Nano cubic liquid crystal dexamethasone preparation for eye and preparation method thereof
A technology of nano-cubic liquid crystals and dexamethasone, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems that the application of nano-cubic liquid crystals has not been reported yet, and achieve the goal of promoting transparency. The effect of passing the corneal barrier, high comfort, and excellent stability
Inactive Publication Date: 2009-08-12
CHINA PHARM UNIV
View PDF1 Cites 23 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, there have been research reports on the carrier in the fields of skin administration, intravenous injection and oral adm
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
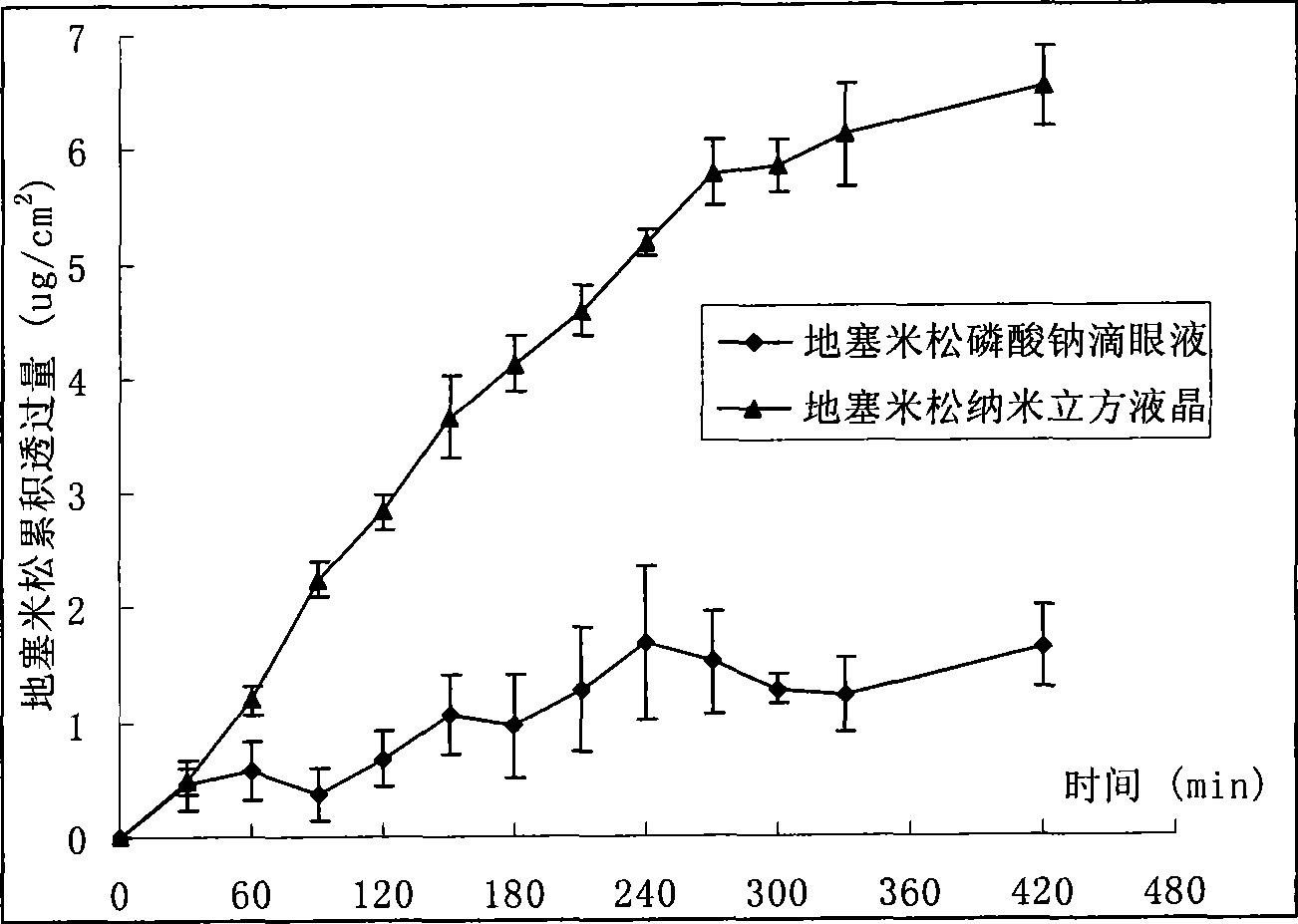
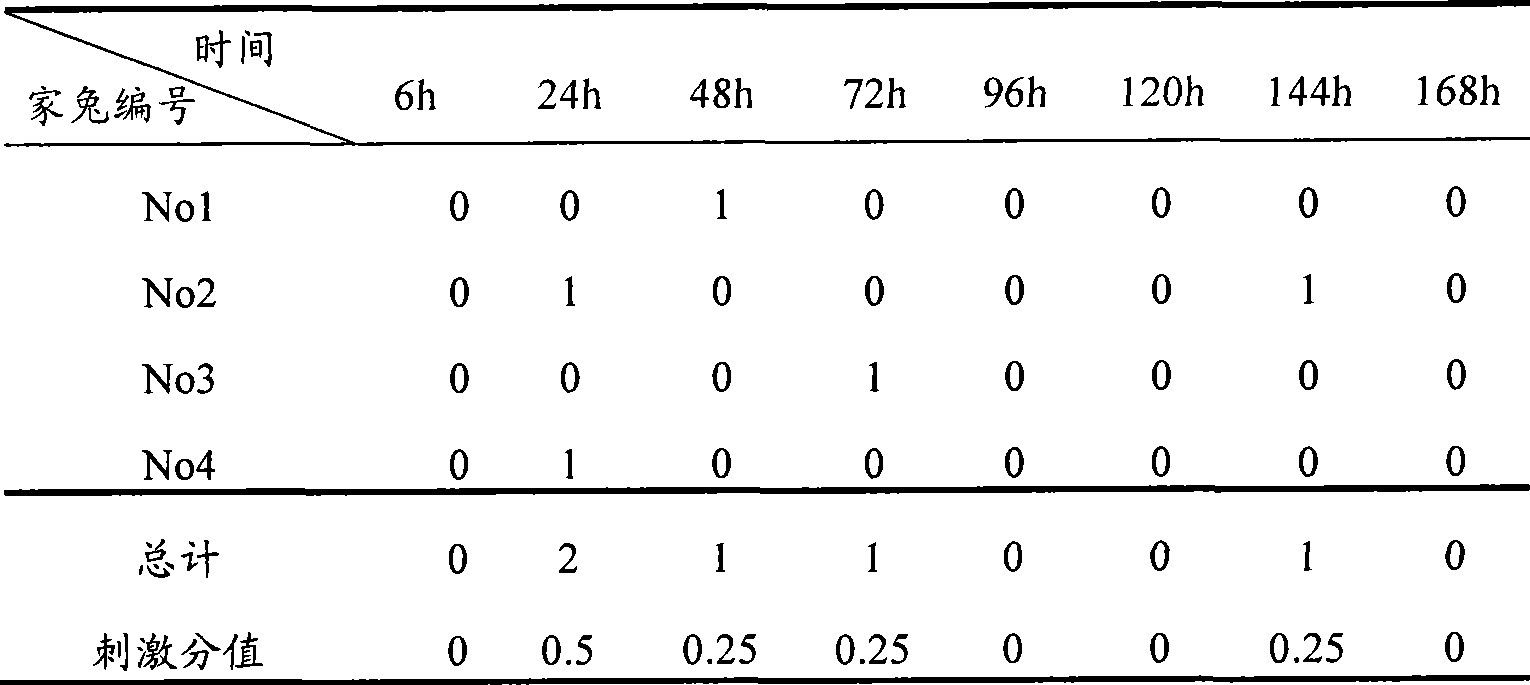
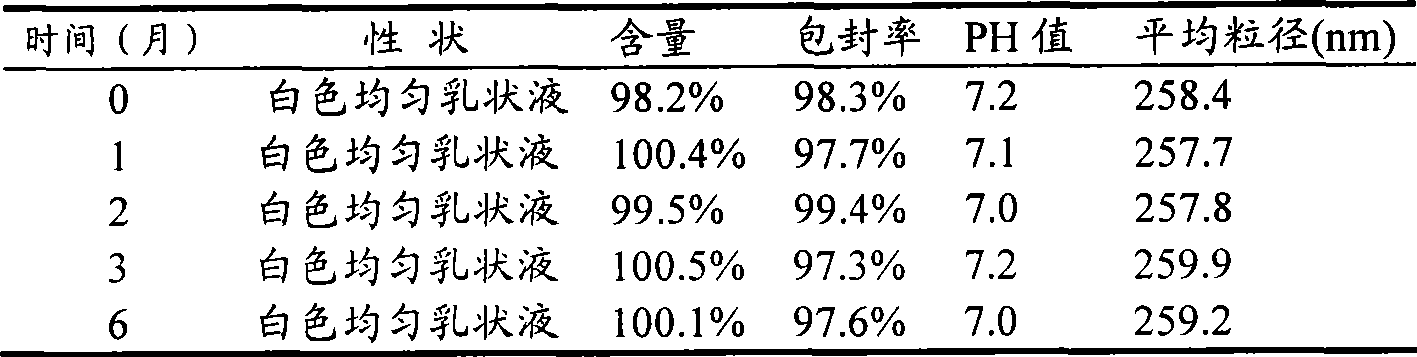
The invention relates to a dexamethasone ophthalmic nano-cubic liquid-crystal preparation and a preparation method thereof. The dexamethasone ophthalmic nanocube crystal preparation is prepared from lipid materials, an emulsifying agent, a thickening agent, an osmotic pressure regulating agent, a bacteriostatic agent, a pH value regulating agent and purified water and characterized in that the amphipathic lipid capable of forming the cubic liquid-crystal morphology, one or more out of glyceryl monooleate (GMO), phytantriol and lecithin are adopted as the lipid materials; and the safe and non-irritant ophthalmic surfactant, one or more out of poloxamer 407 and polyvinyl alcohol (PVA) are adopted as the emulsifying