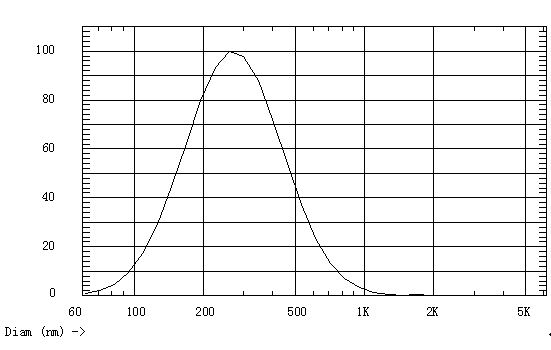
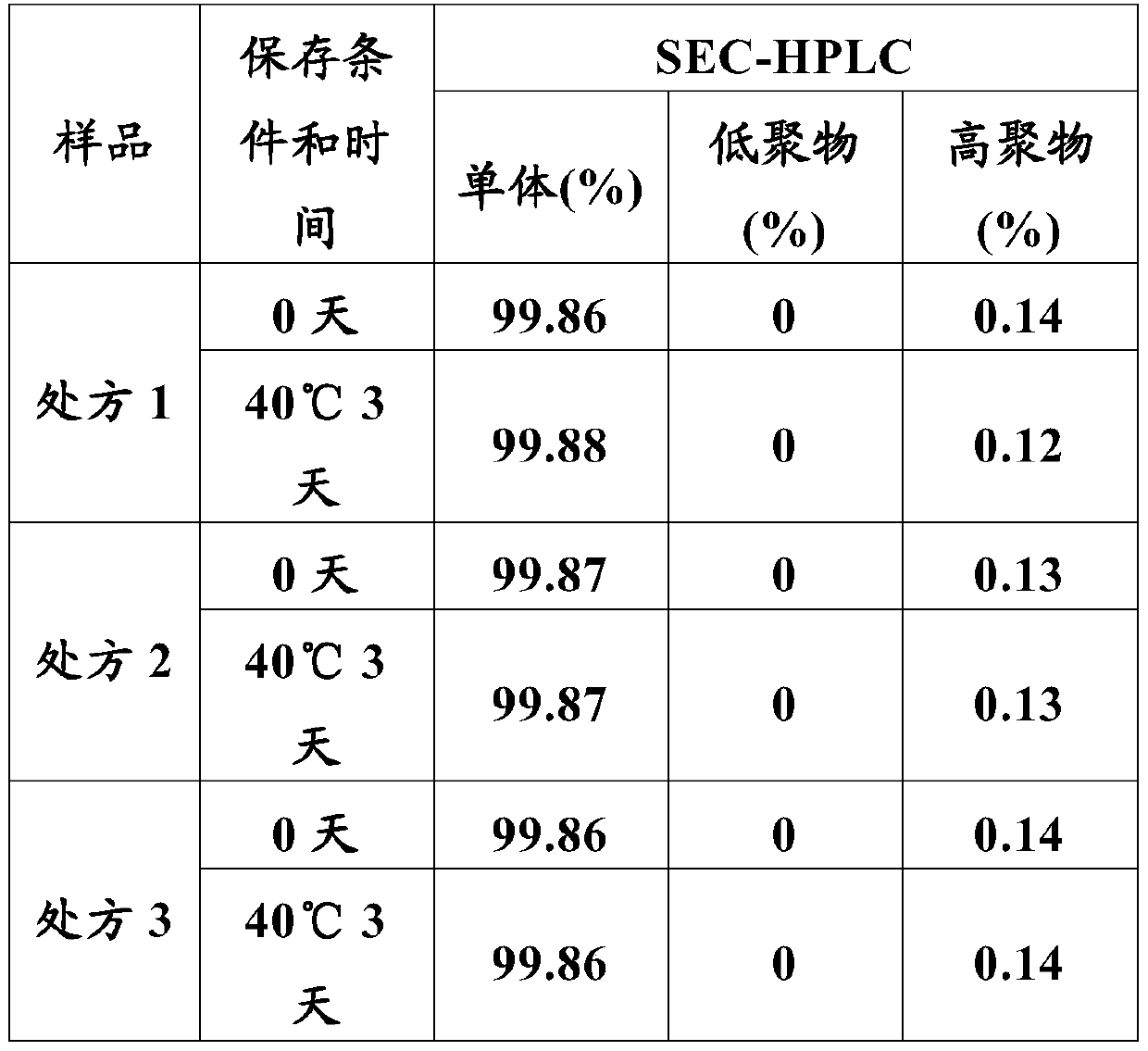
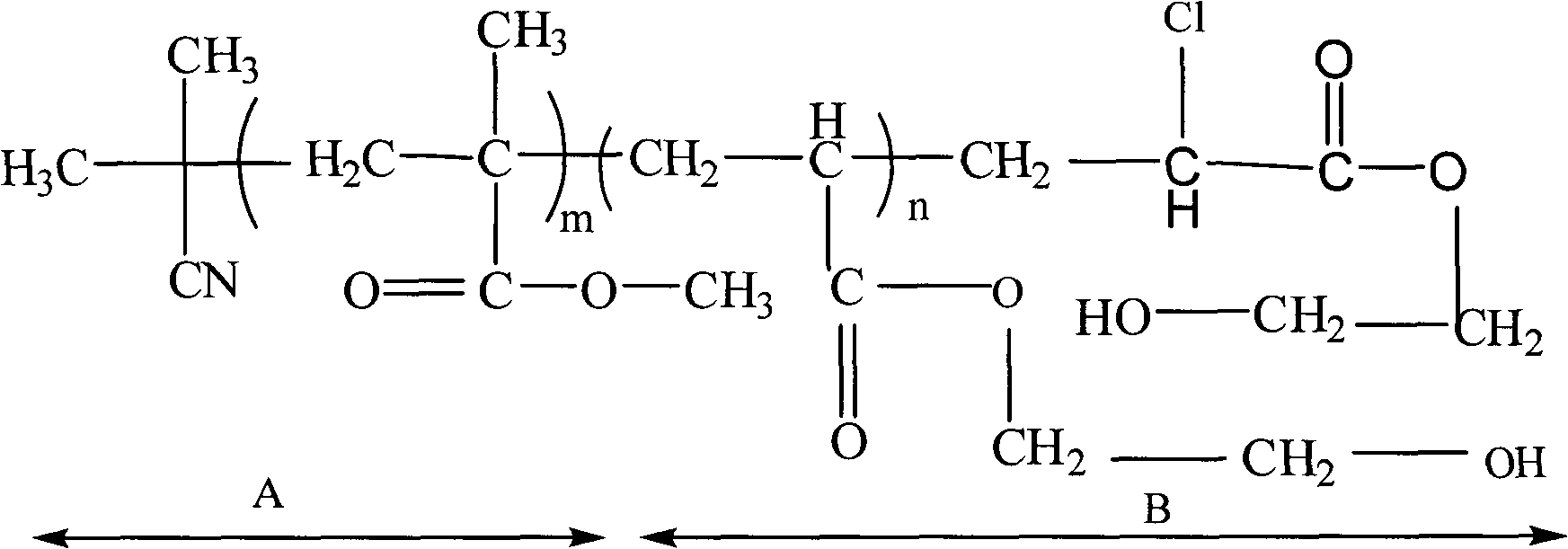
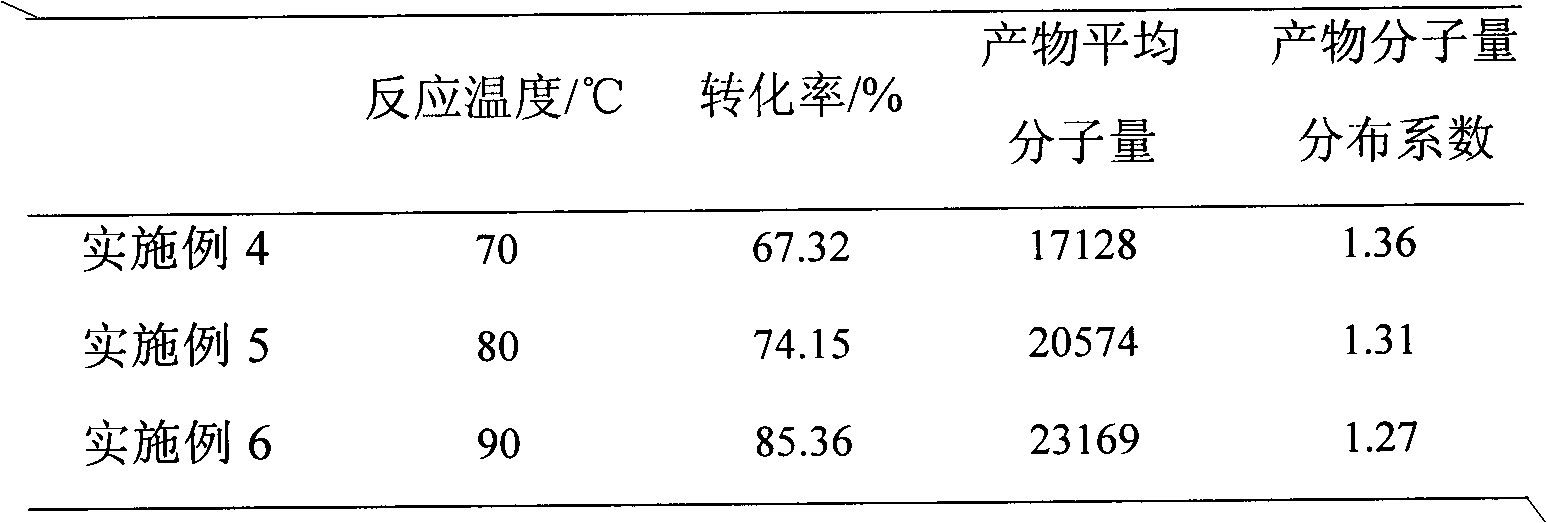
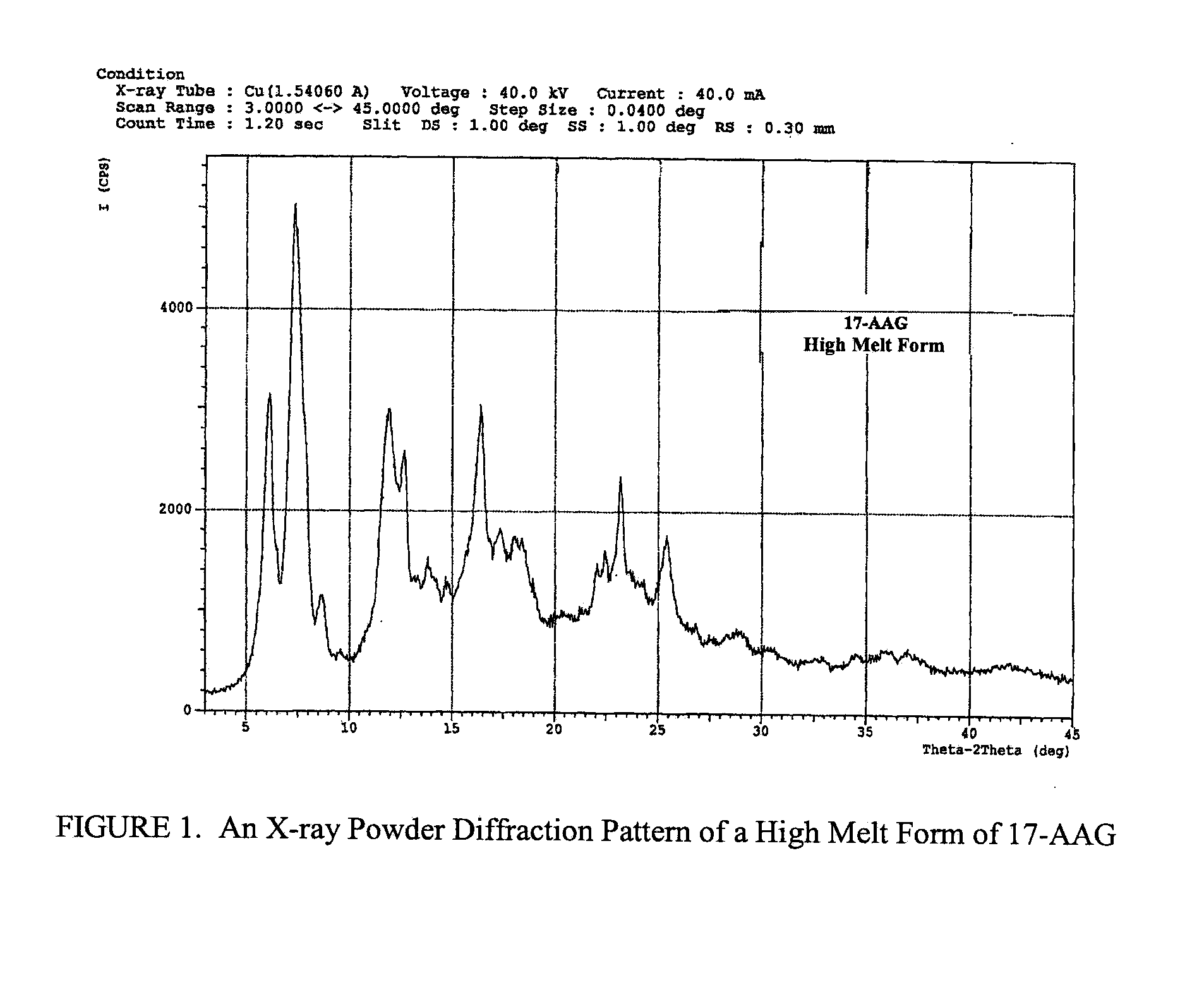
Patents
Literature
62results about How to "Good physiological compatibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
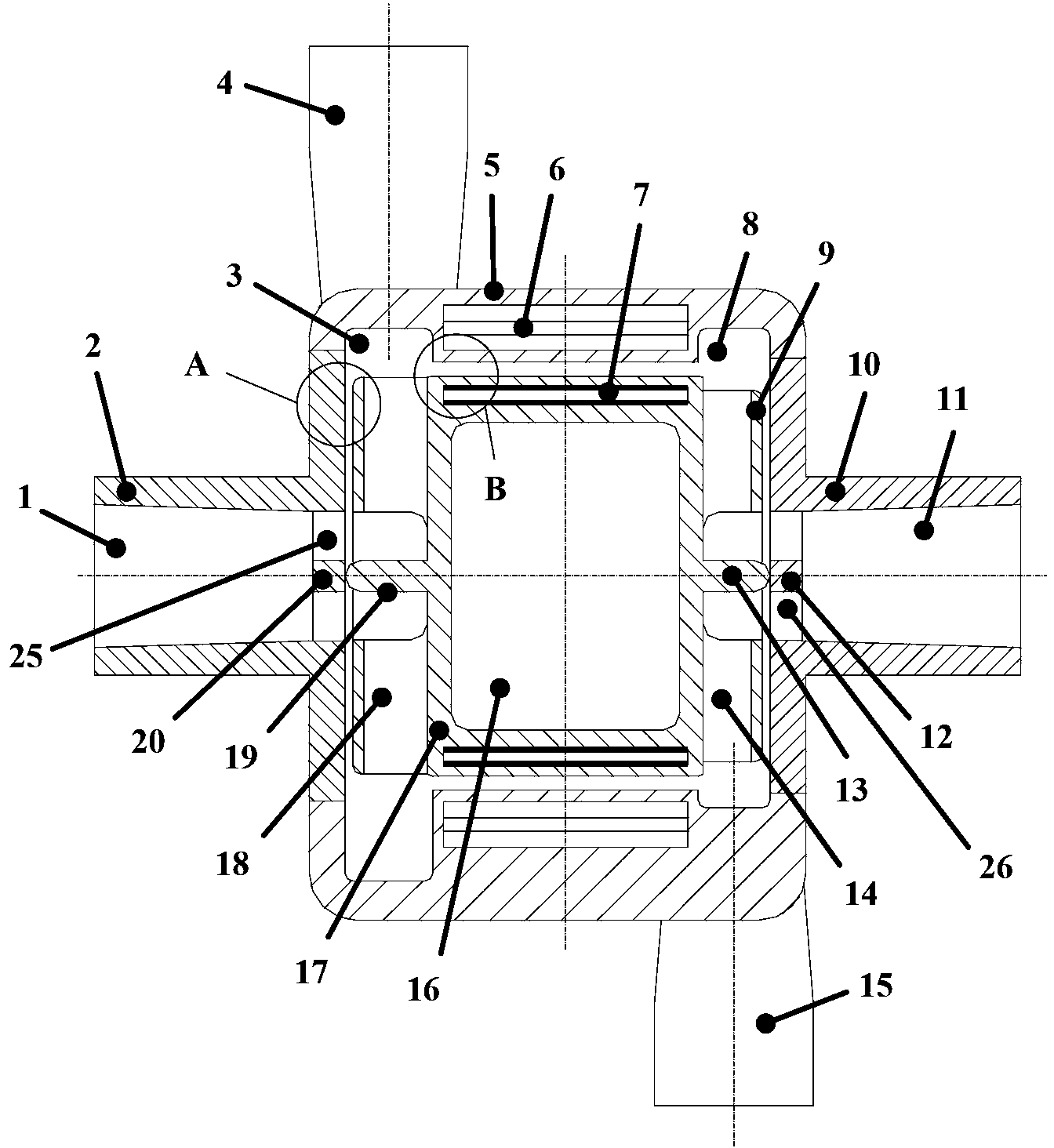
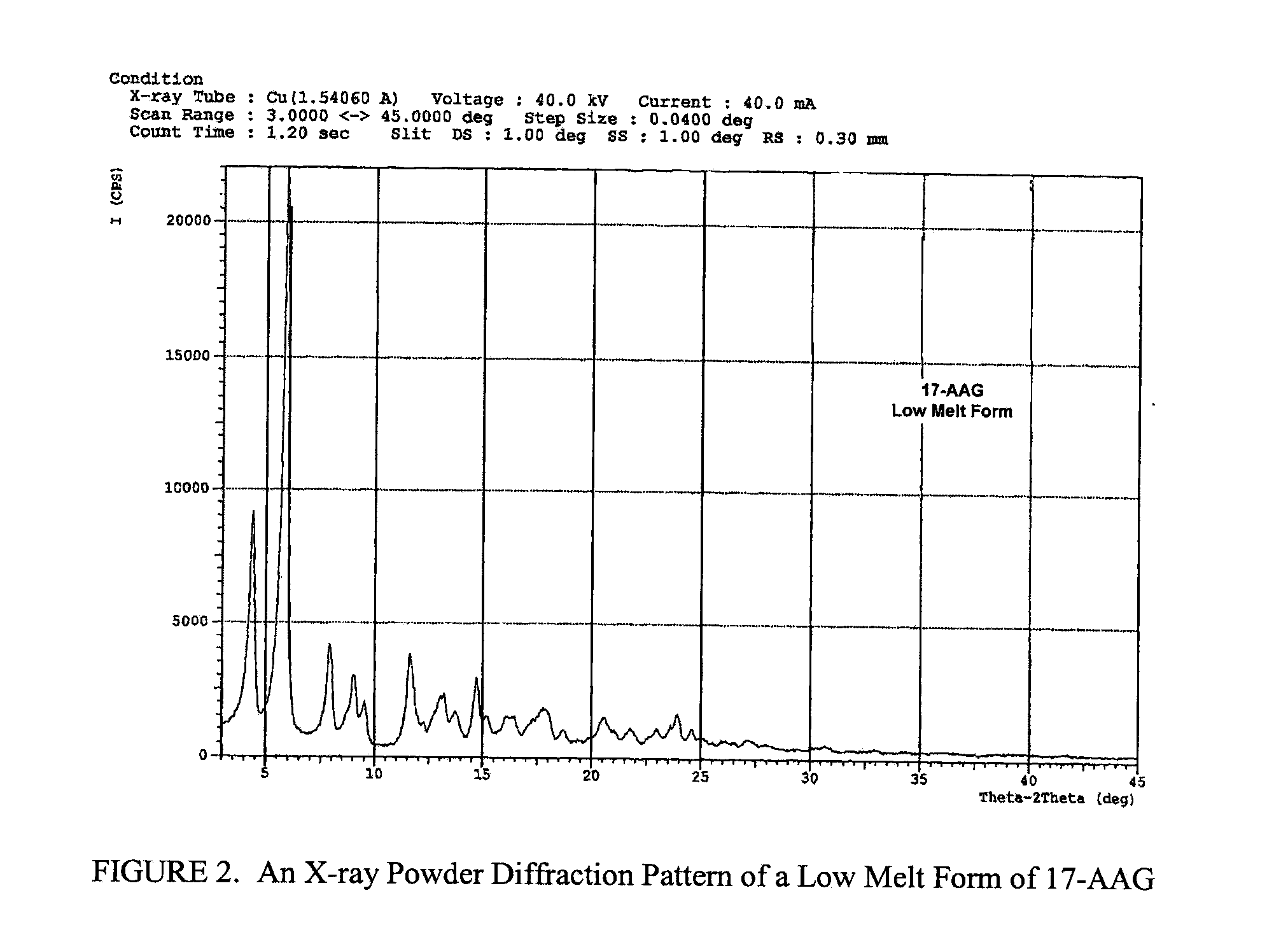
Implant for vessel ligature
InactiveUS20050266041A1Excellent propertyHighly physiological effectSurgeryBlood vesselsLanthanideOrthodontic ligature
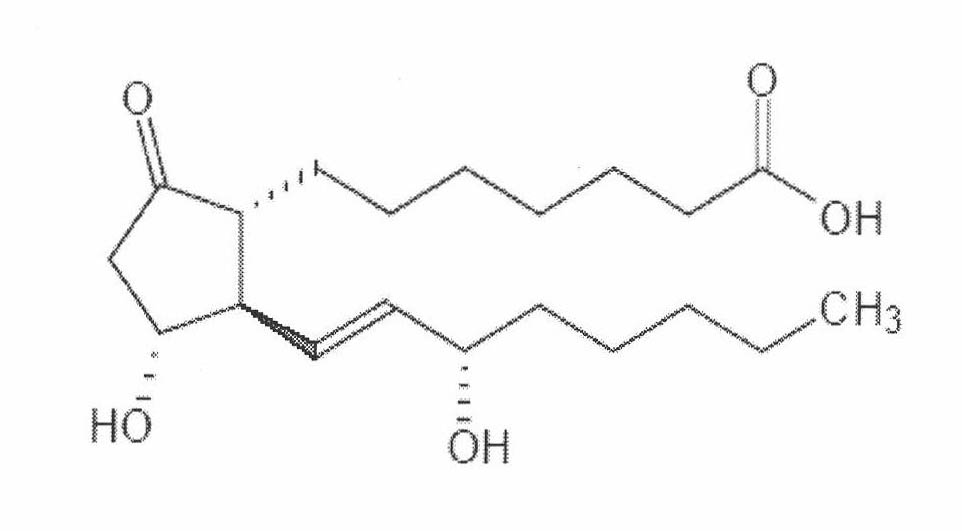
The invention concerns inter alia an implant for vessel ligature, the implant which comprises an alloy, wherein the alloy is at least partially biodegradable, and wherein the alloy comprises: greater than 87% magnesium; from about 3% to about 6% yttrium; from about 1% to about 5% lanthanide; and a balance of about 0.0% to about 2%.
Owner:BIOTRONIK VI PATENT
Mixture composition comprising rhamnolipids
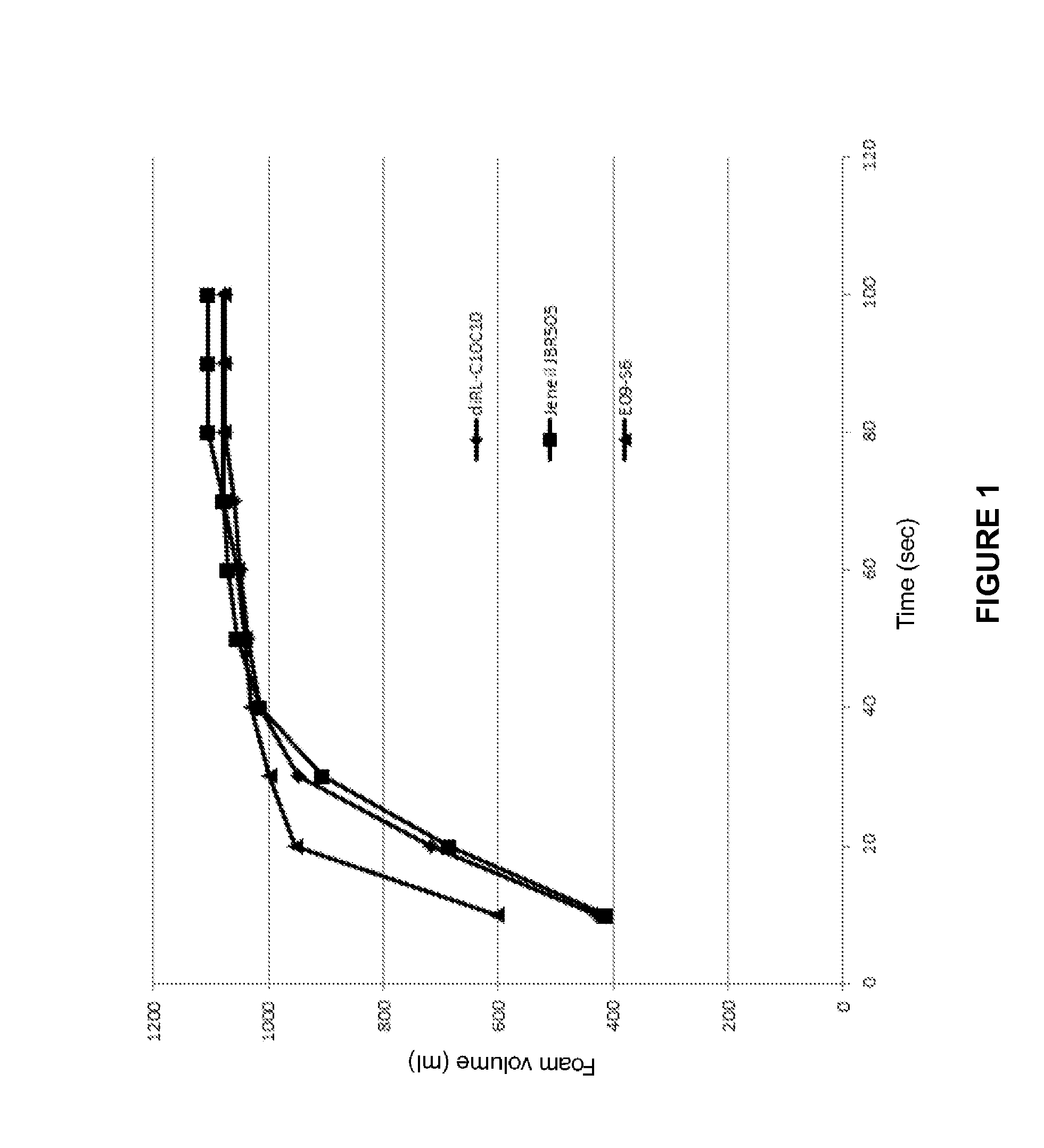
ActiveUS20140296168A1Good physiological compatibilityIncrease valueBiocideCosmetic preparationsRhamnolipidChemistry
Owner:EVONIK OPERATIONS GMBH
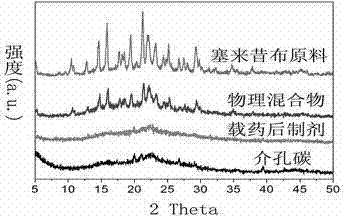
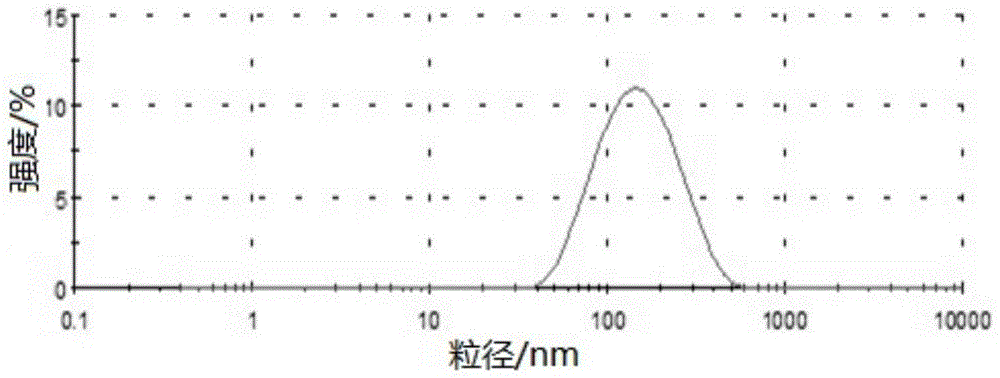
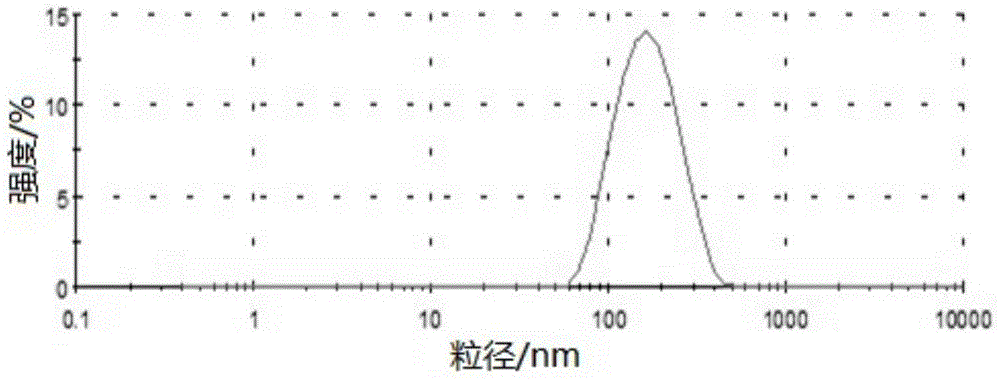
