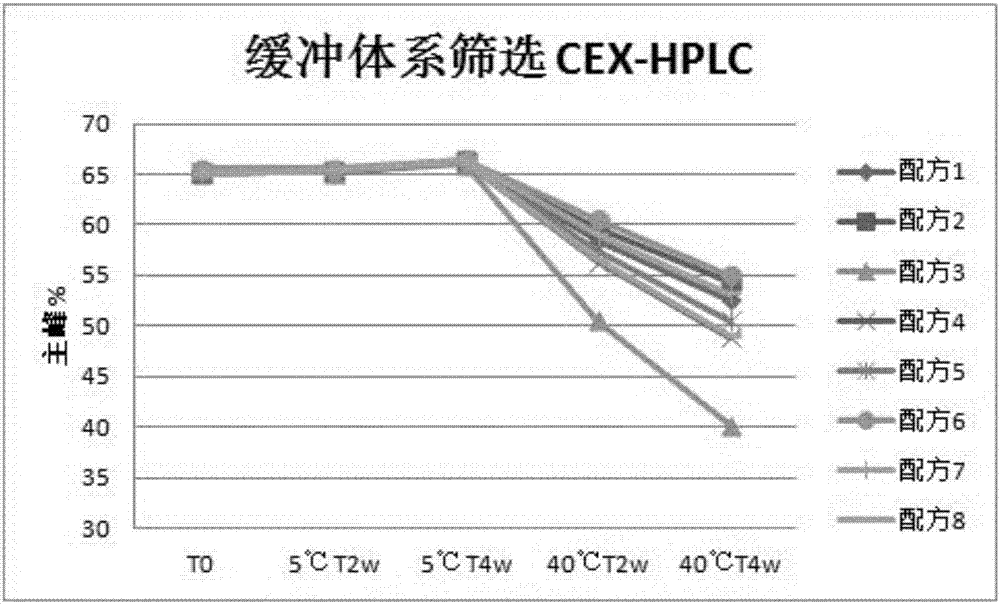
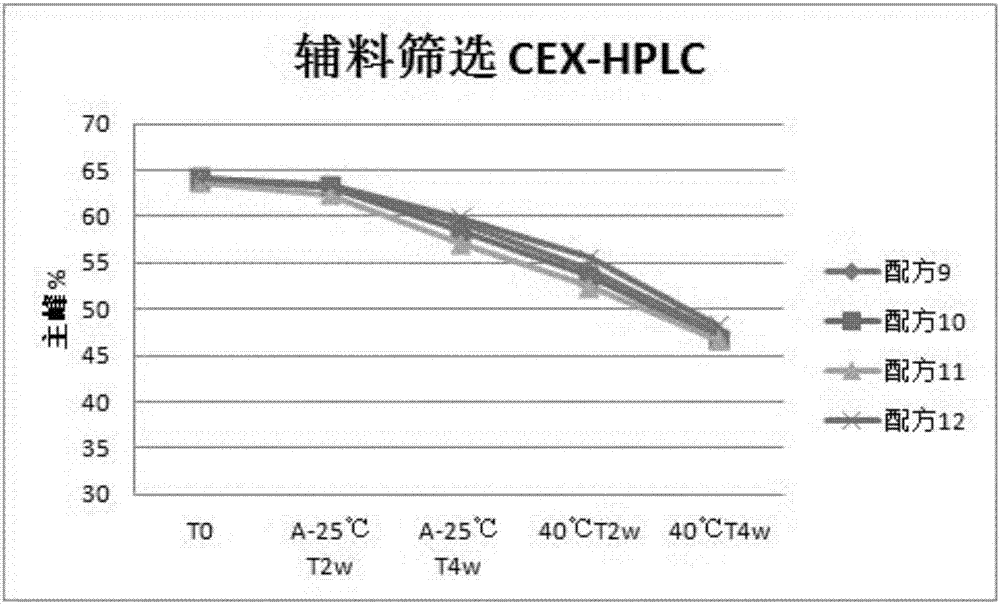
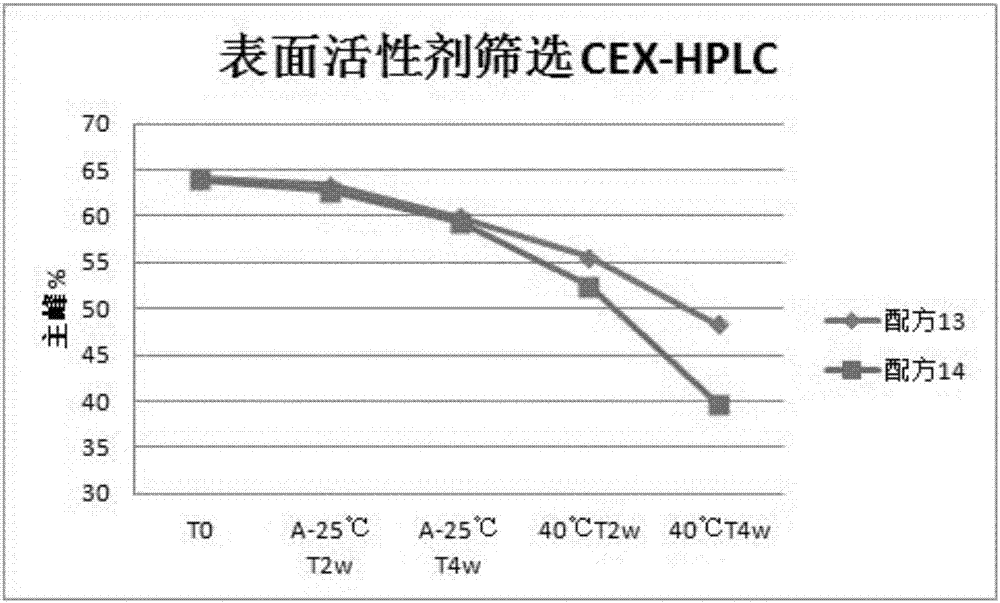
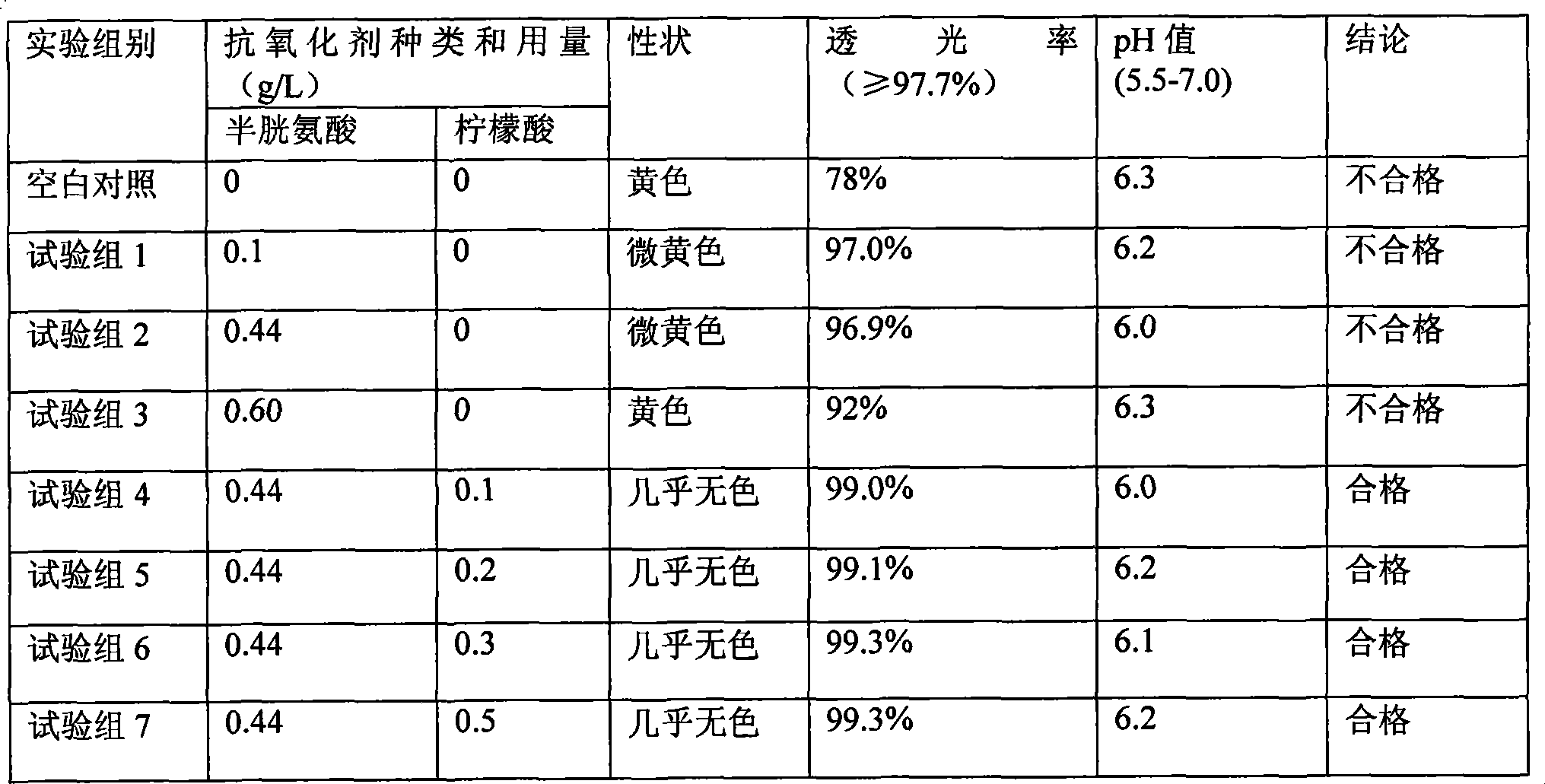
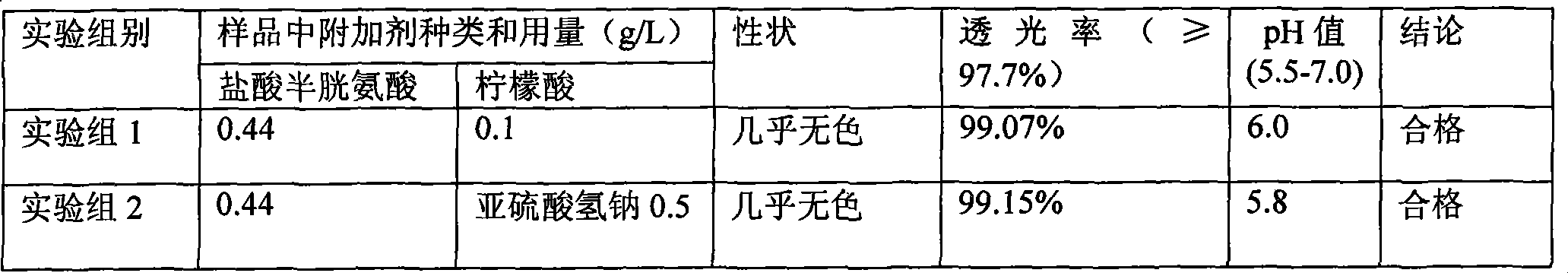
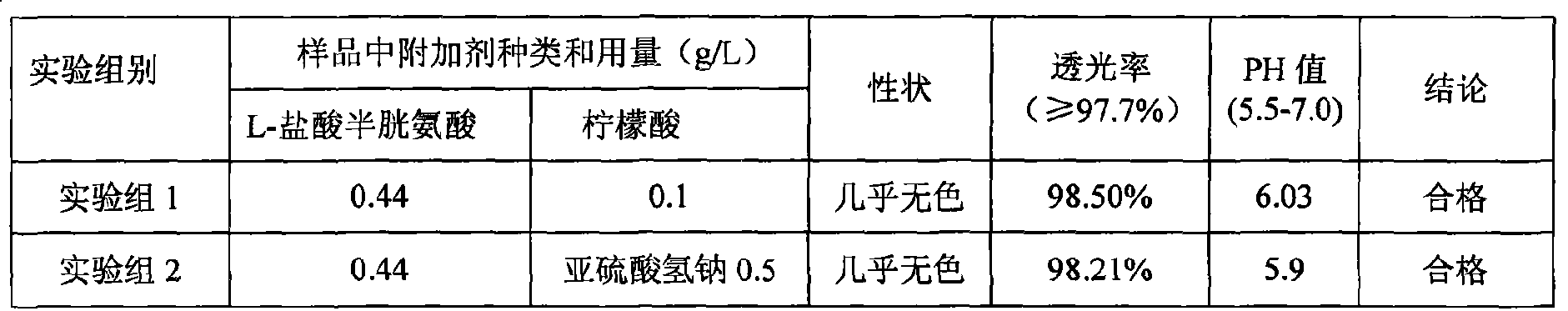
