Hydrobromic acid lappaconitine solid lipid nano particle and preparation method thereof
A technology of urbinine hydrobromide and solid lipid nanotechnology, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, and medical preparations containing active ingredients, etc. Problems such as solid lipid nanoparticles and drug loading limit the wide application of solid lipid nanoparticles, and low drug loading of solid lipid nanoparticles to improve bioavailability, reduce drug dosage and side effects, and have good analgesic effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The percentage in the prescription is the weight percentage that this component accounts for in the whole prescription, and the following examples are the same.
[0051] Prescription: Hovenine Hydrobromide 200mg
[0052] Glyceryl monostearate 2.5g
[0053] Poloxamer 188 1.5g
[0054] Tween-80 1.5g
[0055] water 50mL
[0056] Preparation of homogenate hydrobromide solid lipid nanoparticles:
[0057] Step 1: After mixing Poloxamer 188 and Tween-80 in a beaker, add an appropriate amount of distilled water to ultrasonically disperse until completely dissolved to form an aqueous phase; fully mix urine hydrobromide and glyceryl monostearate Mix and melt to form the oil phase;
[0058] Step 2: Heat the water phase and the oil phase to 75°C respectively, and add the water phase to the oil phase dropwise under stirring conditions to make colostrum;
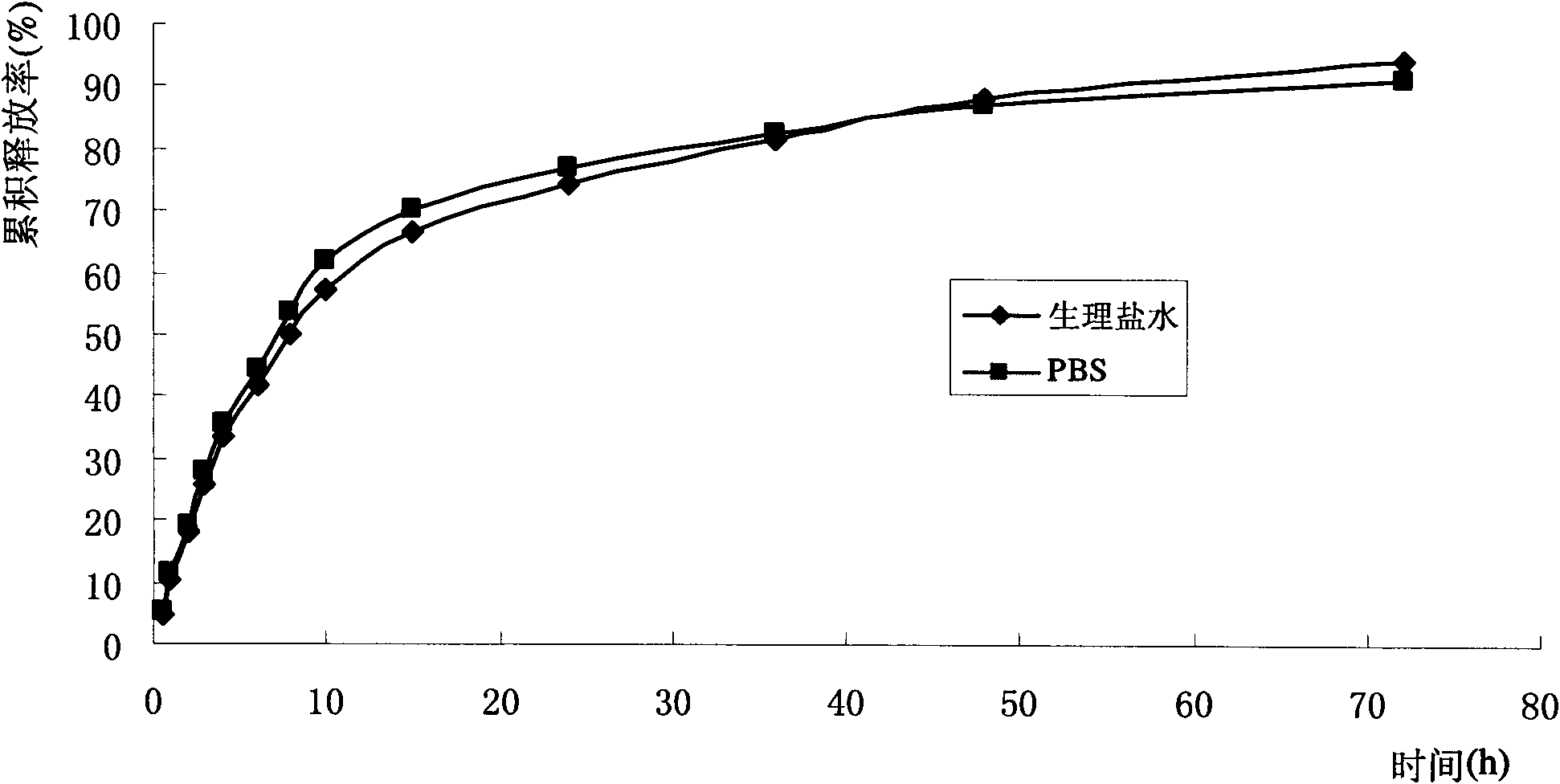
[0059] Step 3: Homogenize colostrum high pressure milk to obtain a transparent suspension with ligh...