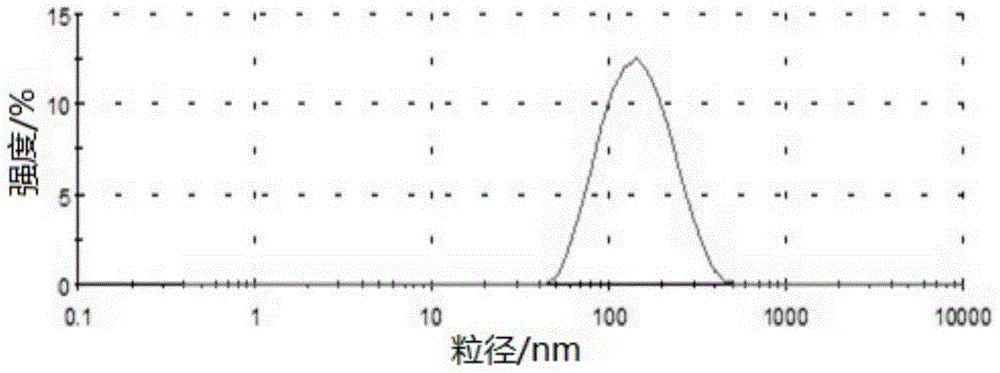
Preparation method and applications of mesoporous carbon
InactiveCN103086346ALarge specific surface areaUniform particle sizePowder deliveryCarbon preparation/purificationSucroseSide effect
The invention belongs to the technical field of medicine, and relates to mesoporous carbon and a preparation process thereof, and an application of the mesoporous carbon in insoluble drug delivery systems. According to the process, a hard template method is adopted to prepare the mesoporous carbon, wherein mesoporous silica is adopted as a template, sucrose is adopted as a carbon source, and sulfuric acid is adopted as a catalyst to prepare the mesoporous carbon, wherein the prepared mesoporous carbon has characteristics of large specific surface area, stable property, no toxic-side effect and good biocompatibility, and can be adopted as a insoluble drug carrier. According to the present invention, a solvent method and a melting method are adopted to carry out drug embedding and adsorption so as to achieve uniform dispersion of the drug in the pores and on the surface of the carrier; with the drug delivery system, water solubility of insoluble drugs can be significantly enhanced, and in vitro dissolution rate and oral bioavailability can be improved.
