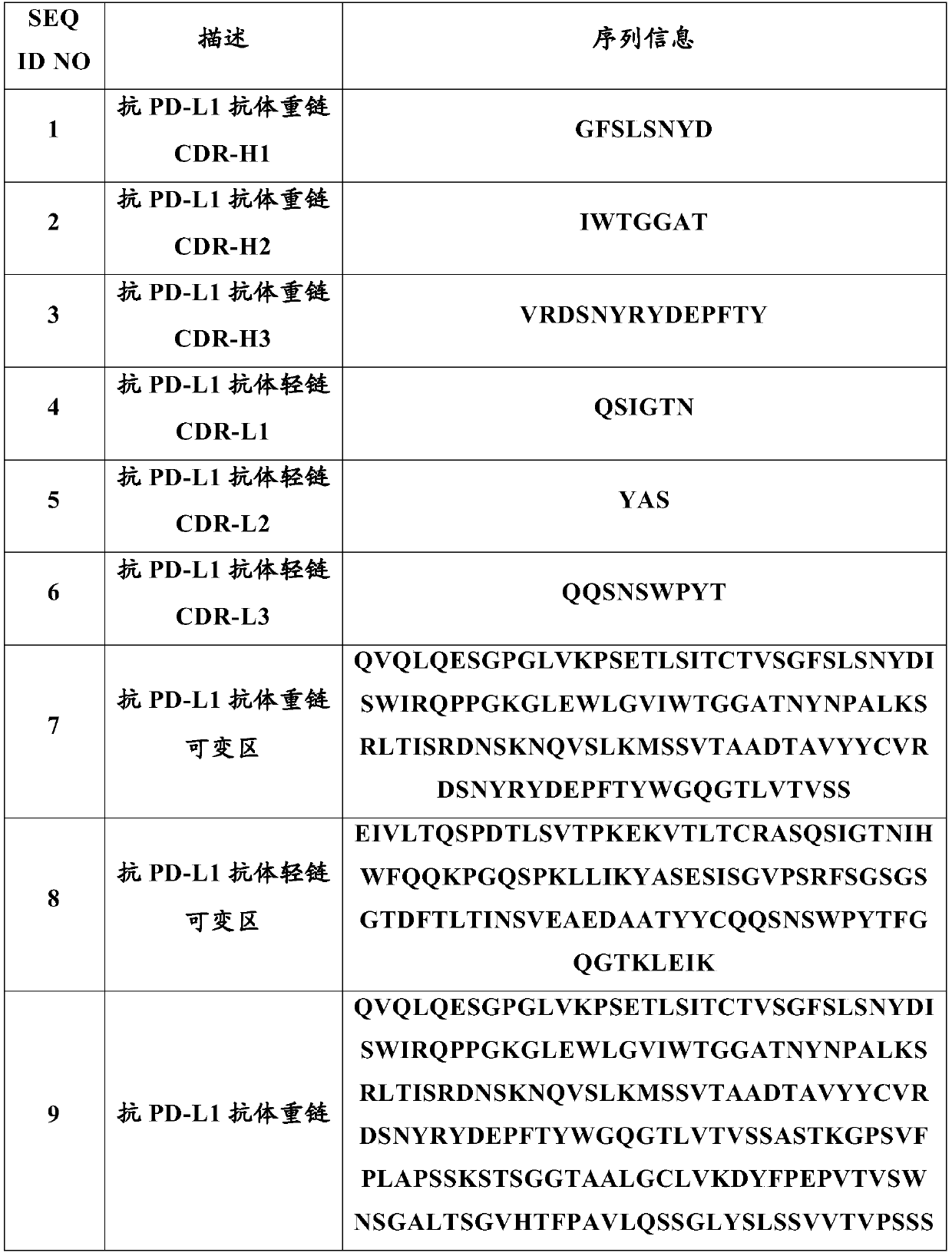
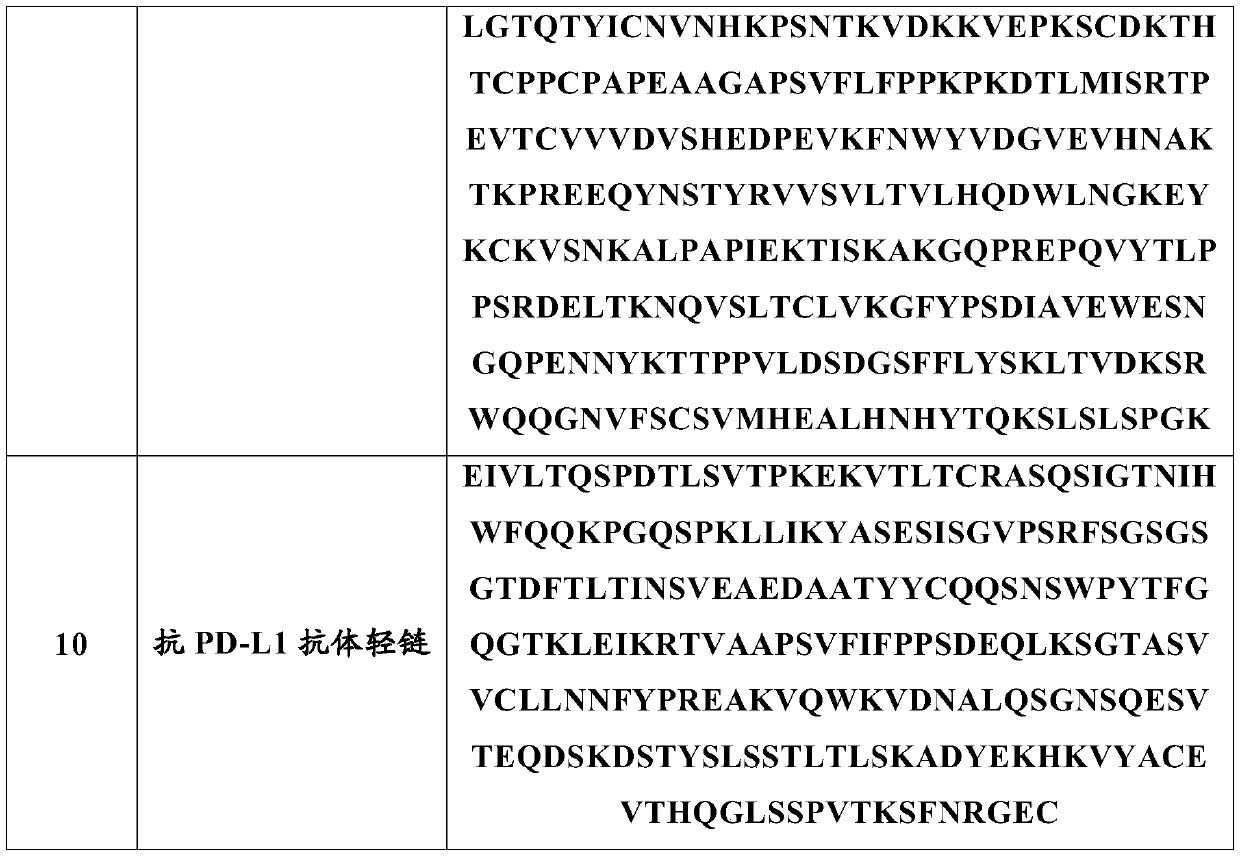
Anti-programmed cell death-ligand 1 (PD-L1) antibody preparation
A technology of PD-L1 and liquid preparations, which is applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., and can solve problems such as easy turbidity, poor stability, and high viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
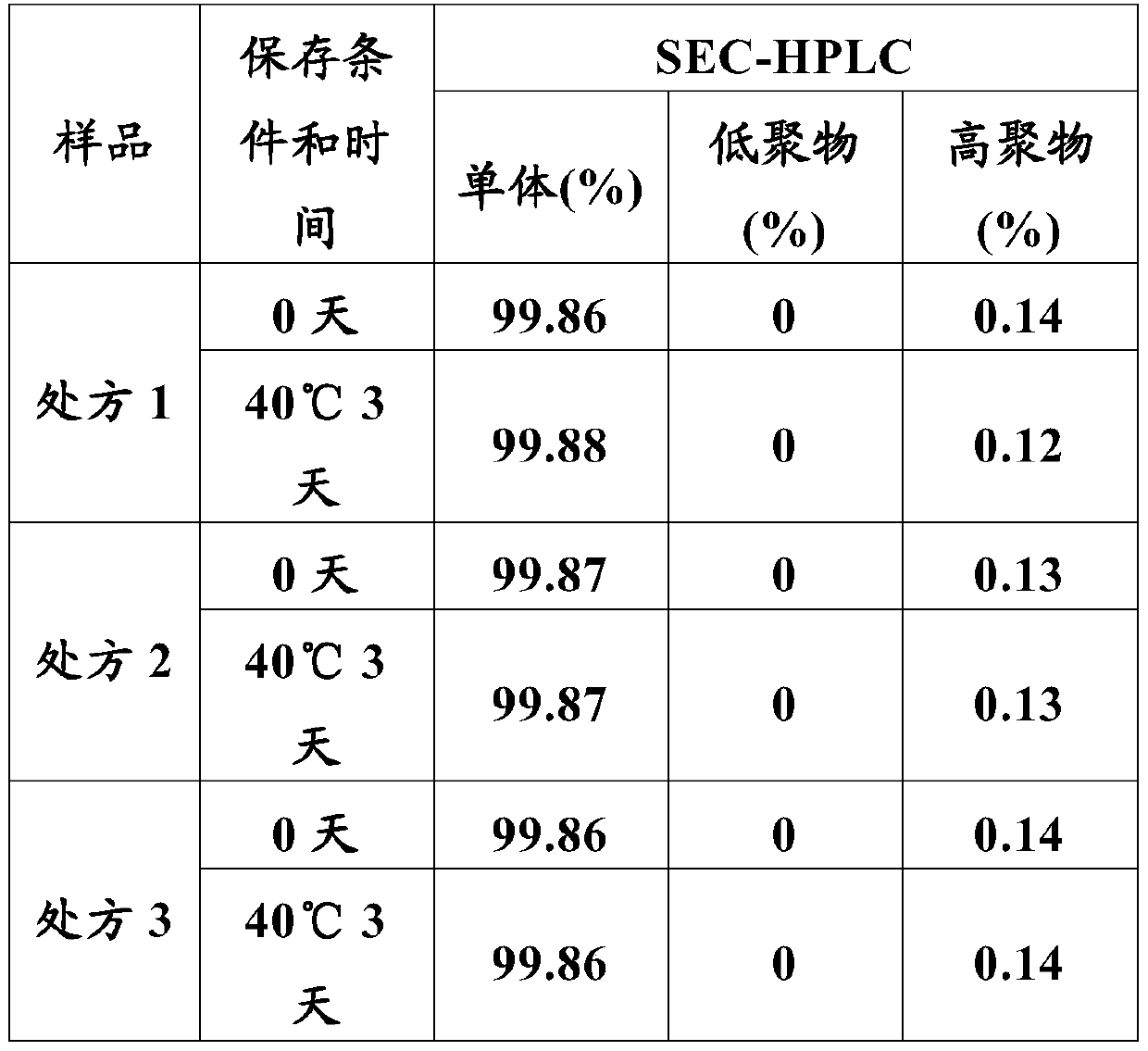
[0123] Prescription 1: anti-PD-L1 humanized monoclonal antibody 20mg / ml, 100mM sodium chloride, 20mM citric acid-sodium citrate, pH5.2.
[0124] Prescription 2: anti-PD-L1 humanized monoclonal antibody 20mg / ml, 100mM sodium chloride, 20mM citric acid-sodium citrate, pH 5.6.
[0125] Prescription 3: Anti-PD-L1 humanized monoclonal antibody 20mg / ml, 100mM sodium chloride, 20mM citric acid-sodium citrate, pH 6.0.
[0126] Prescription 4: anti-PD-L1 humanized monoclonal antibody 20mg / ml, 100mM sodium chloride, 20mM citric acid-sodium citrate, pH 6.4.