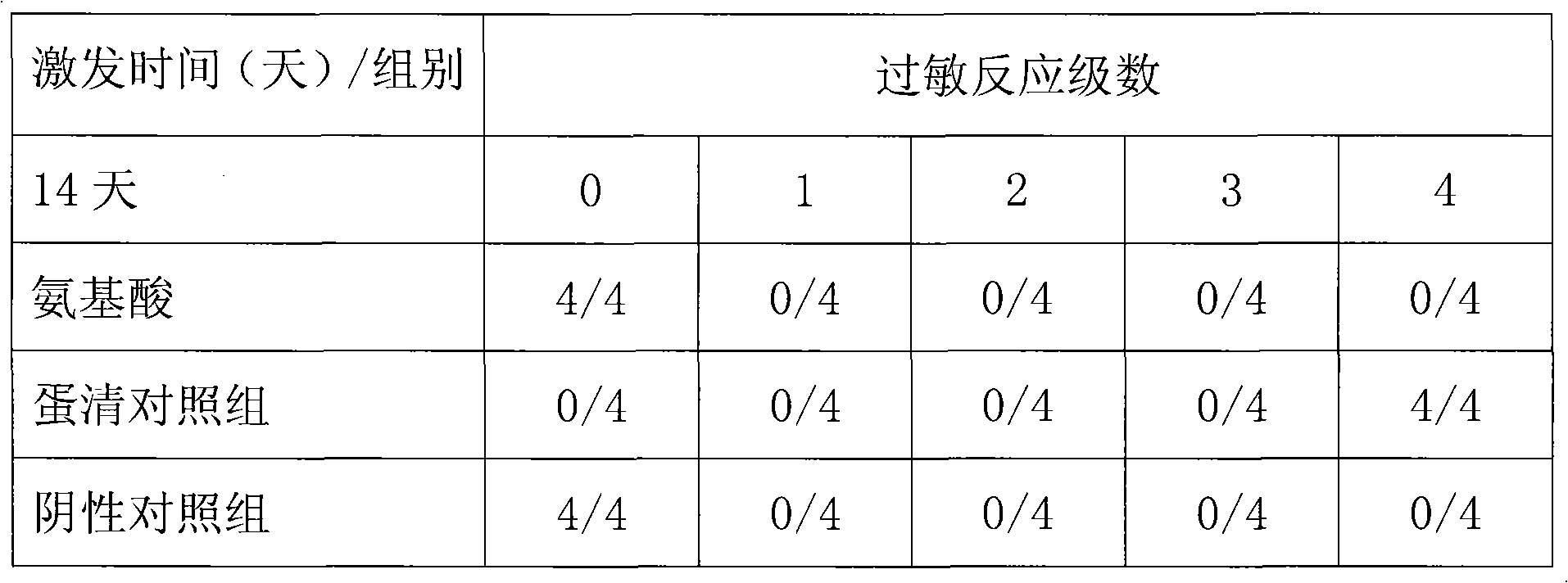
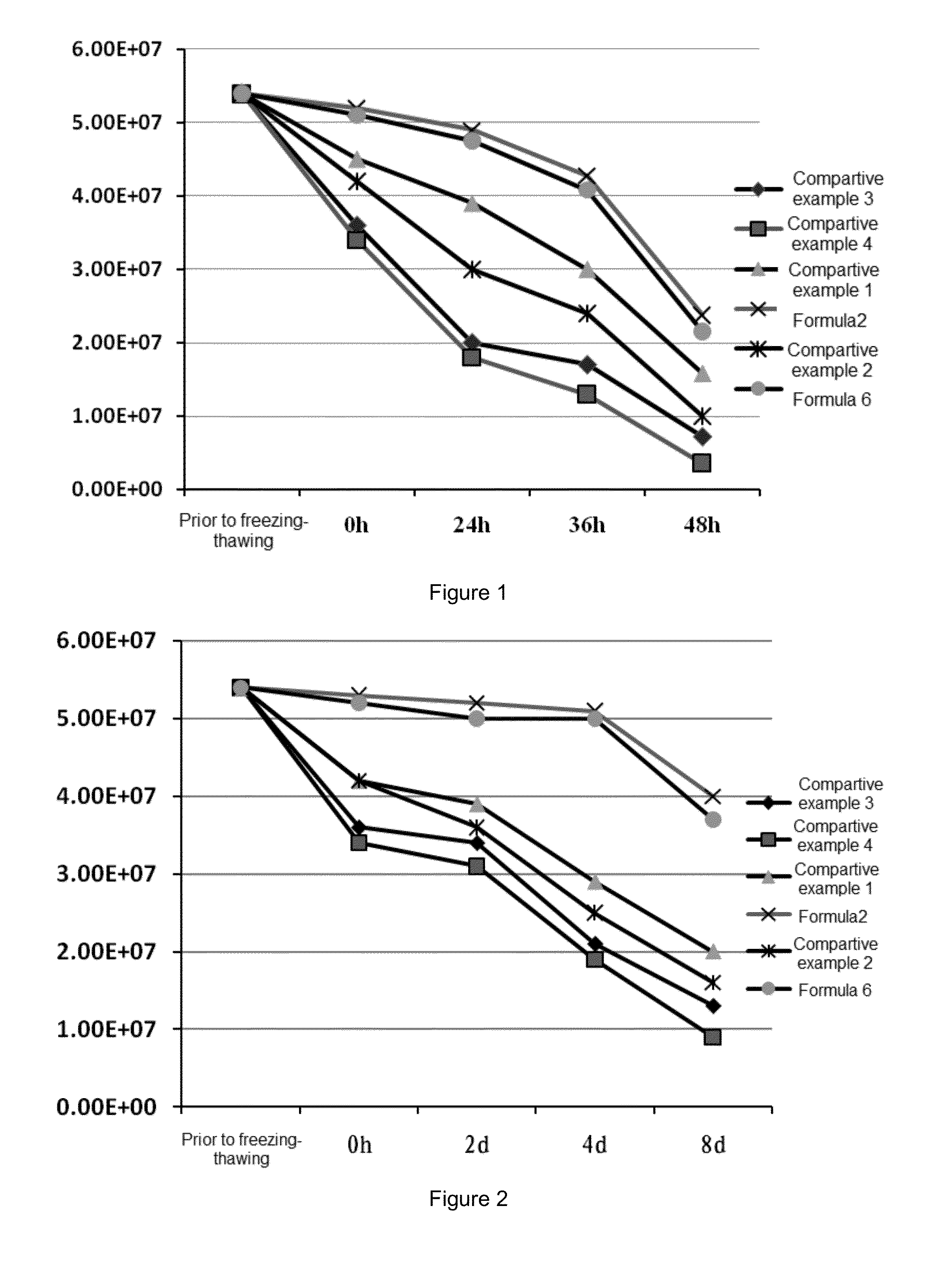
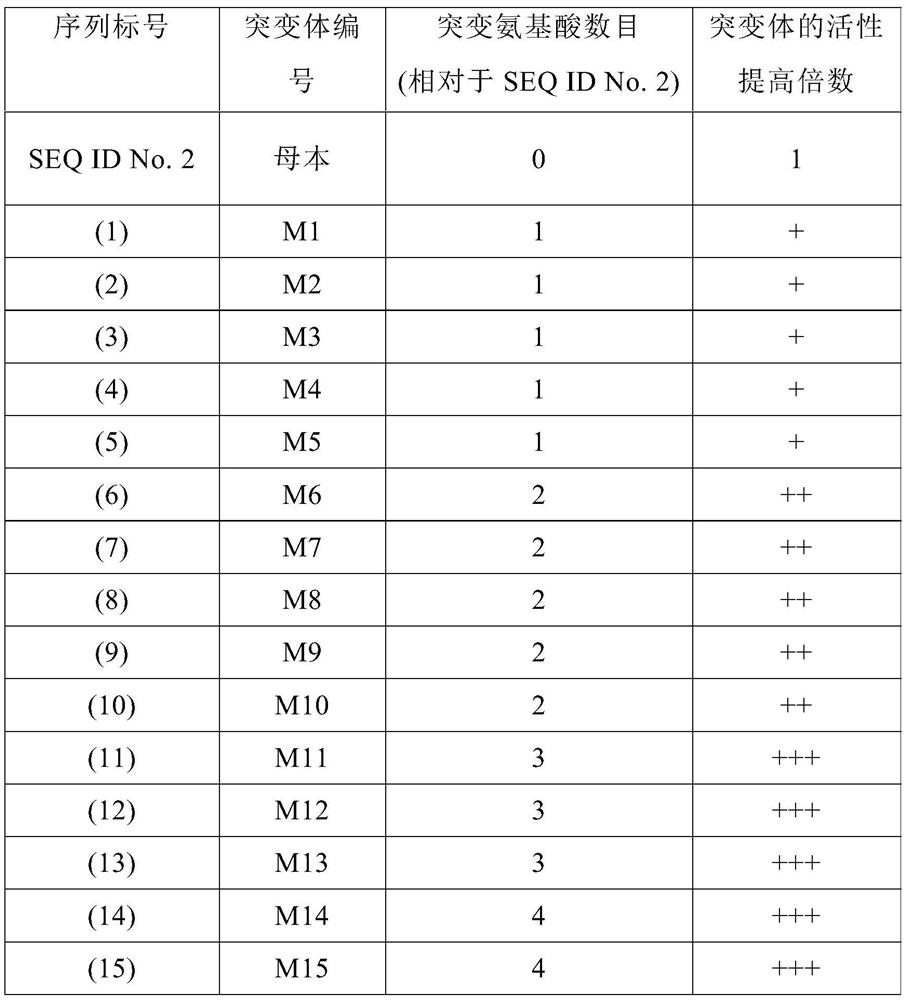
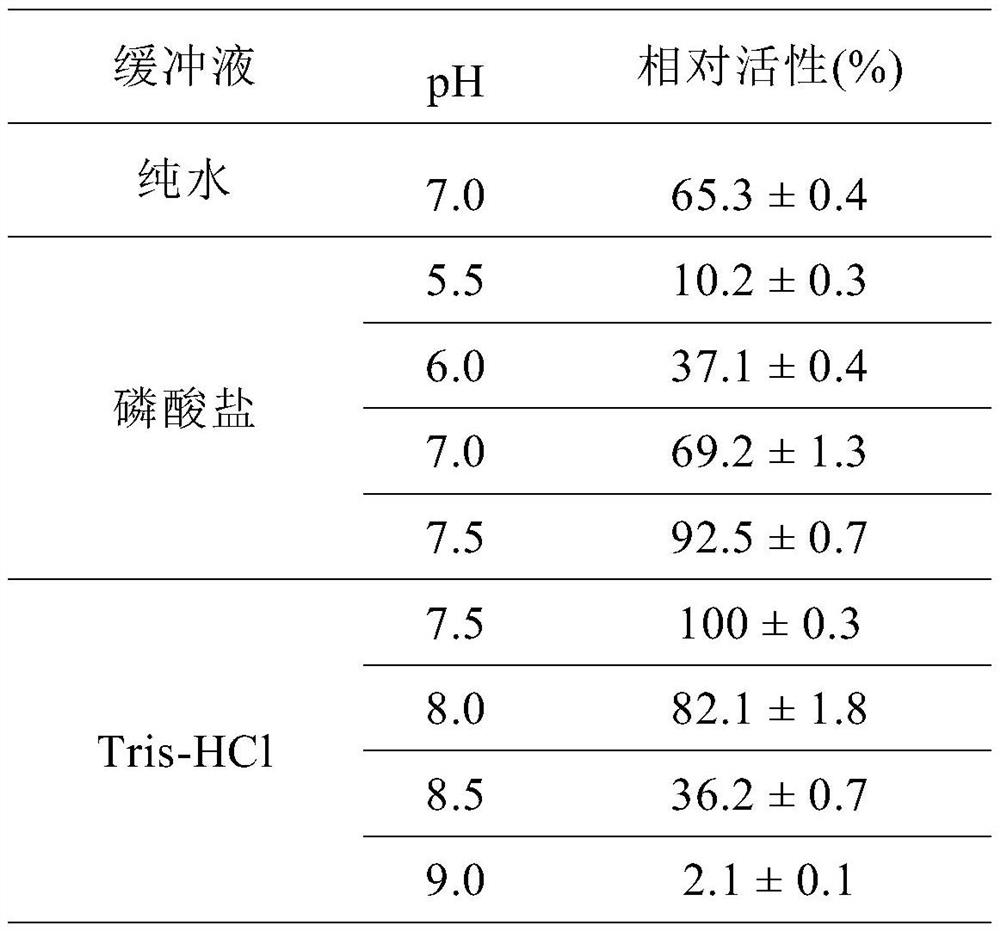
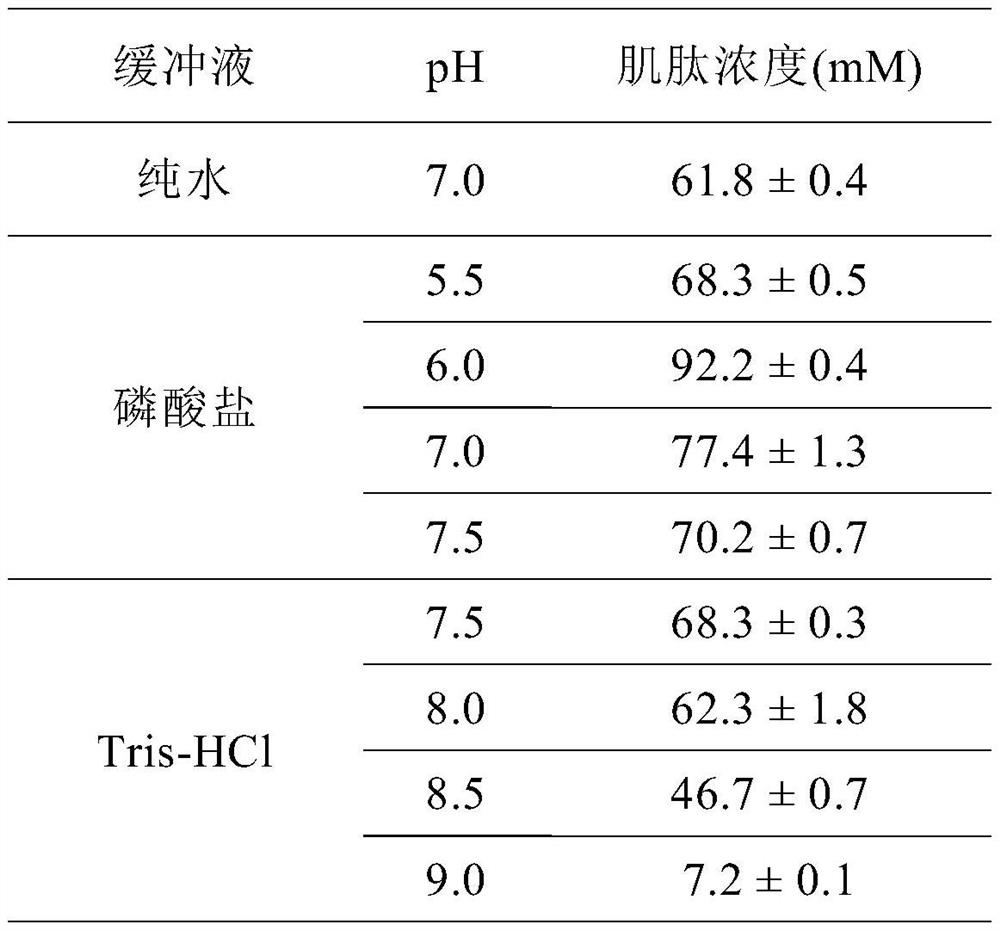