Owner:SHENYANG PHARMA UNIVERSITY
Nano-structural lipid carrier pharmaceutical composition and preparation method thereof
InactiveCN105708799ANo harmGood physiological compatibilityPowder deliveryMetabolism disorderLipid formationLiquid state
The invention discloses a nano-structural lipid carrier pharmaceutical composition and a preparation method thereof. The pharmaceutical composition consists of the following components in percentage by weight: 0.02-1% of an insoluble drug, 0.3-15% of a solid-state lipid material, 0.5-20% of a liquid-state liquid material, 0.8-10% of a fat-soluble emulsifier, 1-15% of a water-soluble emulsifier and 39-97.38% of an aqueous solvent. The nano-structural lipid carrier pharmaceutical composition prepared by the invention can greatly overcome the shortcomings of the insoluble drug which is not easy to dissolve in water, low in oral bioavailability and the like. The preparation method is simple and controllable, good in repeatability, and an available drug delivery system is provided for the insoluble drug.
Owner:金银秀 +1
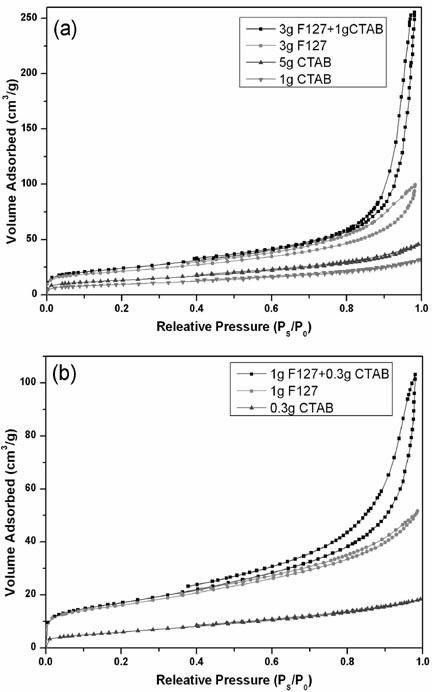
Porous hydroxyapatite and preparation method and application thereof
InactiveCN102583286AIncrease the areaGood dispersionInorganic non-active ingredientsPhosphorus compoundsMeth-Medicine
The invention belongs to the technical field of medicaments and relates to porous hydroxyapatite, a preparation process thereof and application thereof to difficultly soluble medicament feeding system. A hydrothermal template method is adopted in the process, namely, a triblock copolymer F127 and hexadecyl trimethyl ammonium bromide (CTAB) serve as templates to prepare the porous hydroxyapatite under a hydrothermal condition. The porous hydroxyapatite is large in specific surface area, stable in performance and good in biocompatibility, does not have toxic or side effects, has a plurality of hydrophilic groups on the surface, and is suitable to be used as a carrier of a difficultly soluble medicament. The medicament is embedded and absorbed by a solvent method or a melting method, so that the medicament is uniformly dispersed in the aperture of the carrier and on the surface of the carrier. By the system, the water-solubility of the difficultly soluble medicament can be obviously improved, the dissolution speed is improved, and the bioavailability of the medicament is improved when the medicament is orally taken.
Owner:SHENYANG PHARMA UNIVERSITY
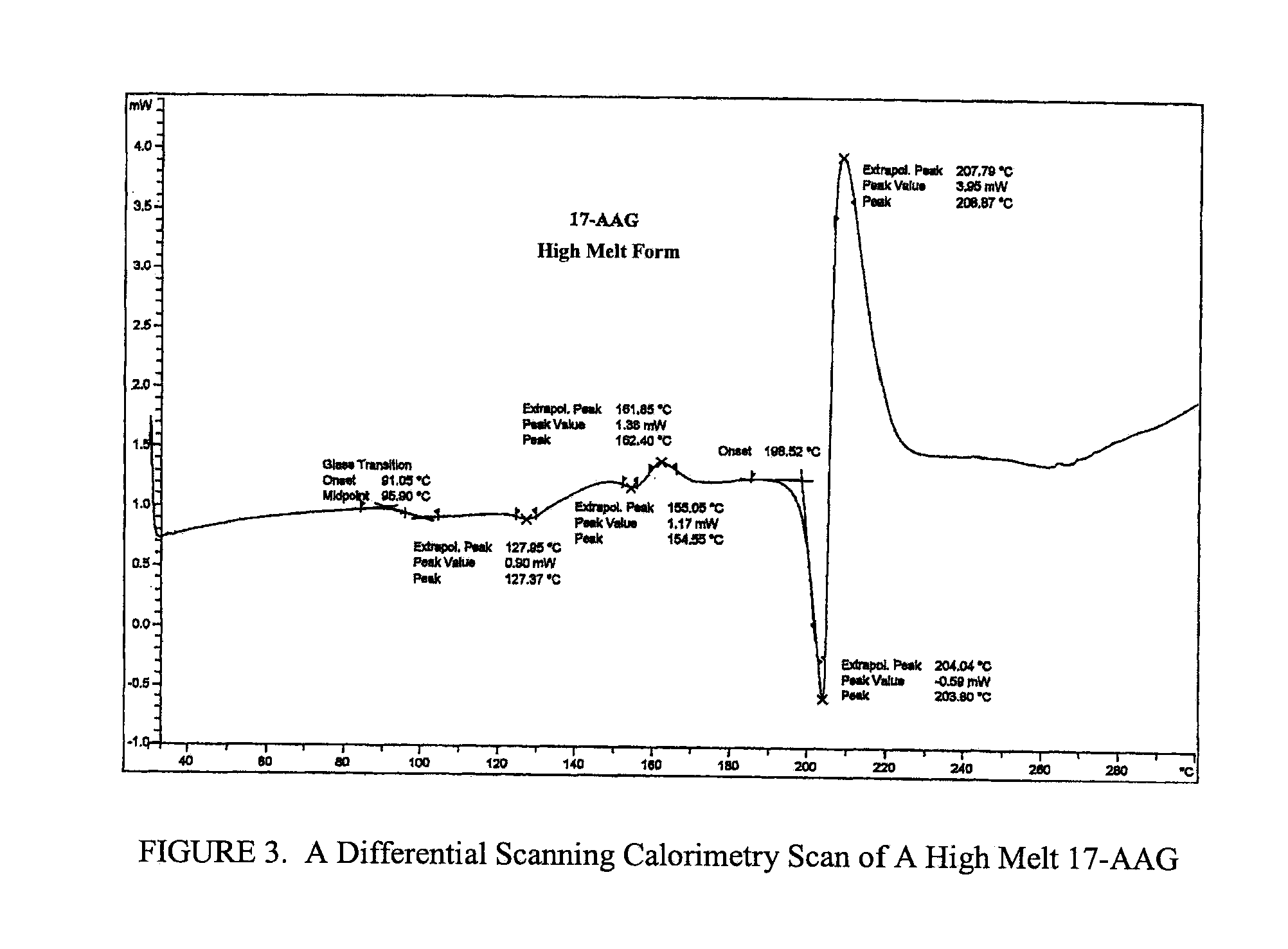
Phospholipid-based pharmaceutical formulations and methods for producing and using same
InactiveUS20060228405A1Good physiological compatibilityImprove abilitiesAntibacterial agentsBiocideCompound (substance)Triglyceride
Pharmaceutical formulations and methods of producing and using the same are described and claimed. The formulations are dispersions of phospholipids and one or more pharmacologically active compounds, pharmaceutically acceptable salts thereof, or prodrugs thereof. In specific embodiments, the pharmaceutically active compounds are ansamycins and the overall formulation is substantially devoid of medium and long chain triglycerides.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Oil-in-water type garlicin-garlic oil sub-microemulsion as well as method for preparing same
InactiveCN101524459AImprove physical stabilityGood chemical stabilityAntibacterial agentsOrganic active ingredientsDiseaseAntioxidant
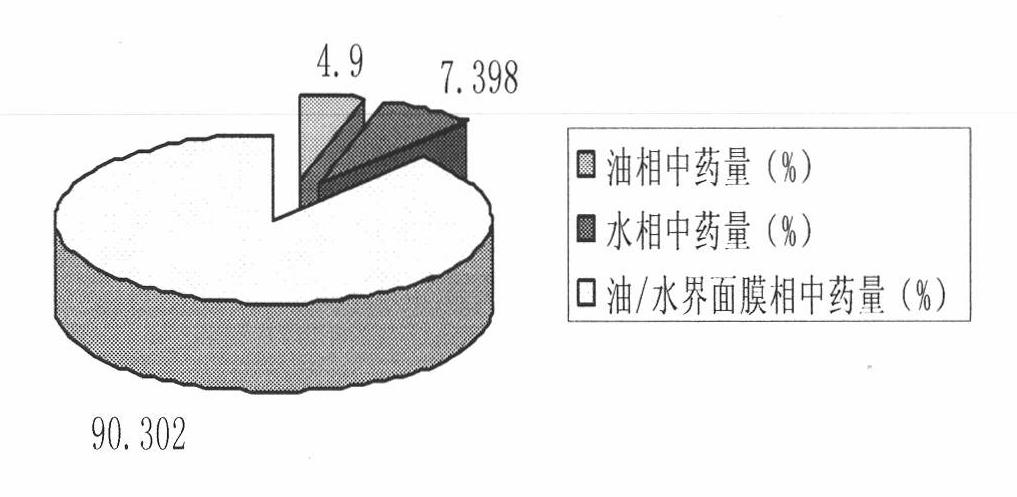
The invention relates to an oil-in-water type garlicin-garlic oil sub-microemulsion distributed in an inner phase (oil phase) as well as a method for preparing the same. The formulation of the emulsion contains 0.5 to 3 weight portions of garlicin or garlic oil as a medicament, and the garlicin or the garlic oil is added with a pharmaceutic adjuvant and is prepared into a garlicin sub-microemulsion or a garlic oil sub-microemulsion, wherein the pharmaceutic adjuvant comprises 5 to 20 weight portions of injection oil, 0.2 to 5 weight portions of emulsifier, 0 to 5 weight portions of coemulsifier, 0.5 to 3 weight portions of isoosmotic adjusting agent, a proper amount of antioxidant, a proper amount of pH regulator and a proper amount of injection water. The invention discusses the medicament distribution in oil, an oil / water interfacial film and water when the garlicin or the garlic oil is in an oil-in-water type dispersion system macroscopically and microscopically; more than 98 percent of the medicaments are enwrapped in the oil phase and the medicament free in the water phase is less than 1 percent; and the problem of a garlicin or garlic oil intravenous injection preparation for a hydrophobic dug is solved, the stability of an emulsion thereof is improved, and the vascular stimulation in an intravenous injection is reduced, thus the adaptability of a patient is greatly improved. The oil-in-water type garlicin-garlic oil sub-microemulsion can be applied to the adjuvant therapy of bacteria resistance, cardiovascular and cerebrovascular diseases and tumors.
Owner:李淑斌
Mesoporous titanium dioxide and preparation method thereof and application thereof
InactiveCN102718254AOptimal Control StructureLow densityInorganic non-active ingredientsNanotechnologyActive agentSurface-active agents
The invention belongs to the field of medicine technology, and relates to a preparation method of mesoporous titanium dioxide with high biological compatibility and a controllable structure and application of the mesoporous titanium dioxide as a hard soluble drug carrier. The method adopts a soft template method, namely, adopts a surfactant as a structure guiding agent, isopropyl titanate as titanium source, and double distilled water as a reaction rate modifier to prepare the mesoporous titanium dioxide having different mesoporous passage structures. The prepared mesoporous titanium dioxide is of large specific surface area, chemical stability, non-toxic effect, no side effect and good biocompatibility, and is suitable for being used as the hard soluble drug carrier. The application of the titanium dioxide as the hard soluble drug carrier can lay a theoretical foundation for uses of multivariant functional carriers. The method adopts a solvent method and a fusion method to perform embedding and adsorption on a drug so as to uniformly disperse the drug inside the mesoporous or on surface of the carrier. The drug carrier system can assist in significantly enhancing water solubility of a hard soluble drug and improving dissolution rate in vitro and oral bioavailability.
