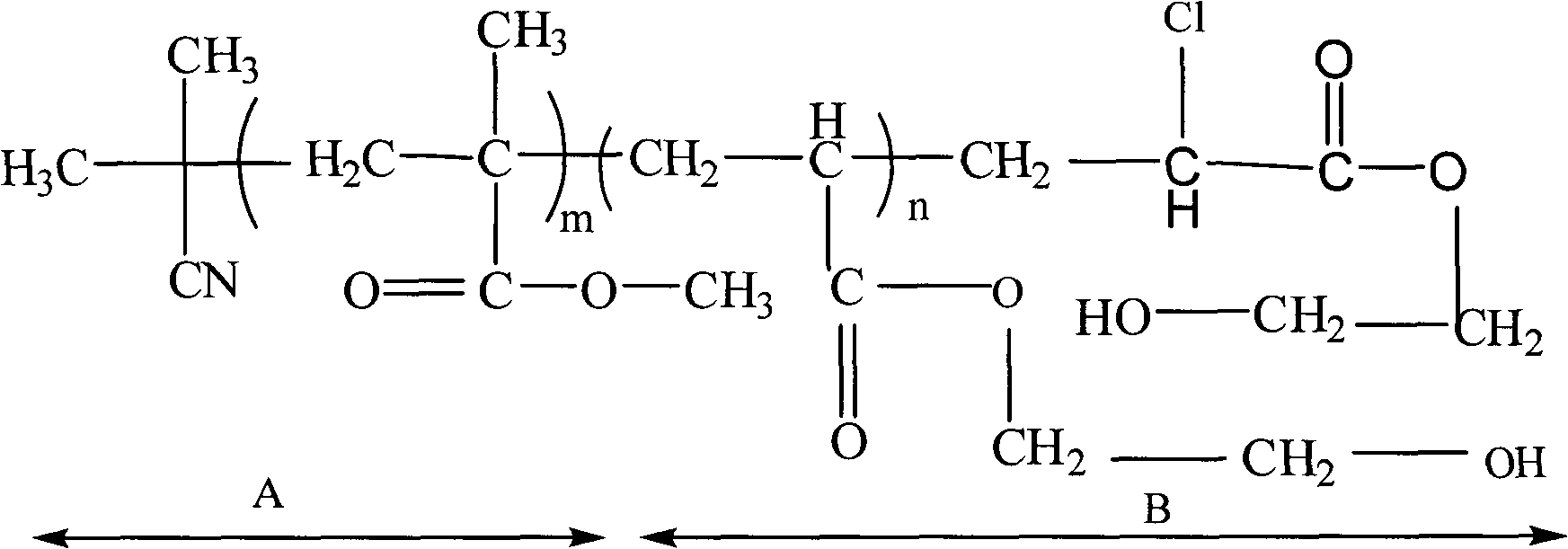
Methyl methacrylate block copolymer and preparation method thereof
A technology of methyl methacrylate and polymethyl methacrylate, which is applied in the field of methyl methacrylate block copolymer and its preparation, can solve the problems of not being disclosed, and achieve strong complementarity, excellent light transmittance, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 3
[0023] In a three-necked flask equipped with a stirrer, add azobisisobutyronitrile, ferric chloride, triphenylphosphine, and methyl methacrylate in sequence at a molar ratio of 1:1:3:200, and then Add xylene solvent at a concentration of 1g / ml, start stirring, and pass nitrogen gas to make it completely dissolved. The reaction process still needs to continue to pass nitrogen protection. React at 80°C for 3 hours and 4.5 hours, stop the reaction after 6 hours, cool naturally, add tetrahydrofuran until the polymer is completely dissolved, pass through a neutral alumina chromatography column, precipitate the filtrate with an appropriate amount of methanol, and filter with suction to obtain the precipitate . Then the precipitate was washed three times with deionized water, and vacuum-dried at 80° C. to constant weight to obtain polymethyl methacrylate containing terminal chlorine.