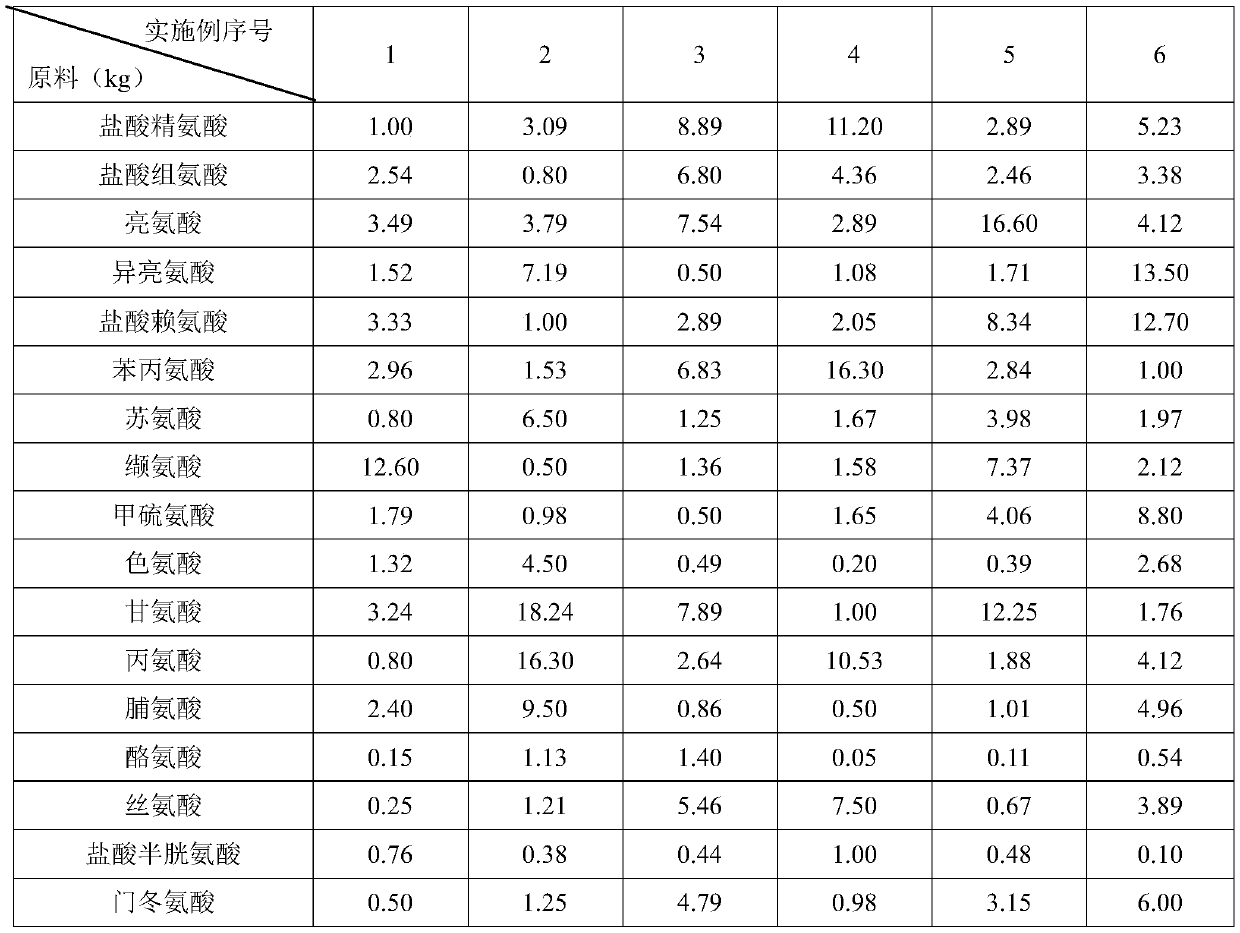
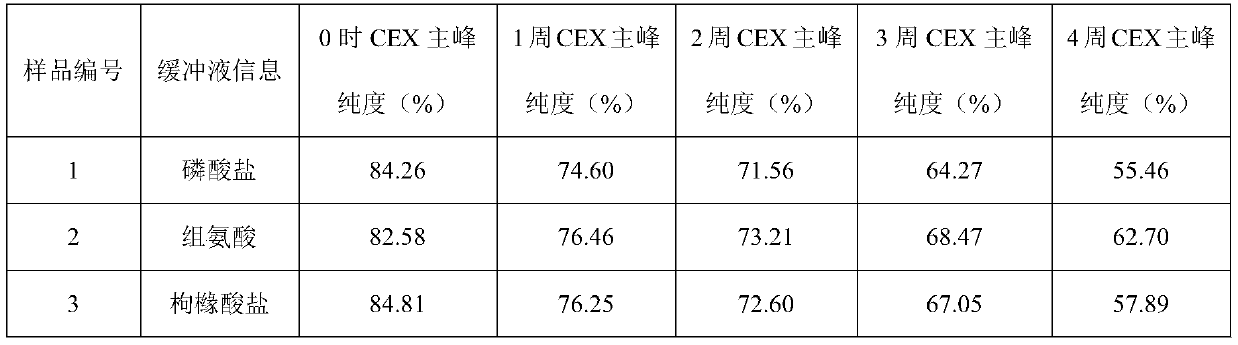
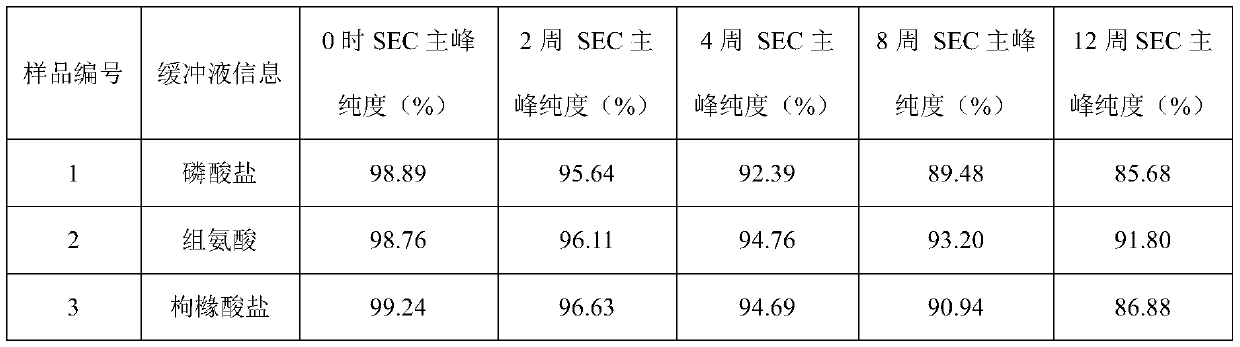
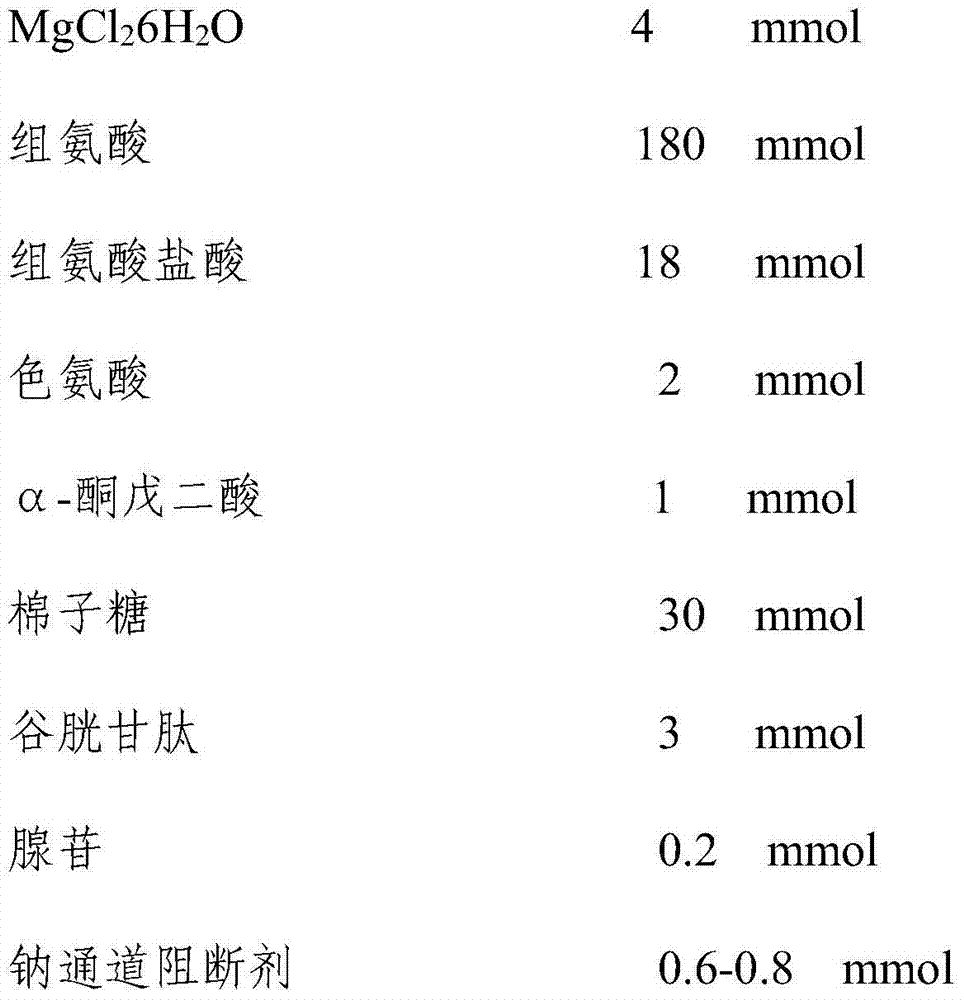
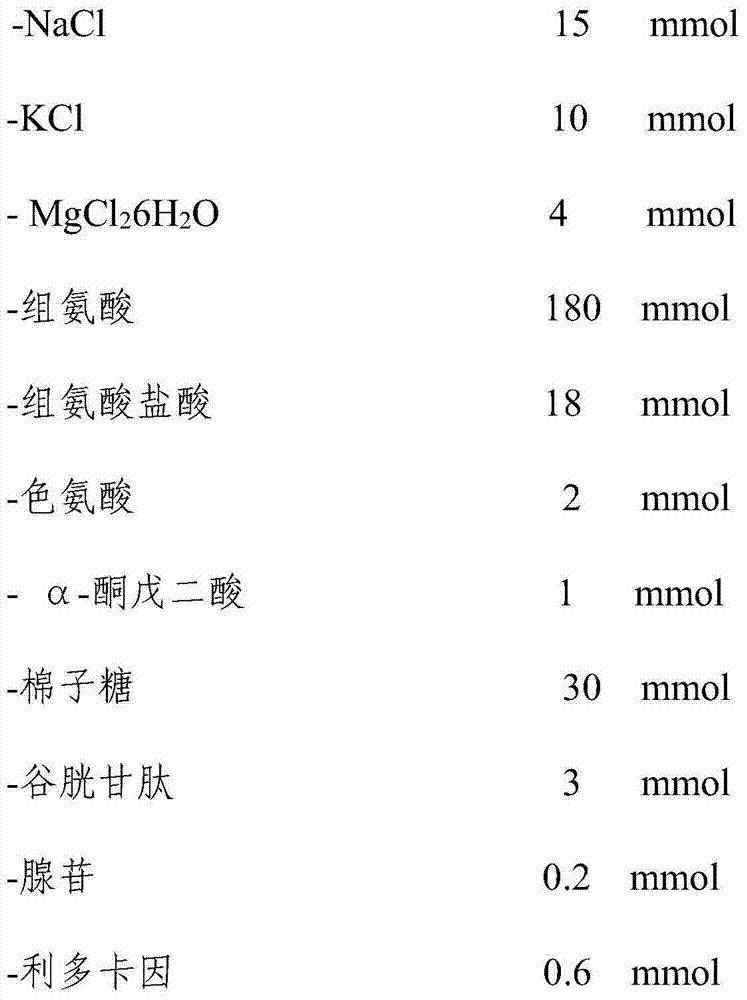
Patents
Literature
53 results about "Histidine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liquid preparation of recombinant anti-PD-L1 whole-human monoclonal antibody
InactiveCN107198773AImprove stabilityEasy to administerAntibody ingredientsImmunoglobulinsMonoclonal antibodyPD-L1
The invention discloses a stable liquid preparation of a recombinant anti-PD-L1 whole-human monoclonal antibody. The liquid preparation is prepared from a recombinant anti-PD-L1 whole-human monoclonal antibody, a buffer solution, a stabilizing agent, an osmotic pressure regulator and a surfactant, wherein the buffer solution is a histidine-histidine hydrochloride buffer solution; the stabilizing agent is mannitol; the osmotic pressure regulator is sodium chloride; the surfactant is polysorbate 80. The liquid preparation of the recombinant anti-PD-L1 whole-human monoclonal antibody has superior stability, and can be stored stably at the temperature of 5+ / -3 DEG C for at least 24 months.