Owner:SHENYANG PHARMA UNIVERSITY
Atorvastatin calcium nano-lipid carrier and preparation method thereof
ActiveCN103690513AGood physiological compatibilityLow toxicityMetabolism disorderPharmaceutical non-active ingredientsEmulsionAqueous solubility
The invention relates to an atorvastatin calcium nano-lipid carrier and a preparation method thereof. The atorvastatin calcium nano-lipid carrier comprises the following components: 1 wt% to 2 wt% of atorvastatin calcium, 12 wt% to 24 wt% of a lipid material, 10 wt% to 30 wt% of phospholipid and 30 wt% to 50 wt% of an emulsifier. The preparation method comprises the following steps: weighing the atorvastatin calcium, the lipid material and the phospholipid, ultrasonically dissolving in an organic solvent, heating to 60-75 DEG C in a water bath, and using an obtained product as an organic phase; dissolving the emulsifier in water, heating to 60-75 DEG C in the water bath, and using an obtained product as a water phase; dropwise adding the organic phase into the water phase stirred at the speed of 90-1,200 r / min, continuously stirring for fully volatilizing the organic solvent, and concentrating to form a primary emulsion; pouring the primary emulsion into ice water with the volume being 2-5 times that of the primary emulsion, and continuously stirring and solidifying in an ice bath to obtain the atorvastatin calcium nano-lipid carrier. The atorvastatin calcium nano-lipid carrier is internally degradable and high in encapsulation efficiency; after drugs are prepared into nano-lipid carriers, the stability, the water solubility and the bioavailability of the drugs can be improved.
Owner:RUNZE PHARMACEUTICAL (SUZHOU) CO LTD
Glabridin-cladded nano solid lipid carrier and preparation method and application thereof
InactiveCN105581911AGood compatibilityImproved release propertiesCosmetic preparationsToilet preparationsInorganic saltsPolyol
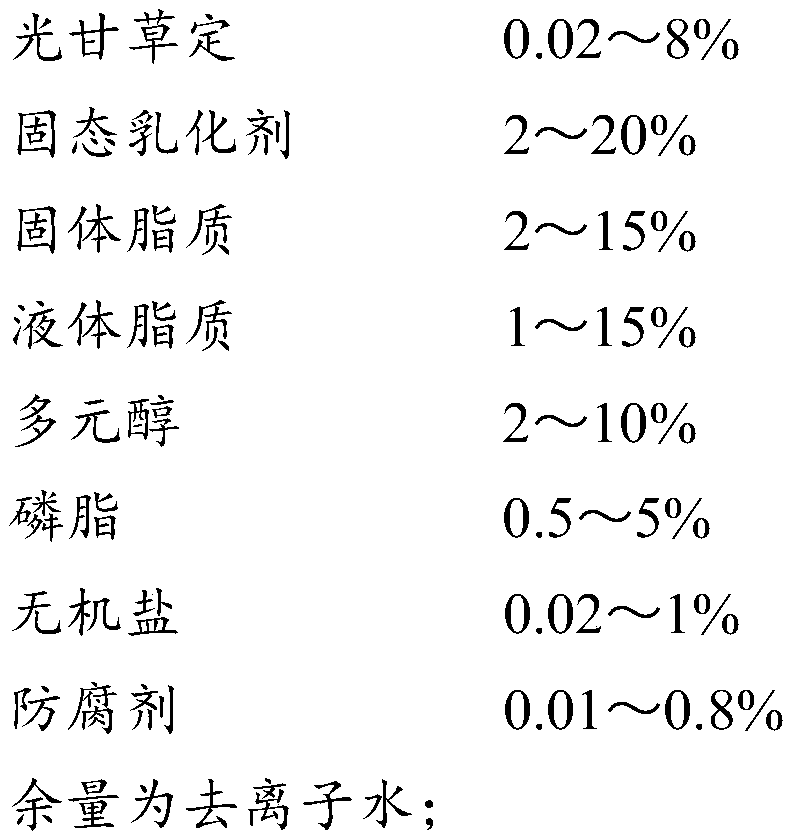
A glabridin-cladded nano solid lipid carrier contains 0.02-8% of glabridin, 2-20% of a solid emulsifier, 2-15% of solid lipid, 1-15% of liquid lipid, 2-10% of polyols, 0.5-5% of phospholipid, 0.02-1% of inorganic salt, 0.01-0.8% of a preservative and deionized water. The invention further provides a preparation method of the glabridin-cladded nano solid lipid carrier. The stability of the glabridin is improved by the glabridin-cladded nano solid lipid carrier, the infiltration of the glabridin into the skin is promoted, and the skin whitening and anti-oxidation effects are improved.
Owner:INFINITUS (CHINA) CO LTD
Curcuma zedoary oil solid lipid nano-particle and its preparation method
A lipid nanoparticle of zedoary oil used for preparing solid, liquid, injection, ointment, suppository, film, aerosol, etc is prepared from zedoary oil, medicinal phosphatide, emulsifier, solid lipid, liquid lipid and deoxidant through multiple methods.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of textile electrode material and textile electrode material
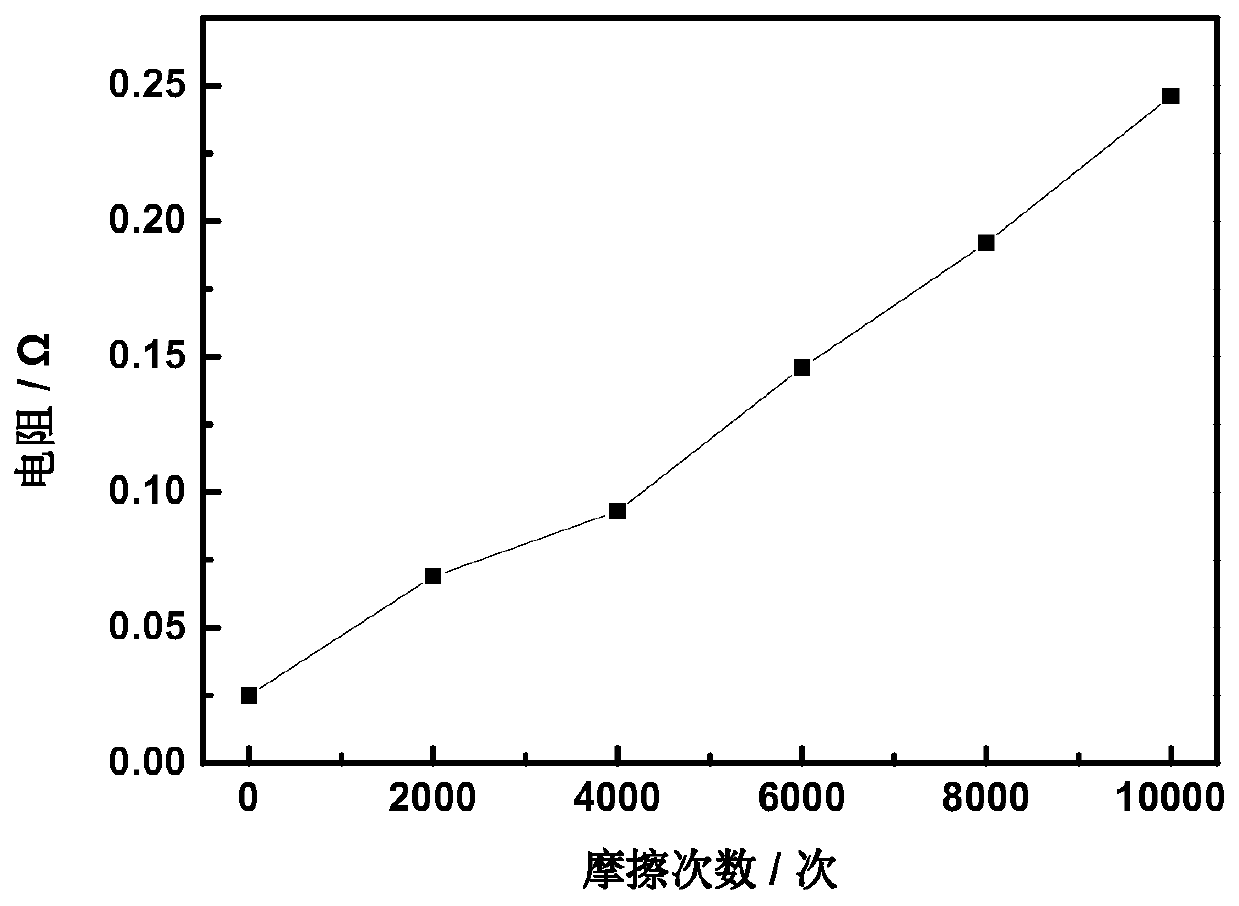
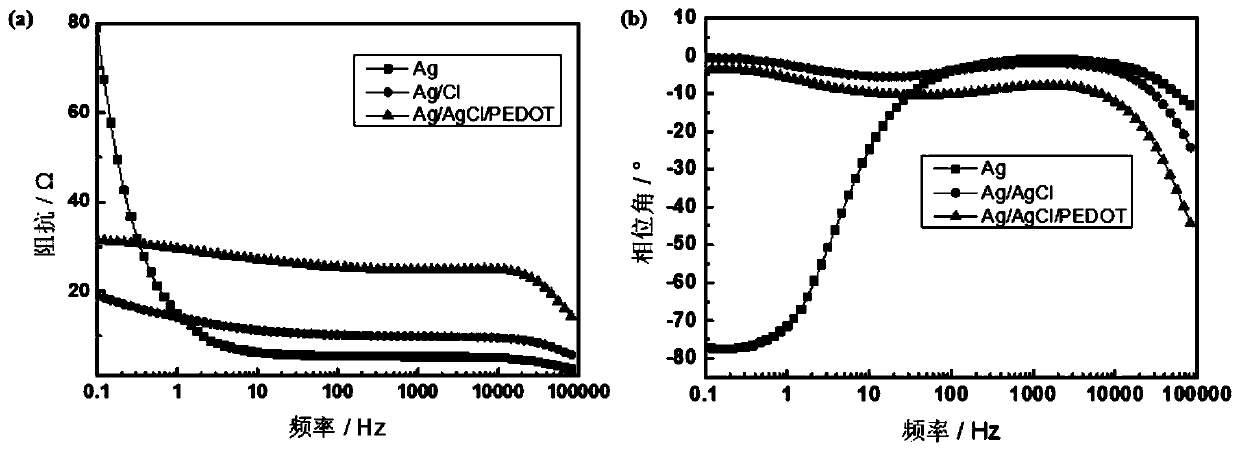
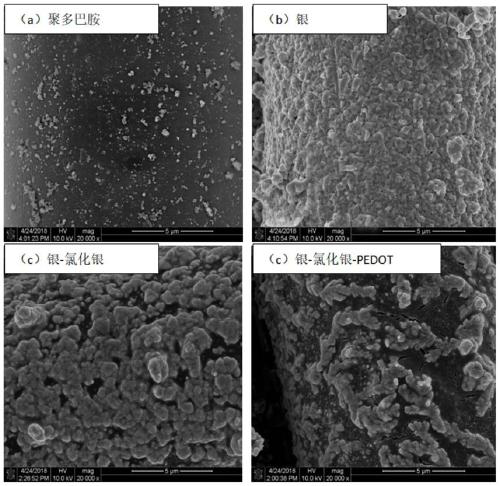
ActiveCN111395002AImprove conductivitySimplified coarseningLiquid/solution decomposition chemical coatingDiagnostic recording/measuringConductive polymer compositeSilver chloride
The invention discloses a preparation method of a textile electrode material and the textile electrode material. The preparation method comprises the steps that firstly, pretreated fabric is soaked ina dopamine hydrochloride solution, and polydopamine coated fabric is obtained; then, the polydopamine coated fabric is soaked in a silver-ammonia solution, a glucose solution is added after a certainperiod of time, and a silver-plated fabric is obtained; the silver-plated fabric is subjected to chlorination treatment in a sodium chloride solution through an electrochemical method, and a silver-silver chloride composite coating fabric is formed; finally, the silver-silver chloride composite coating fabric is modified in a conductive polymer monomer solution through an electrochemical method,and the silver-silver chloride-conductive polymer composite coating fabric is obtained. The surface specific resistance of the textile electrode material is 0.01-5 omega, the polarization impedance ina 0.9% sodium chloride solution is 10-1000 omega, the skin interface impedance is 1K omega-100M omega at 1Hz, and the phase angle variation within the frequency range of 0.5-50Hz is 0-30 degrees.
Owner:MINJIANG UNIV
Resveratrol solid lipid nano-particles and preparation method thereof
InactiveCN104688715AStrong bioavailabilityStrong drug loadingHydroxy compound active ingredientsMetabolism disorderLipid formationOrganic solvent
The invention relates to resveratrol solid lipid nano-particles and a preparation method thereof. The preparation method comprises the following steps: (a) weighing resveratrol, an emulsifying agent and a lipid material, heating and melting the lipid material, then uniformly mixing with the emulsifying agent and resveratrol, then adding into an organic solvent, and uniformly mixing to obtain an oil phase; and (b) putting the oil phase into a container, distilling at reduced pressure to obtain an oil film, putting a water phase into the container, and also treating in an ultrasonic cleaner of which the ultrasonic power is 40-60 KHz for 30-60 minutes to obtain the resveratrol solid lipid nano-particles. The resveratrol solid lipid nano-particles prepared by using the method disclosed by the invention have the advantages of small particle sizes, high medicine loading capacity, high medicine absorption speed, high bioavailability, convenience in taking and small toxicity, and have strong practicability.