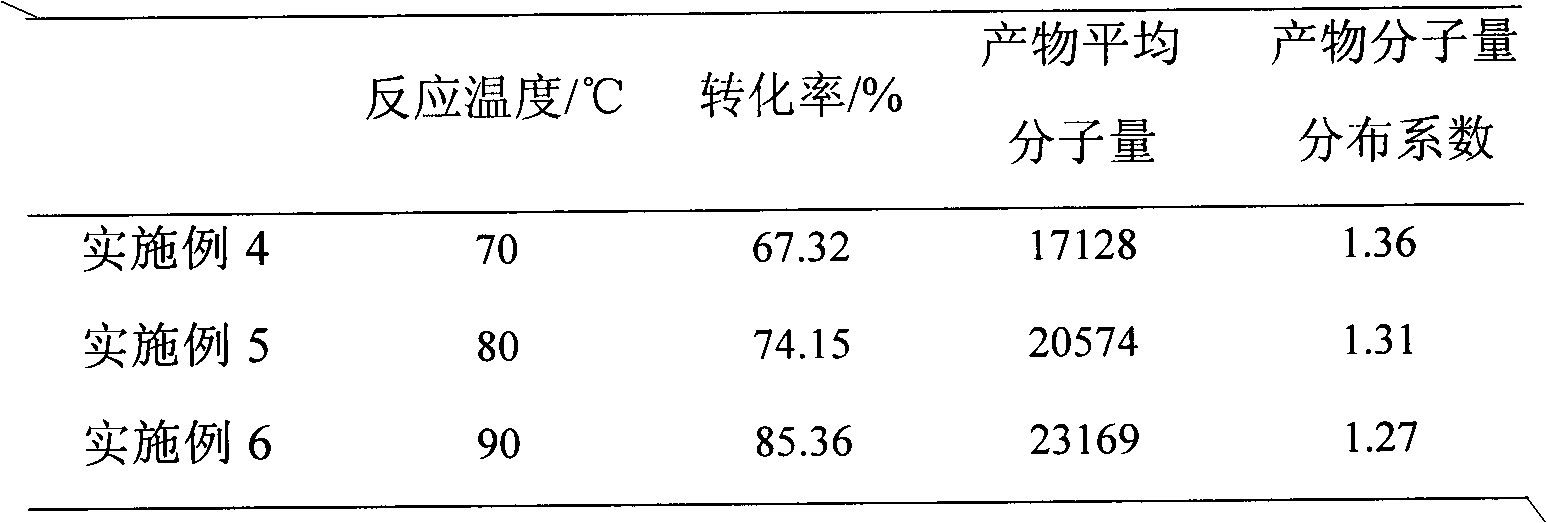
Embodiment 4 to 6
[0025] In a three-necked flask equipped with a stirrer, azobisisobutyronitrile, ferric chloride, triphenylphosphine, and methyl methacrylate were sequentially added in a molar ratio of 1:1:3:500, and then Add xylene solvent at a concentration of 1g / ml, start stirring, and pass nitrogen gas to make it completely dissolved. The reaction process still needs to continue to pass nitrogen protection. React at 70°C, 80°C, and 90°C for 5 hours to stop the reaction, cool naturally, add tetrahydrofuran until the polymer is completely dissolved, and pass through a neutral alumina chromatography column. The filtrate is precipitated with an appropriate amount of methanol, and the precipitate is obtained by suction filtration. Then the precipitate was washed three times with deionized water, and dried in a vacuum oven at 80° C. to constant weight to obtain polymethyl methacrylate containing terminal chlorine.
Embodiment 7 to 9
[0027] In a three-necked flask equipped with magnetic stirring and an inert gas conduit, polymethyl methacrylate containing terminal chlorine, ferrous chloride, triphenylphosphine, acrylic acid- 2-Hydroxyethyl ester, polymethyl methacrylate containing terminal chlorine is synthesized according to the above-mentioned Example 3, the stirring is started, and nitrogen gas is passed through to make it completely dissolved, and the reaction process still needs to continue to pass nitrogen gas for protection. React at 80°C for 3 hours and 4.5 hours, stop the reaction after 6 hours, cool naturally, add tetrahydrofuran to dissolve the polymer completely, and pass through the neutral alumina chromatography column, the filtrate is precipitated with an appropriate amount of methanol, filtered with suction, A precipitate was obtained. Then the precipitate was rinsed three times with deionized water, and vacuum-dried at 80° C. to constant weight to obtain a block copolymer polymethyl methac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com