agent. .The invention realizes the ophthalmic administration of dexamethasone, a fat-soluble drug, and has the advantages of high comfort level and non-irritant administration; and the invention can promote the drug to permeate into the corneal barriers after the ophthalmic administration, thereby achieving the effect of improving the ophthalmic bioavailability.
Description
Technical field [0001] The invention relates to a novel ophthalmic preparation of dexamethasone, in particular to a nano cubic liquid crystal preparation for ophthalmic use of dexamethasone. The invention also relates to a preparation method of the dexamethasone ophthalmic nano cubic liquid crystal preparation. Background technique [0002] Uveitis is a common eye disease. In the western eye diseases that cause blindness, uveitis accounts for 10%-15%. The main clinical manifestations are iridocyclitis, choroiditis and so on. The pathogenesis of uveitis is complicated. Except for infectious factors, most of them are caused by autoimmune response. Uveitis is regarded as a "difficult disease" in ophthalmology, and its treatment is quite difficult. [0003] The front of the eyeball is dense corneal tissue. The normal corneal epithelial layer is composed of 6-7 layers of epithelial cells, and there are close connections between epithelial cells. While performing normal physiological p...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/00A61K31/573A61K47/38A61K47/36A61K47/34A61K47/32A61P27/02A61K47/10
Inventor 朱家壁甘莉甘勇
Owner CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com