Owner:SHANGHAI HOSPITAL OF TRADITIONAL CHINESE MEDICINE
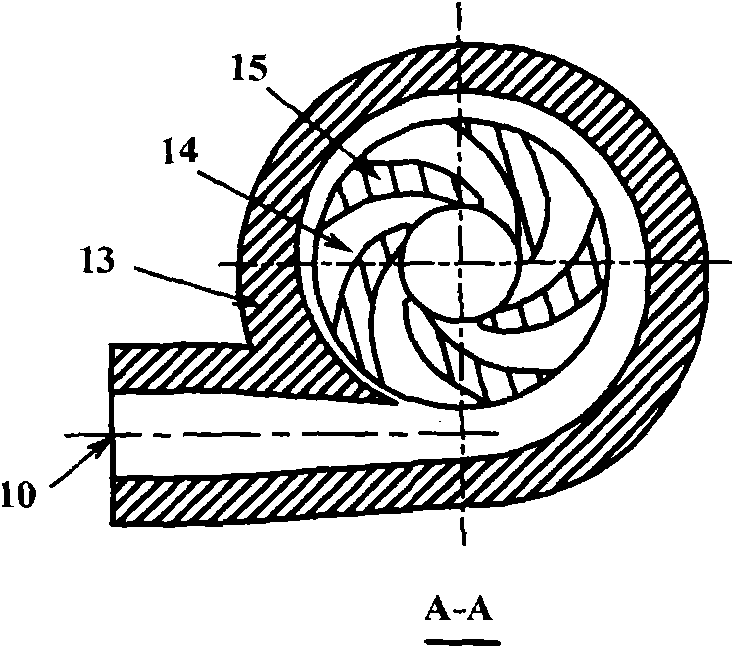
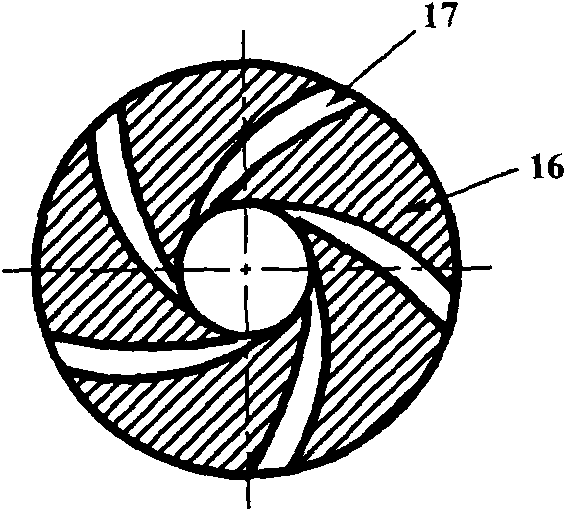
Hydrodynamic pressure suspension double-flow pump
InactiveCN103216453APlay the role of heat dissipationAchieve levitationPump componentsBlood pumpsImpellerEngineering
The invention provides a hydrodynamic pressure suspension double-flow pump, relating to an impeller ultra-small pump which has no external mechanism axis and is driven magnetically. The pump comprises two mutually separate flowing channels; each flowing channel consists of an inlet, an impeller, a pressure water chamber and an outlet; and the geometrical parameters of the flowing channels are the same or different; a stator of the pump is embedded in a pump shell, a permanent magnet is embedded in a rotor; and the stator and the permanent magnet are arranged opposite along a radial direction; a radial gap is formed between the internal surface of the pump shell and the external surface of the rotor, so that liquid hydrodynamic support for restricting axial movement is formed during normal operation of the pump; an axial gap is formed between the front cover plate of each impeller and the corresponding pump cover, so that liquid hydrodynamic support for restricting rotor axial movement is formed during the normal operation of the pump. In such a way, the rotor can be suspended in the pump cavity during normal operation. The pump provided by the invention can be used for meeting double-flow use requirement of different flows and pressures, and good flowing condition can be effectively and favorably formed in the internal cavity of the pump shell, and moreover, the operation reliability of the pump can be improved.
Owner:TSINGHUA UNIV
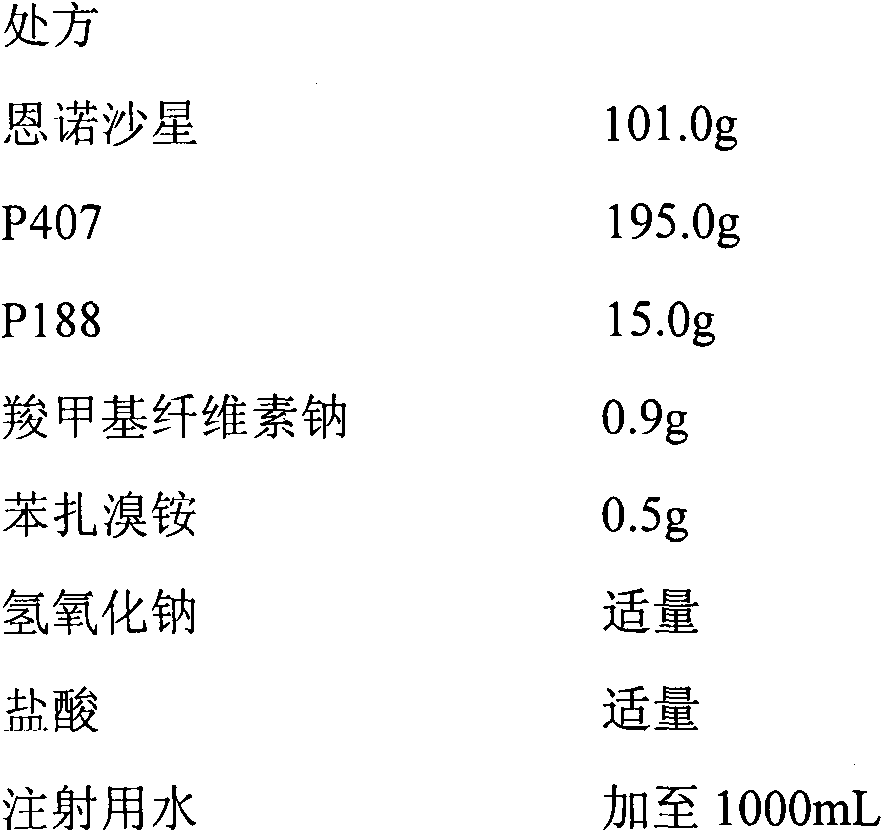
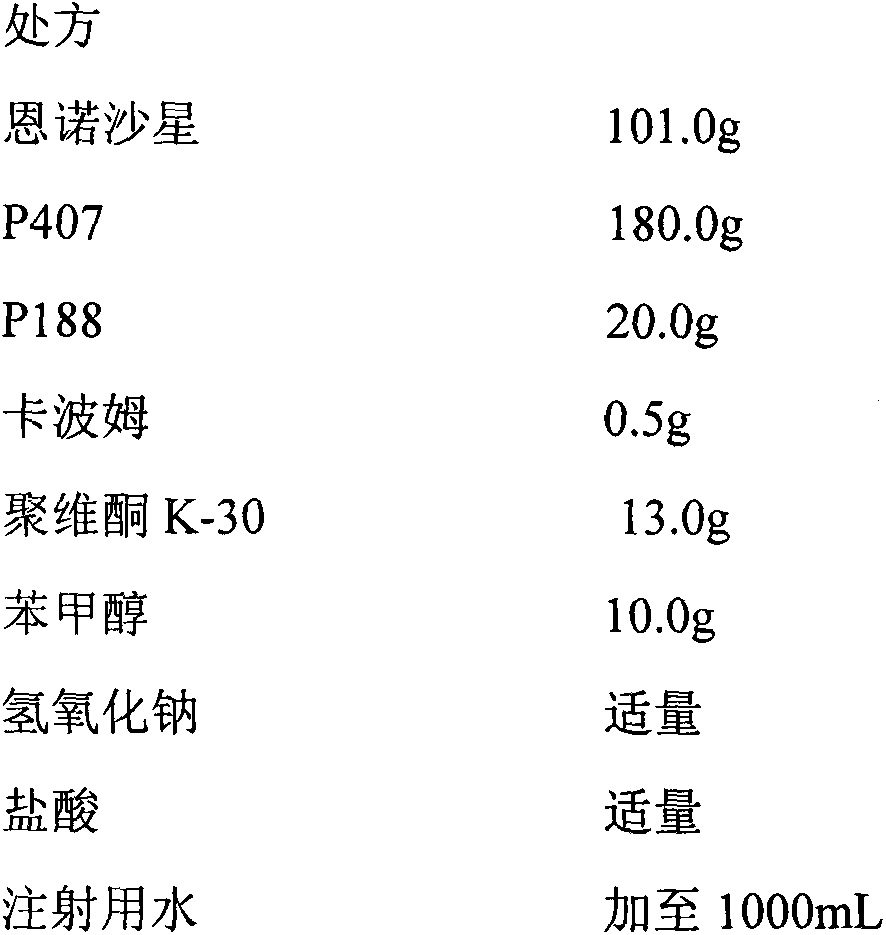
In-situ gel for enrofloxacin injection and preparation method thereof
InactiveCN103385850AImprove complianceSlow drug releaseAntibacterial agentsOrganic active ingredientsDiseaseGel preparation
The invention belongs to the field of special pharmaceutical preparations for animals, and relates to an in-situ gel preparation for enrofloxacin injection and a preparation method thereof. The preparation comprises the following components in parts by weight: 5.0-20.0 parts of enrofloxacin, 14.5-32.5 parts of P407, 0.5-10.5 parts of P188, 25.0-90.0 parts of water, 0.01-10.0 parts of a high molecular retardant, 0.01-3.0 parts of a preservative and appropriate amount of a pH adjustor. The preparation provided by the invention is a freely flowing liquid at room temperature, and forms a semi-solid gel through dosage by intramuscular injection or subcutaneous injection, so that the preparation is just delivered once in a course of treatment. The preparation has the advantages of stable property, long lasting time for delivery, exact curative effect, strong animal adaptability, no toxic and side effects and adverse reaction and the like, and is simple to prepare and convenient to delivery. The preparation provided by the invention has broad-spectrum antibacterial effect, and can be used as a medicine for preventing and treating diseases of animals such as pigs, sheep, dogs, cats and cattle.
Owner:NANJING AGRICULTURAL UNIVERSITY
Mixture composition comprising rhamnolipids
ActiveCN104095765ASuperior Foaming BehaviorSimple ConfigurabilityCosmetic preparationsHair cosmeticsRhamnolipidChemistry
Owner:EVONIK OPERATIONS GMBH
Glycyrrhetinic acid solid lipid nanoparticles and preparation method for same
InactiveCN102512369AUniform particle sizeHigh encapsulation efficiencyOrganic active ingredientsDigestive systemDiseaseActive agent
The invention relates to glycyrrhetinic acid solid lipid nanoparticles and a preparation method for the same, belonging to the field of medicinal preparation. The main ingredients of the glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention comprise active raw material glycyrrhetinic acid, medicinal phospholipid, a lipid material and a surfactant. The glycyrrhetinic acid solid lipid nanoparticle solution and the freeze-dried powder injection thereof prepared by the preparation method disclosed by the invention are small in particle diameter, high in entrapment efficiency, good in stability and capable of being used for a plurality of administration routes such as oral administration and injection administration. The glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention can reduce dosage, enhance curative effect and reduce the toxic and side effects of medicine, as well as are suitable for treating a plurality of diseases such as hepatitis, liver cancer, lung cancer, ovarian cancer, gastritis, gastric cancer, leukaemia and aids.
Owner:WUHAN UNIV
Silicon-based modified fadable pigment and preparation method thereof
ActiveCN103756361AGood physiological compatibilityStrong coloring powerOrganic dyesPigment treatment with organosilicon compoundsSulfite saltPrinting ink
The invention provides a silicon-based modified fadable pigment and a preparation method thereof. The fadable pigment is prepared by modifying silicon dioxide through an amino and modifying by carboxyl, and carrying out a condensation reaction with a dye containing the amino under the effect of a catalyst. The pigment takes the silicon dioxide as a carrier, has good physiological compatibility and has no pollution to the environment; the pigment can be faded at a normal temperature and a normal pressure under the effects of sodium sulfite, formamidine sulphinic acid, sodium borohydride and the like; the pigment provided by the invention has good tinting strength, color and luster and brightness, can be used for preparing fadable writing printing ink, printing ink and printing ink for an ink-jet printer, and can also be used for fields including coating, oil pant and the like.
Owner:安徽省智力猴文化科技有限公司
Hydrobromic acid lappaconitine solid lipid nano particle and preparation method thereof
ActiveCN101658495AGood physiological compatibilityImprove stabilityOrganic active ingredientsPowder deliveryLipid formationOrganic solvent
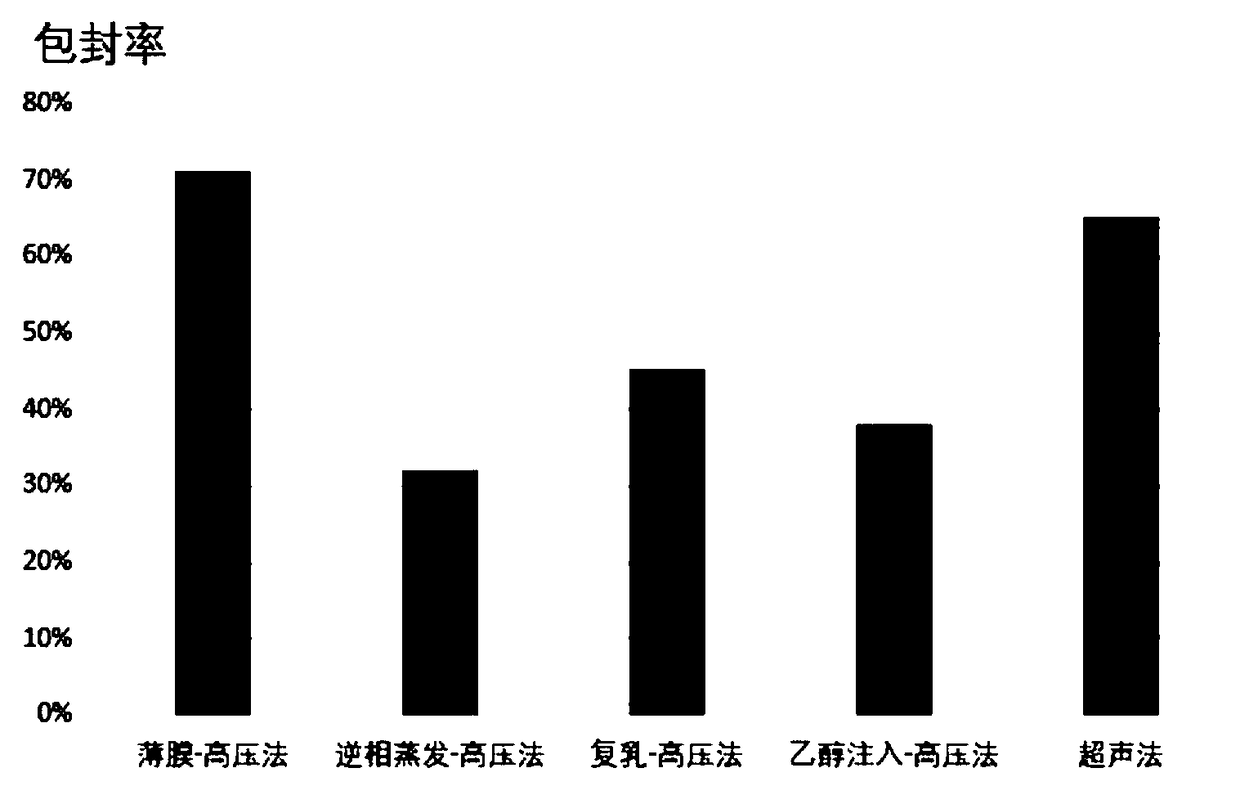
The invention provides a hydrobromic acid lappaconitine solid lipid nano particle and a preparation method thereof. The hydrobromic acid lappaconitine solid lipid nano particle is prepared from the following components, by weight percent: 0.1-1% of hydrobromic acid lappaconitine, 1-8% of lipid material, 0-5% of phospholipid, 0.5-10% of emulsifying agent and the balance water. The preparation method comprises the following steps: mixing the emulsifying agent and the water fully and evenly and preparing a water phase; mixing the hydrobromic acid lappaconitine, the lipid material and the phospholipid fully, melting and preparing an oil phase; heating the water phase and the oil phase respectively to 65-85 DEG C, adding the water phase into the oil phase under a stirring condition and preparing primary emulsion; emulsifying the primary emulsion at high pressure evenly and obtaining suspensoid liquid; and putting the suspensoid liquid under a condition of 0-4 DEG C, cooling, solidifying andpreparing the hydrobromic acid lappaconitine solid lipid nano particle. The invention adopts a high-pressure even emulsification method to prepare the hydrobromic acid lappaconitine solid lipid nanoparticle, does not need to use an organic solvent and is suitable for large industrialized production.