[0127] Prescription 5: anti-PD-L1 humanized monoclonal antibody 20mg / ml, 100mM sodium chloride, 20mM citric acid-sodium citrate, pH 6.8.
[0128] The preparation method of the antibody preparation of prescription 1-5 is as follows:
[0129] 1. Prepare buffer: add 100mM sodium chloride and 20mM citric acid to the water for injection, and adjust to the pH value required in the above prescription with sodium hydroxide.
[0130] 2. T...
Embodiment 2
[0139] Prescription 6: Anti-PD-L1 humanized monoclonal antibody 20mg / ml, 140mM sodium chloride, 20mM histidine-histidine hydrochloride, polysorbate 80 with a mass volume ratio of 0.02%, pH 5.8.
[0140]Prepare 100ml of the antibody preparation of prescription 6, the preparation method is as follows:
[0141] 1. Prepare the buffer solution: add 140mM sodium chloride and 20mM histidine to the water for injection, and adjust the pH to 5.8 with hydrochloric acid.
[0142] 2. The antibody stock solution (batch number 1) was replaced by ultrafiltration, dialyzed to the above buffer solution, adjusted to 20 mg / ml antibody concentration, and an appropriate amount of polysorbate 80 stock solution was added.
[0143] 3. The prepared sample is sterilized and filtered through a filter with a pore size of 0.22 μm under laminar flow conditions, filled into a vial, and the sample is obtained by plugging and capping.
[0144] The sample stability of prescription 6 was investigated at 40°C fo...
Embodiment 3
[0146] Prescription 7: Anti-PD-L1 humanized monoclonal antibody 20mg / ml, 140mM sodium chloride, 20mM histidine-histidine hydrochloride, polysorbate 80 with a mass volume ratio of 0.02%, pH 5.0.
[0147] Adjust the pH of the buffer to 5.0, and the rest of the preparation method is the same as in Example 2.
[0148] The sample stability of prescription 7 was investigated at 40°C for 28 days, and the test results are shown in Tables 5-9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com