Embodiment 2
[0063] Prescription: Hovenine Hydrobromide 320mg
[0064] Palmitic acid 1g
[0065] Poloxamer 407 3.5g
[0066] water 50mL
[0067] Preparation of homogenate hydrobromide solid lipid nanoparticles:
[0068] Step 1: Put Poloxamer 407 in a beaker, add an appropriate amount of distilled water to ultrasonically disperse until completely dissolved, and form a water phase; fully mix and melt homogenin hydrobromide and palmitic acid to form an oil phase;
[0069] Step 2: Heat the water phase and the oil phase to 85°C respectively, and add the water phase to the oil phase dropwise under stirring conditions to make colostrum;
[0070] Step 3: Homogenize colostrum high pressure milk to obtain a transparent suspension with light blue opalescence;
[0071] Step 4: placing the translucent suspension at 0-4° C., cooling and solidifying to form an aqueous dispersion of solid lipid nanoparticles.
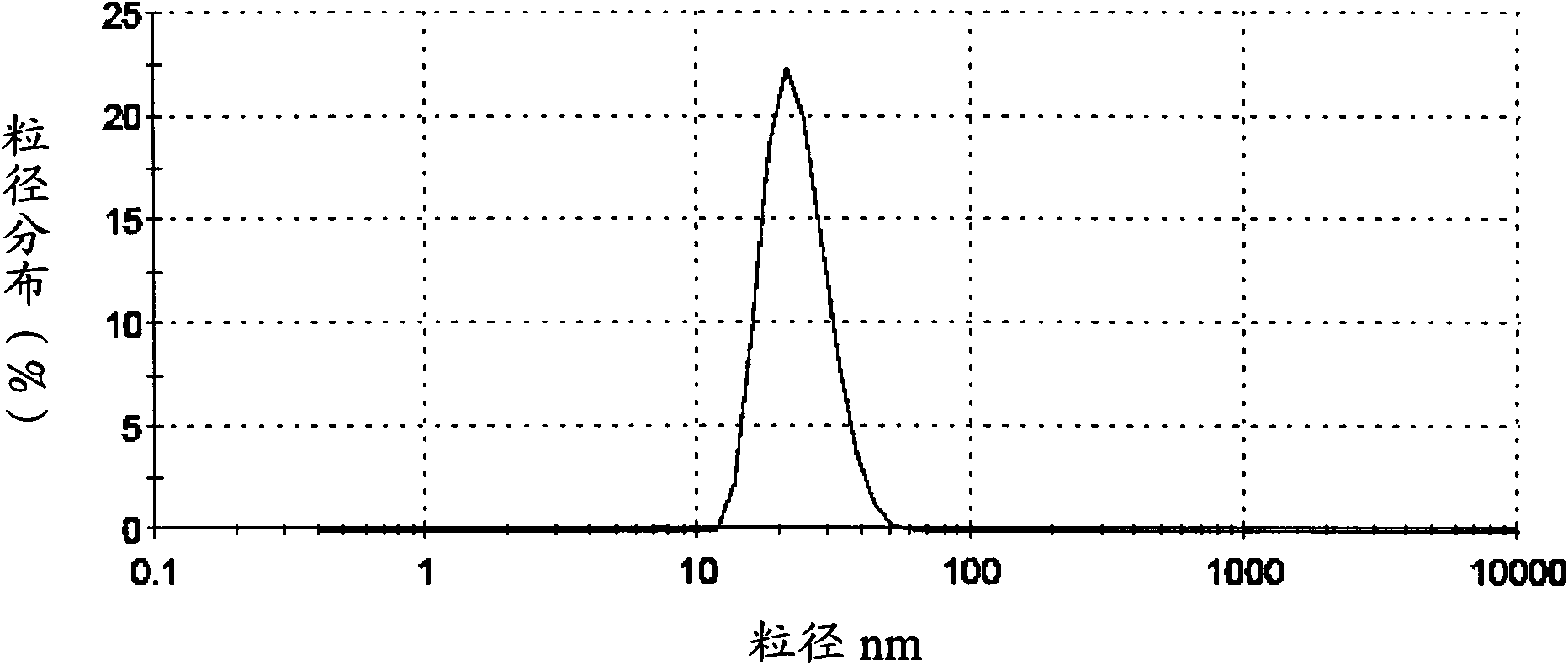
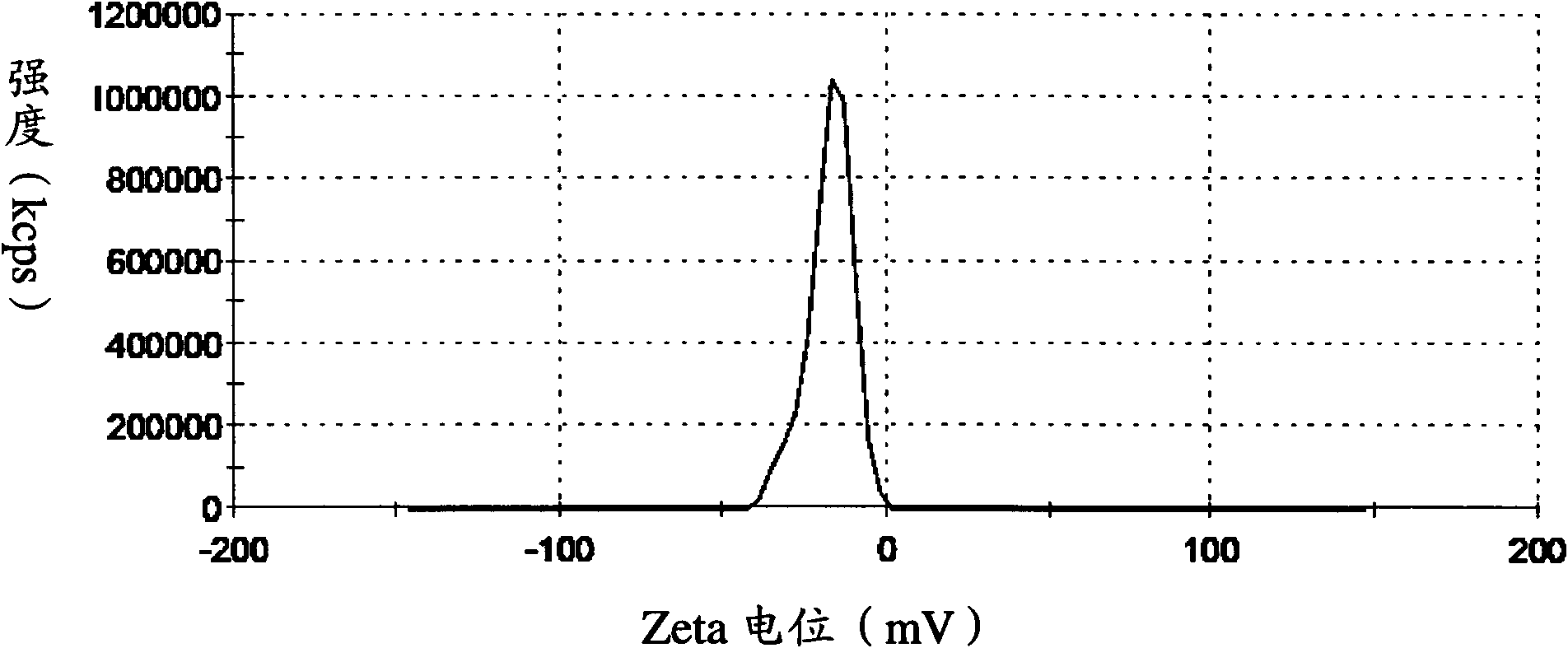
[0072] Detection: the average particle size of the solid lipid nanoparti...
Embodiment 3
[0074] Prescription: Hovenine Hydrobromide 500mg
[0075] Glyceryl Behenate 1.5g
[0076] Poloxamer 188 0.5g
[0077] Egg yolk phospholipids 2g
[0078] water 50mL
[0079] Preparation of homogenate hydrobromide solid lipid nanoparticles:
[0080] Step 1: Put Poloxamer 188 in a beaker, add an appropriate amount of distilled water to ultrasonically disperse until completely dissolved, and form a water phase; fully mix and melt homogenin hydrobromide, glyceryl behenate, and egg yolk phospholipids to form an oil Mutually;
[0081] Step 2: Heat the water phase and the oil phase to 65°C respectively, and add the water phase to the oil phase dropwise under stirring conditions to make colostrum;
[0082] Step 3: Homogenize colostrum high pressure milk to obtain a transparent suspension with light blue opalescence;
[0083] Step 4: placing the translucent suspension at 0-4° C., cooling and solidifying to form an aqueous dispersion of solid lipid nanoparti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com