Owner:GUANGDONG PHARMA UNIV
Decitabine sustained release microsphere and preparation method thereof
ActiveCN101966157AExtended half-lifeImprove efficacyOrganic active ingredientsPharmaceutical non-active ingredientsDrug administrationEntrapment
The invention discloses a Decitabine sustained release microsphere, which comprises Decitabine and a carrier material, wherein the weight percentage of the Decitabine and the carrier material is 1-8%, the partical size of the Decitabine sustained release microsphere is below 10 mu m, and the entrapment rate of the Decitabine sustained release microsphere is more than 75%. The Decitabine sustainedrelease microsphere provided by the invention has higher loading rate, no in-vivo burst effect, stable blood concentration, and no drug release delivery deadtime, thus greatly reducing clinical interval of drug administration, reducing dosage, improving compliance of patients, and reducing hazard rating of adverse reaction.
Owner:苏州科耐尔医药科技有限公司
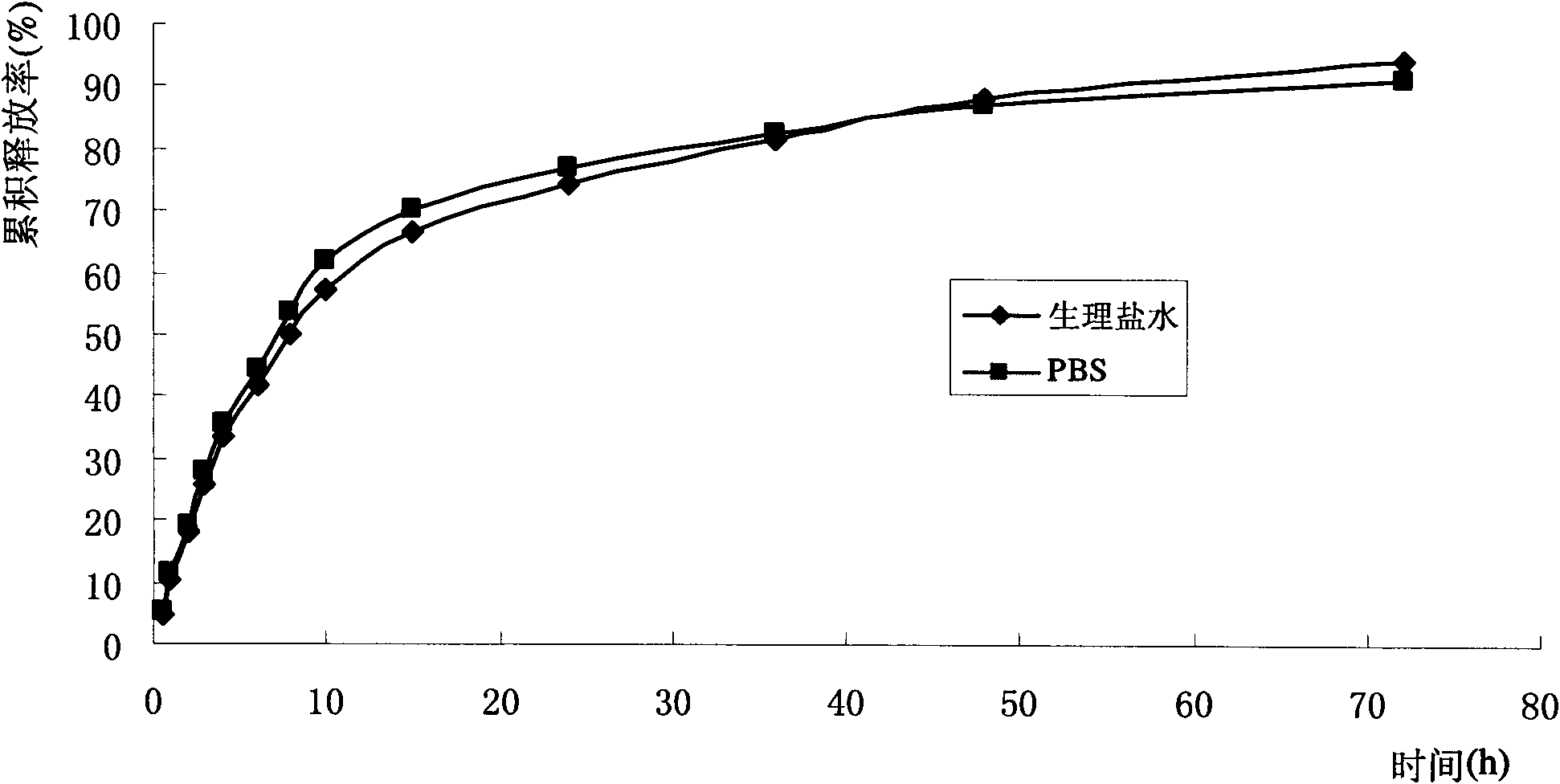
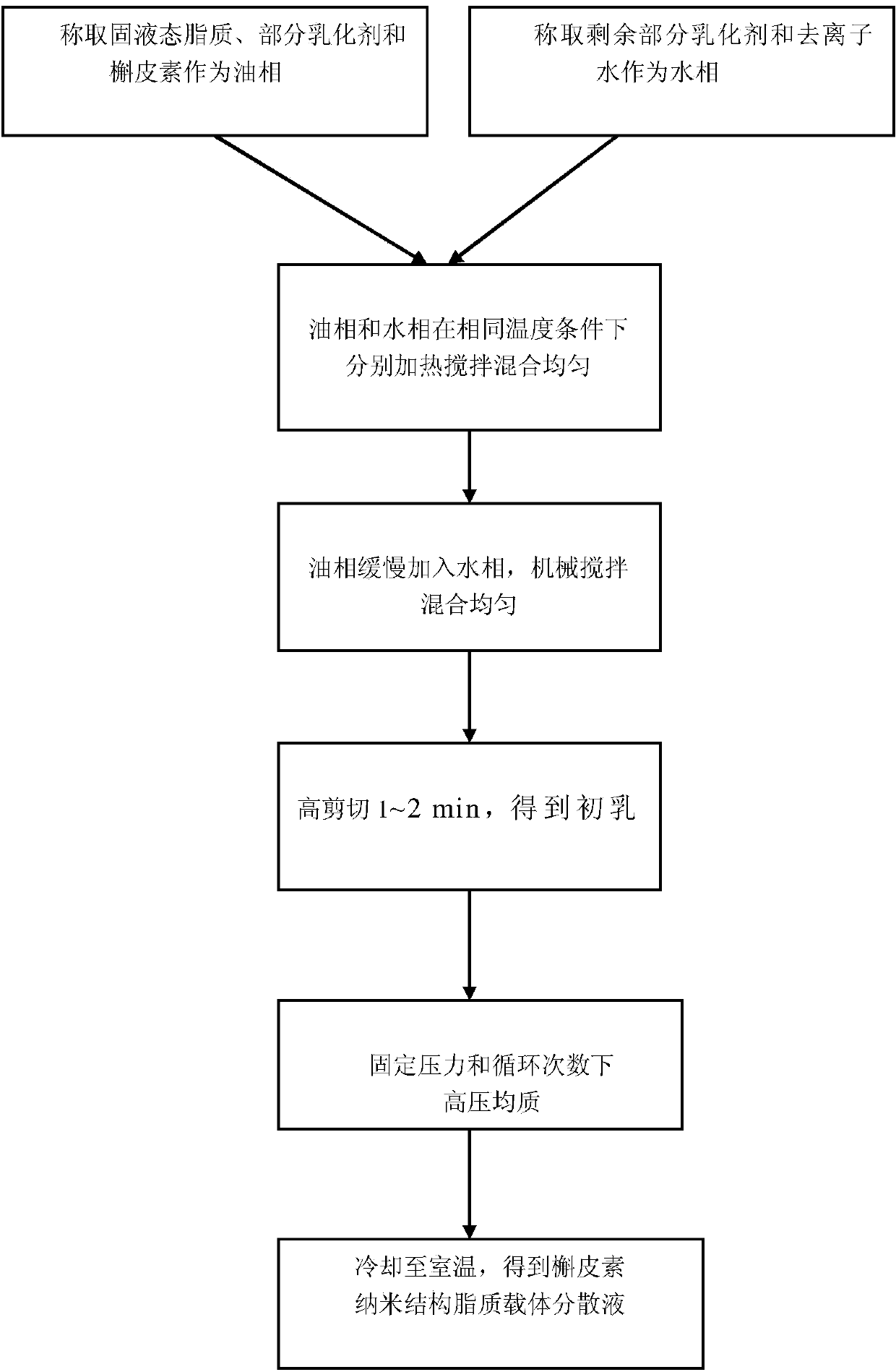
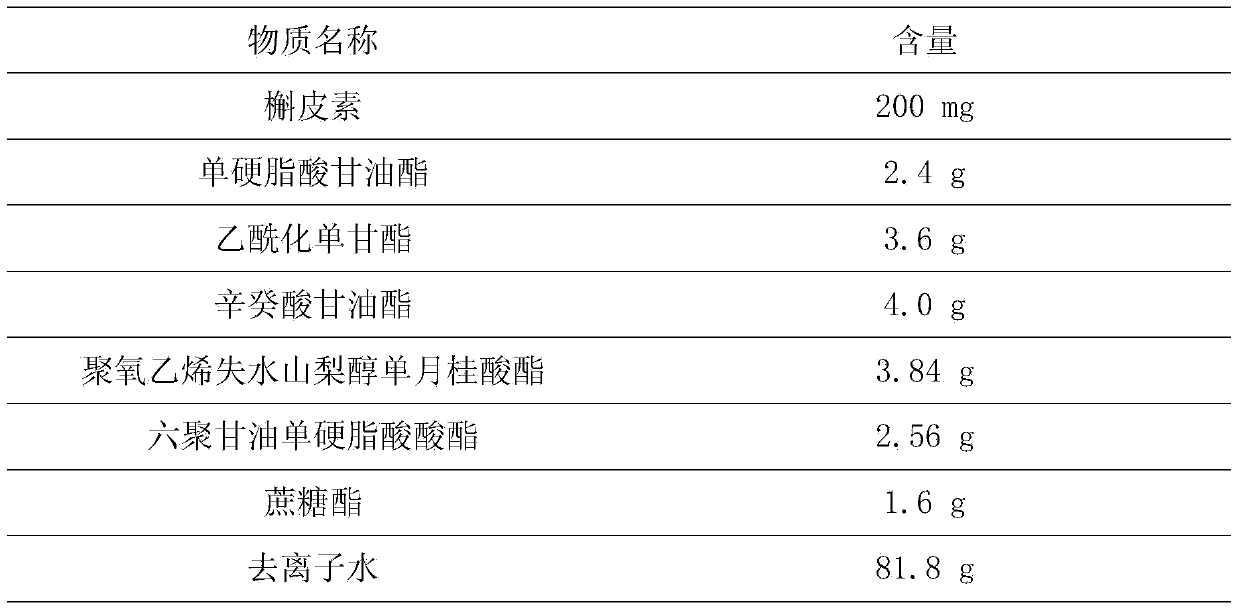
Quercetin nanostructured lipid carrier and preparation method thereof
InactiveCN104172184AThe preparation process is easy to controlGood chemical stabilityFood preparationFood ingredient as emulsifierSolubilityMonoglyceride
The invention discloses a quercetin nanostructured lipid carrier and a preparation method thereof, wherein the quercetin nanostructured lipid carrier comprises the following components by weight percent: 0.1-1% of quercetin, 2%-15% of an emulsifying agent, 2%-15% of compound lipid material and the balance being water. The compound lipid material is a solid-liquid state lipid mixture; the solid lipid is one or more of acetylation monoglyceride, stearate, glyceryl monostearate and monolaurate; the liquid lipid is one or more of decanoyl / octanoyl-glycerides, camellia oleosa seed oil, soybean oil and peanut oil. The quercetin is loaded by utilizing a nanostructured lipid carrier technique, on the one hand, the sensibility of the quercetin to the environmental factors such as illumination and temperature can be reduced, and the chemical stability is improved, and on the other hand, the solubility of the quercetin in water can be improved, thereby improving the bioavailability. The preparation method is simple and controllable, and is good in reproducibility; the quercetin nanostructured lipid carrier emulsion prepared by the method is suitable for being added into functional food beverages.
