a spray, that is, ready.
Embodiment 2
[0247] Embodiment 2 geniposide nasal administration gel preparation
[0248] 1) Weigh 2.5g of geniposide, 18g of poloxamer 407, 5g of poloxamer 188, 0.9g of sodium chloride, and 0.2g of ethylparaben;
[0249] 2) Dissolve geniposide, sodium chloride, and ethylparaben in water, add Poloxamer 407 and Poloxamer 188 while stirring to make the dispersion uniform, and place it at 4°C for 24 hours to make the gel Fully swell and disperse to obtain a clear solution, put it into a nasal cavity inhalation device, and make it into a spray.
Embodiment 3
[0250] The preparation of embodiment 3 gardenia total iridoid glycosides
[0251] Take 1 kg of gardenia coarse powder, add 6 times of water to decoct 3 times, each time for 30 minutes, filter, combine the decoction, concentrate to 1000 ml of thick paste (each 1 ml is equivalent to 1 g of medicinal materials), add 730 ml of 95% ethanol to contain Alcohol content 40%, stand for 24 hours, take the supernatant, recycle ethanol, concentrate to 500ml of thick cream with no alcohol smell (each 1ml is equivalent to 2g of medicinal materials), add 2700ml of 95% ethanol to alcohol content of 80%, stand for 24 hours, Filter, the filtrate is concentrated to a relative density of 1.05 to 1.10 (60° C.), and dried to obtain 130 g of dry cream. The content of total iridoid glycosides of geniposide is determined to be 55% by ultraviolet spectrophotometry, and the content of total iridoid glycosides of geniposide is determined by high-performance liquid phase. The glycoside content is 26.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com