Owner:SOUTHEAST UNIV
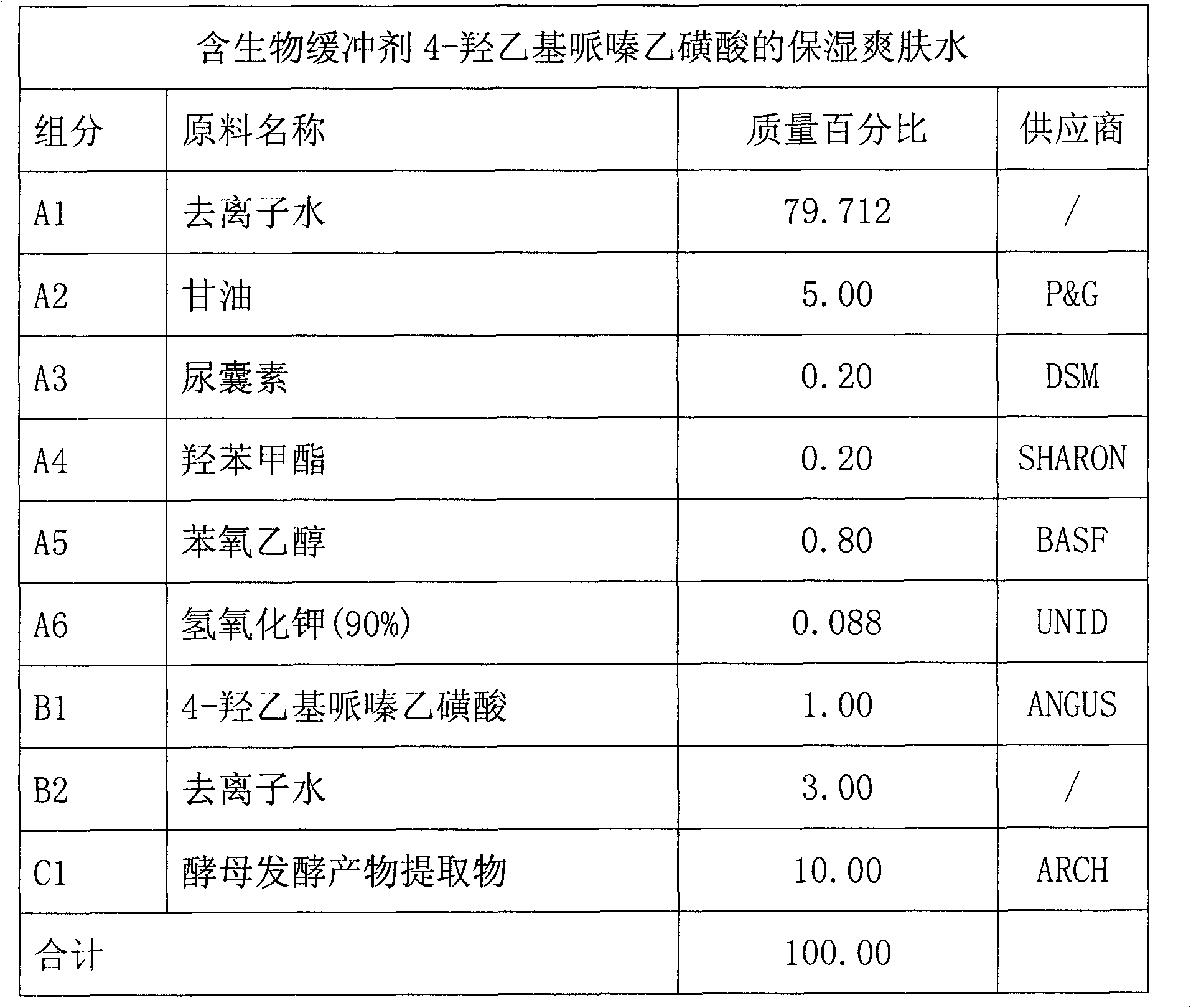
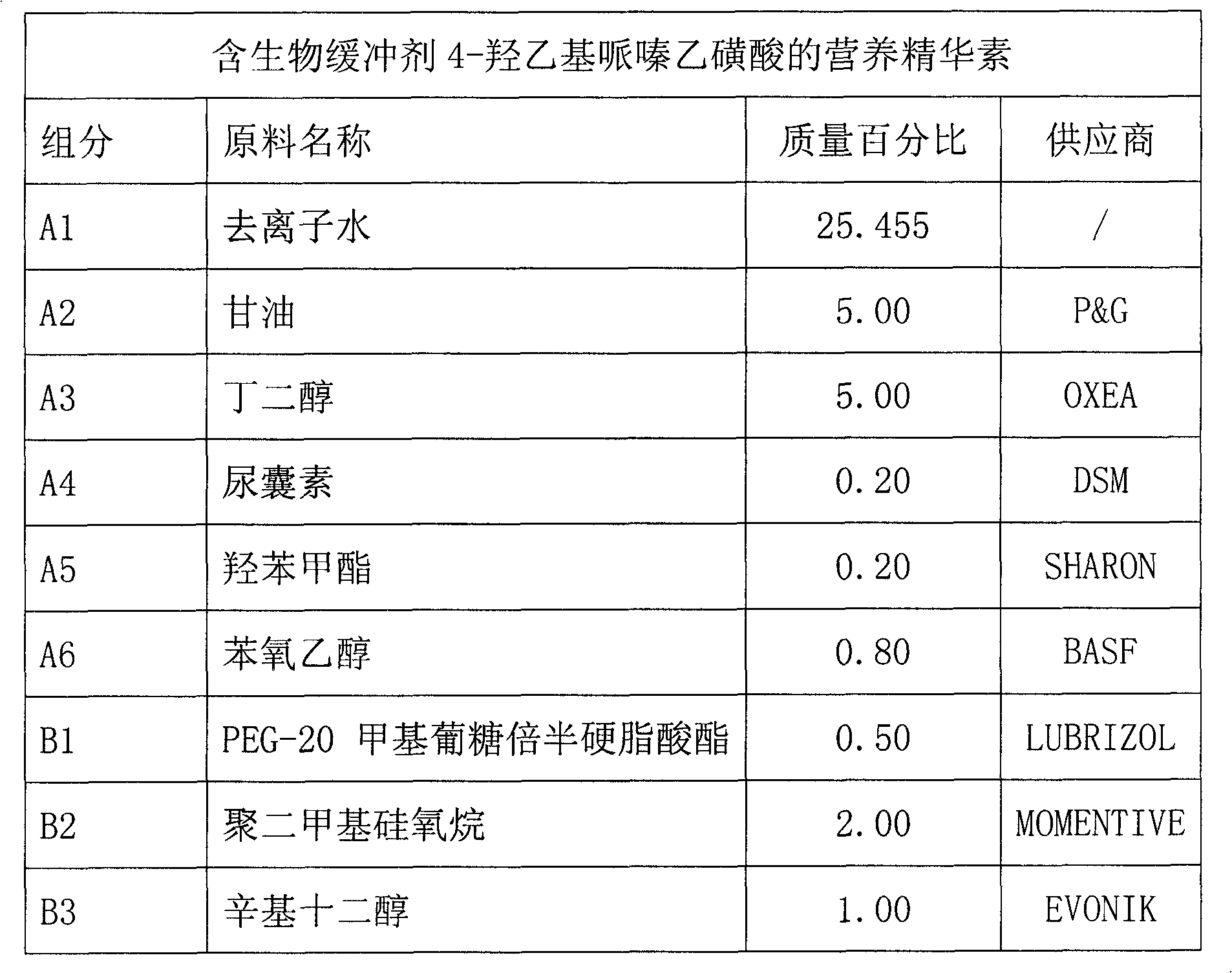
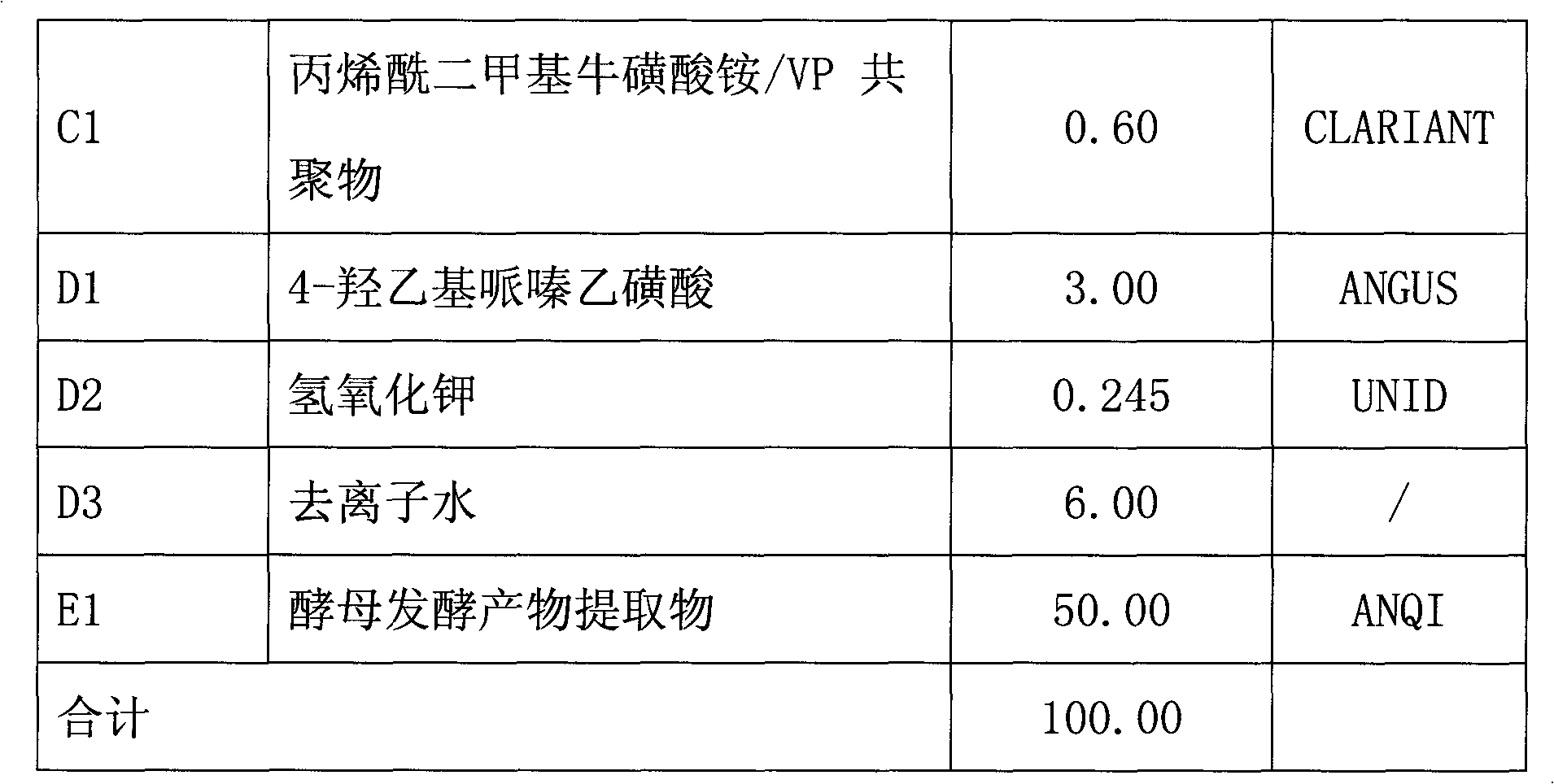
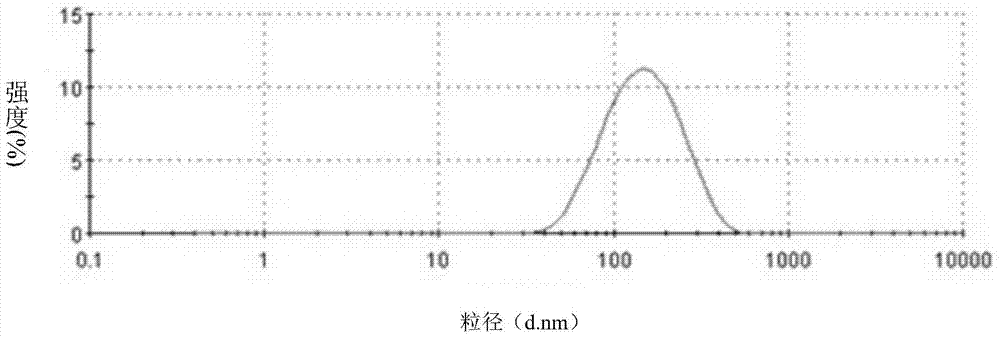
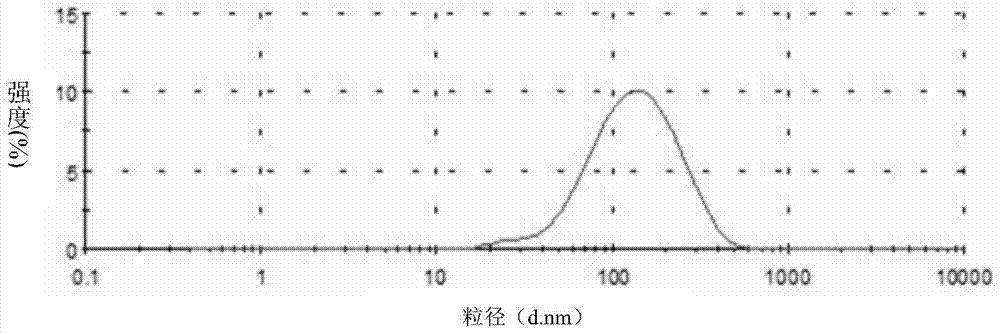
Skin care product containing biological buffering agent
ActiveCN102793657AKeep aliveGood physiological compatibilityCosmetic preparationsToilet preparationsSmall amplitudeMedicine
The invention relates to a skin care product containing a biological buffering agent. The product includes microbial fermentation product extracts, and is characterized by further including the biological buffering agent with a mass percentage of 0.1-3.0%. The skin care product has the advantages that: the pH value of the product drifts in a small amplitude, the pH value stabilizes at 6.0-8.0 in three years after preparation of the skin care product, good protection is applied on the microbial fermentation product extracts added in the product, the microbial fermentation product extracts are not easy to be hydrolyzed, and anti-aging effects of the product lasts a long time.
Owner:PROYA COSMETICS
Lactoferrin modified solid lipid nanoparticles, as well as preparation method and application thereof
InactiveCN103585639AGood biocompatibilityLow toxicityAntipyreticAnalgesicsMedicinePharmaceutical drug
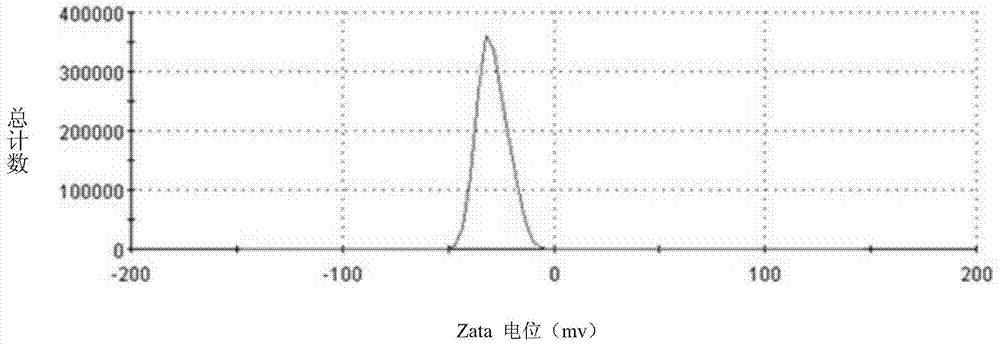
The invention belongs to the technical field of medicines, and in particular relates to lactoferrin modified solid lipid nanoparticles, as well as a preparation method and application thereof. The lactoferrin modified solid lipid nanoparticles are characterized in that lactoferrin is modified on the surfaces of solid lipid nanoparticles. The lactoferrin modified solid lipid nanoparticles can wrap medicines therein and can be used as a medicine transfer carrier. The preparation method disclosed by the invention comprises the following steps: synthesising mal-sac-PEG-MS through DCC dehydration reaction, and preparing a nanosuspension by using mal-sac-PEG-MS; then, carrying out lactoferrin sulfhydrylation (Lf-SH) by virtue of a Traut's agent, adding Lf-SH into the nanosuspension, and stirring at a room temperature to carry out protein modification. The lactoferrin modified solid lipid nanoparticles disclosed by the invention have good particle size, Zeta potential and stability. Experimental results show that the lactoferrin modified solid lipid nanoparticles disclosed by the invention are capable of transferring model medicines, such as loperamide and coumarin 6, into brain.
Owner:YANGZHOU UNIV
Polyglutamic acid and human epidermal growth factor nano-liposome and preparation method thereof
ActiveCN109316446AAvoid influenceImprove placement stabilityOrganic active ingredientsCosmetic preparationsLiposome membraneCuticle
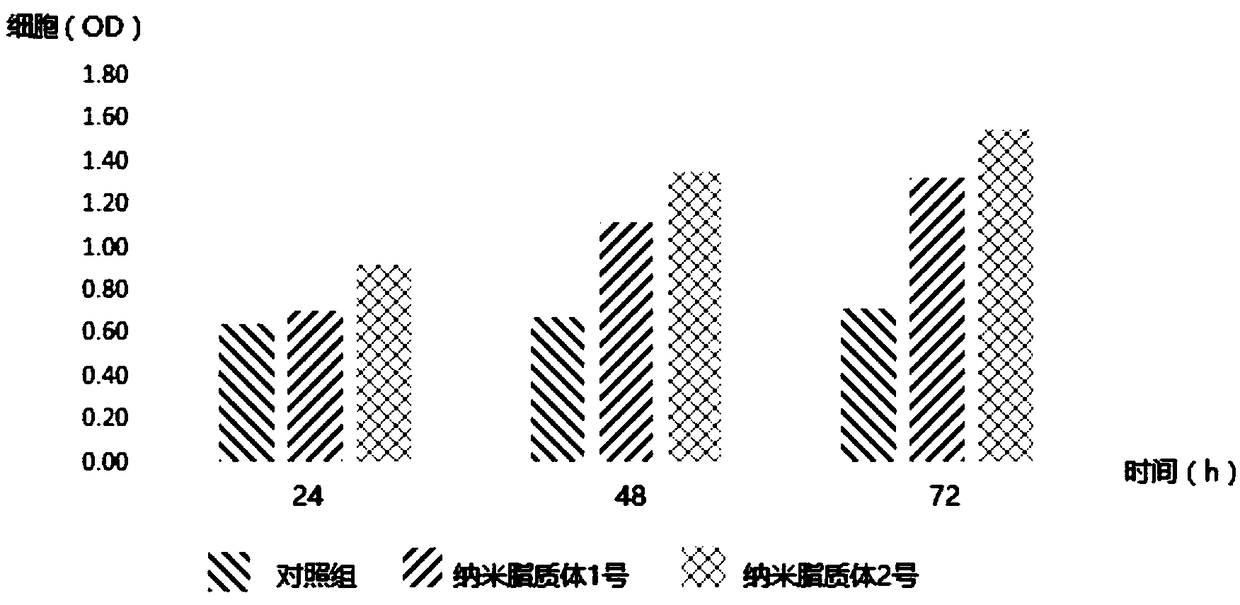
The invention belongs to the technical field of bioengineering and discloses a polyglutamic acid and human epidermal growth factor nano-liposome and a preparation method thereof. The nano-liposome comprises high molecular weight polyglutamic acid, low molecular weight polyglutamic acid, a human epidermal growth factor and a liposome membrane material. According to the polyglutamic acid and human epidermal growth factor nano-liposome, the high molecular weight polyglutamic acid and the low molecular weight polyglutamic acid are compounded with the human epidermal growth factor and the preservative-free nano-liposome is prepared by utilizing a bacterium-inhibition effect of epsilon-polylysine; the nano-liposome has an addition and multiplying effect on skin repairing; the transdermal performance and skin absorption rate of active components are greatly improved; the nano-liposome has stable performance, good utilization compatibility and relatively high encapsulation rate.
Owner:NANJING SHINEKING BIOTECH CO LTD
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787BGood physiological compatibilitySolve the problem of high doseOrganic active ingredientsDigestive systemLipid formationChemical structure
Owner:李淑斌
Hydrodynamic float vane type microminiature pump
ActiveCN101581307BImprove hydraulic efficiencySafe and reliable operationPump componentsRadial flow pumpsImpellerElectric machine
The invention relates to a hydrodynamic float vane type microminiature pump, in particular to a vane type microminiature pump with no external mechanical axis, no leakage or with fluid in no contact with the external world. The invention has the technical characteristics that a main pump impeller and an electric machine rotor are integrated as a whole; auxiliary impellers are arranged at the two ends of the electric machine rotor and are symmetrically distributed with respect to the main impeller; a hydrodynamic suspension bearing is formed by the vane tops of both first and second auxiliary impellers and the corresponding inner walls of pump shells, which can realize suspended propping separately or by combining a magnetic suspension bearing. The invention can effectively form symmetrical, even and smooth flow conditions in the inner chamber of the pump shell, thus improving the hydraulic efficiency of the pump and the controllability of the flow design and further decreasing the structural size of the microminiature pumps.
Owner:TSINGHUA UNIV +1
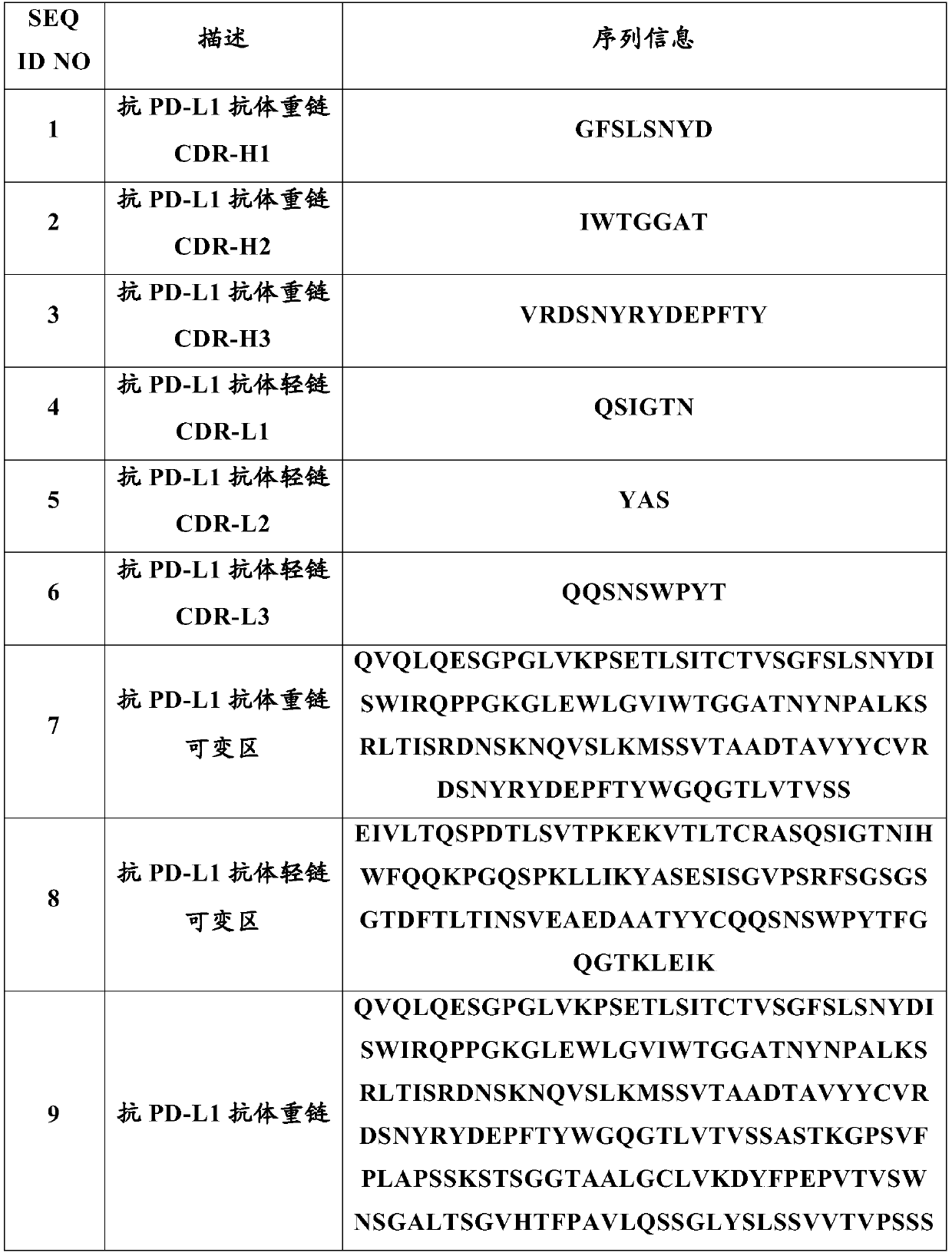
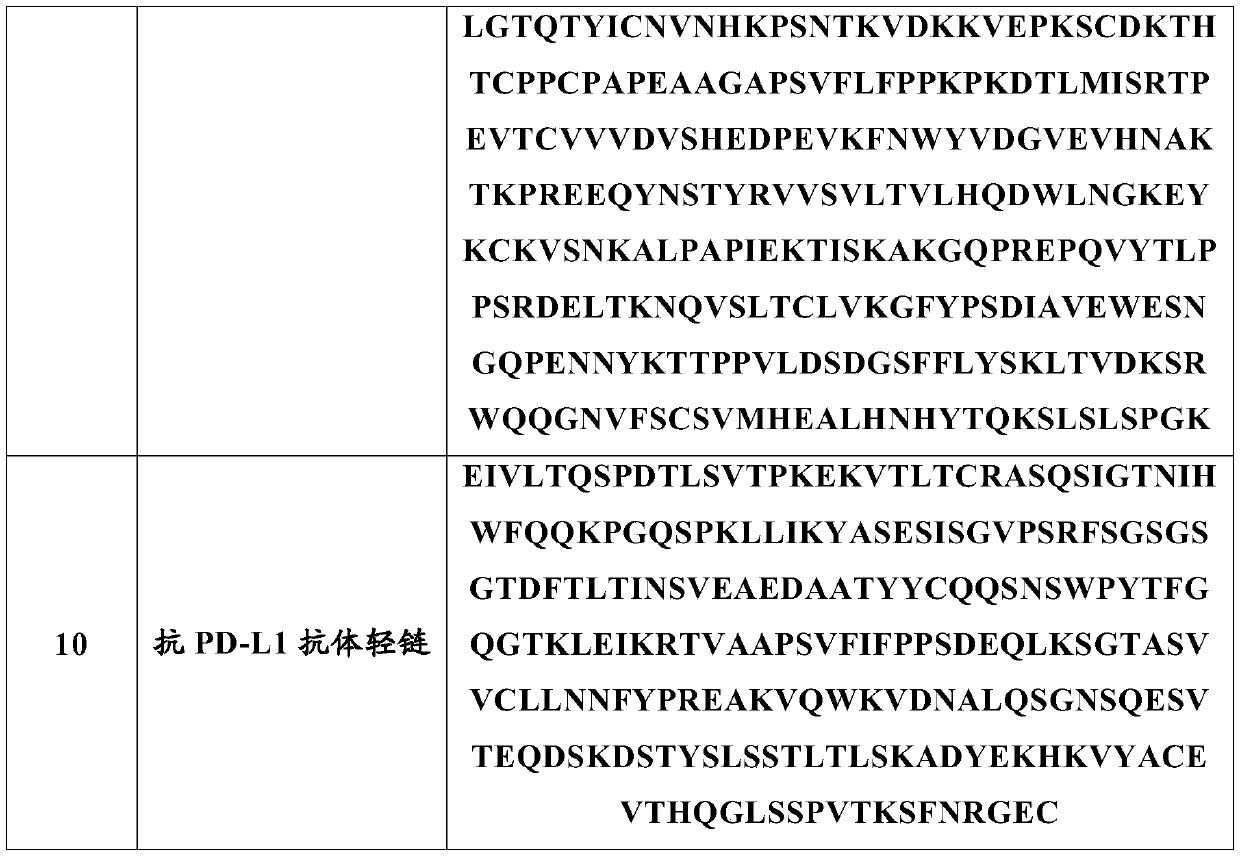
Anti-programmed cell death-ligand 1 (PD-L1) antibody preparation
ActiveCN111228479AGuaranteed physical stabilityMaintain chemical stabilityInorganic non-active ingredientsPharmaceutical delivery mechanismTherapeutic antibodyAntiendomysial antibodies
The invention relates to the field of therapeutic antibodies, in particular relates to an anti-programmed cell death-ligand 1 (PD-L1) antibody preparation. The preparation comprises an anti-PD-L1 antibody, a buffer solution and sodium chloride, and added sugar and / or sugar alcohols are absent. The anti-PD-L1 antibody preparation of the invention can maintain antibody stability after long-term storage. In addition, the invention further relates to a use of the anti-PD-L1 antibody preparation in preparing drugs used for the prevention and / or treatment and / or adjuvant treatment and / or diagnosis of tumors or anemia
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Methyl methacrylate block copolymer and preparation method thereof
InactiveCN101935382AImprove hydrophilicityGood compatibilityEnd-groupAtom-transfer radical-polymerization
The invention discloses a methyl methacrylate block copolymer and a preparation method thereof. The copolymer is polymethylmethacrylate-b-polyacrylic acid-2-ethyl ester serving as an AB block copolymer. The polymethylmethacrylate-b-polyacrylic acid-2-ethyl ester is synthesized by a fractional step method which comprises the following steps of: obtaining end group chlorine-containing polymethylmethacrylate by adopting azodiisobutyronitrile as an initiator and ferric trichloride / triphenylphosphine as a catalytic system through the reverse atom transfer radical polymerization of methyl methacrylate; and obtaining the two-block polymer polymethylmethacrylate-b-polyacrylic acid-2-ethyl ester by adopting the end group chlorine-containing polymethylmethacrylate as the macromolecular initiator and the ferric trichloride / triphenylphosphine as the catalytic system through the atom transfer radical polymerization. The block copolymer has the structure shown as the description.
Owner:GUANGDONG UNIV OF TECH
Phospholipid-based pharmaceutical formulations and methods for producing and using same
InactiveUS20090238880A1Good physiological compatibilityImprove abilitiesAntibacterial agentsBiocidePhospholipidPharmaceutical formulation
Owner:CONFORMAL THERAPEUTICS CORP (US)
Preparation method and application of reduced glutathione lipidosome
InactiveCN103976961AImprove stabilityImprove physical stabilityPowder deliveryTripeptide ingredientsWater bathsFreeze-drying
The invention discloses a preparation method and application of reduced glutathione lipidosome, relates to a preparation method and application of lipidosome, and aims at solving the technical problem of low inclusion rate of the lipidosome prepared by the existing method. The method comprises the following steps: 1, weighing soya bean lecithin and fatty acid glycerides, dissolving into absolute ethyl alcohol, and heating in a water bath to prepare an oil phase; 2, weighing reduced glutathione and a surfactant to be dissolved into distilled water, and heating in the water bath to prepare a water phase; 3, mixing the prepared oil phase with the water phase, emulsifying, carrying out vacuum rotary evaporation to remove absolute ethyl alcohol, adding distilled water, pre-cooling after ice-bath, and then carrying out freeze drying to obtain lipidosome powder. The reduced glutathione lipidosome disclosed by the invention is applied to intravenous injection, oral medication, transdermal drug delivery, and a drug delivery system for the lung and eyes. The encapsulation efficiency of the reduced glutathione lipidosome disclosed by the invention is 68.15%+ / -1.53%. The preparation method is applied to the field of preparation of the lipidosome.
Owner:李永吉
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com