Owner:CSTONE PHARM (SUZHOU) CO LTD +1
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439036AInhibition of oxidative decomposition reactionsQuality assuranceOrganic active ingredientsMetabolism disorderAntioxidantTryptophan
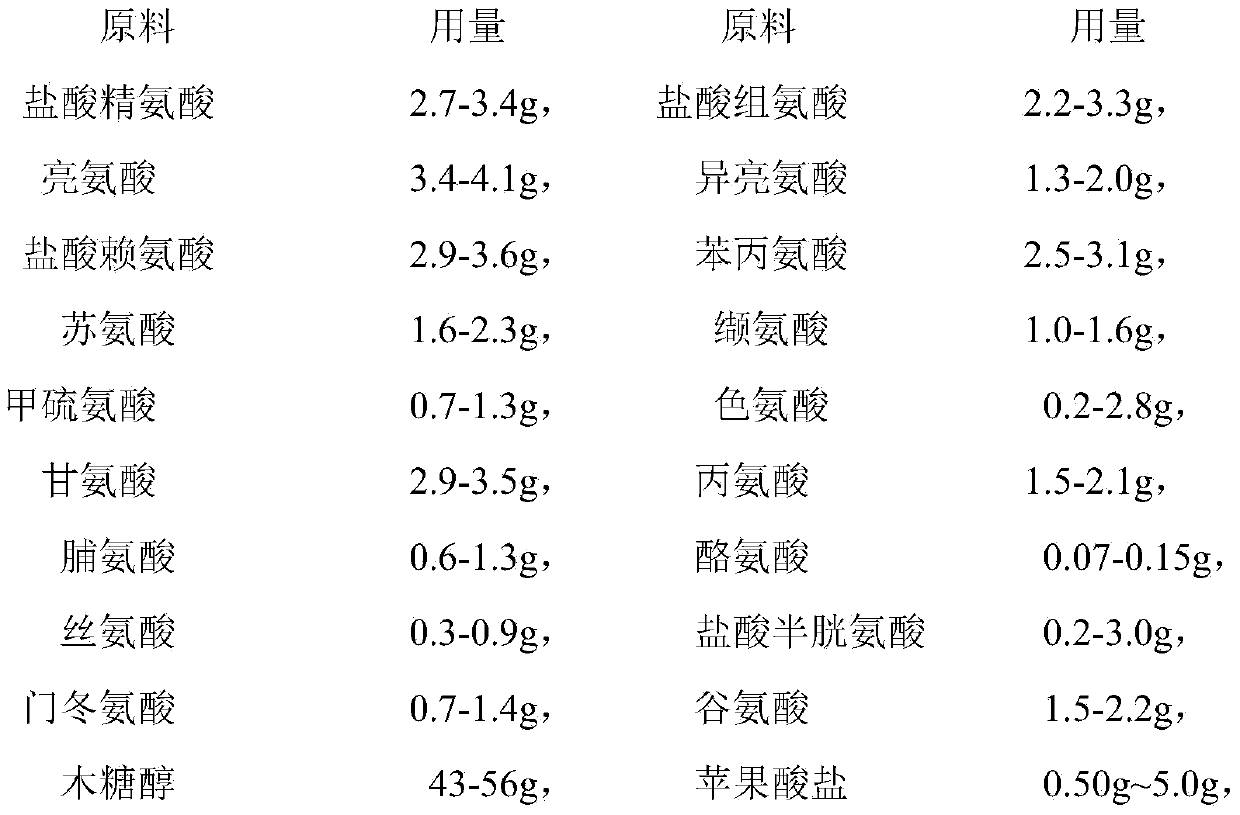
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-V) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 2.89 of arginine hydrochloride, 2.46 of histidine hydrochloride, 3.79 of leucine, 1.70 of isoleucine, 3.33 of lysine hydrochloride, 2.83 of phenylalanine, 1.97 of threonine, 1.36 of valine, 1.06 of methionine, 0.39 of tryptophan, 3.24 of glycine, 1.88 of alanine, 1.00 of proline, 0.11 of tyrosine, 0.67 of serine, 0.44 of cysteine hydrochloride, 1.15 of aspartic acid, 1.97 of glutamic acid, 50 of xylitol, 0.10 to 0.30 of citric acid and injection water with proper amount. The pharmaceutical composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test and a quality test, results show that the pharmaceutical composition is as stable as or more stable than like products (18AA-V) which are sold in the markets and contain sulfites.
Owner:福州凯瑞医药咨询有限公司
Amino acid injection and preparation method thereof
ActiveCN101401785APain reliefReduce clinical adverse reactionsOrganic active ingredientsMetabolism disorderAntioxidantThreonine
The invention discloses an amino acid injection, commonly known as propranolol, which relates to a pharmaceutical preparation containing 18 kinds of amino acids. The amino acid injection contains the following components per 1,000 milliliters: isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, valine, glycine, alanine, glutamic acid, aspartic acid, proline, serine, cystine, lysine hydrochloride or lysine acetate, arginine hydrochloride or arginine, histidine hydrochloride or histidine, tyrosine or acetyl tyrosine, and sodium bisulfite or sodium meta-bisulphite. The osmotic pressure ratio of the amino acid injection is lower than 1.8, which effectively reduces the pain for a patient in infusion; the added quantity of antioxidant is small, which can reduce the clinical adverse reactions; and an infusion bottle body is large, which is convenient to mix liquid in clinical application.
Owner:GUANGDONG LITAI PHARM CO LTD
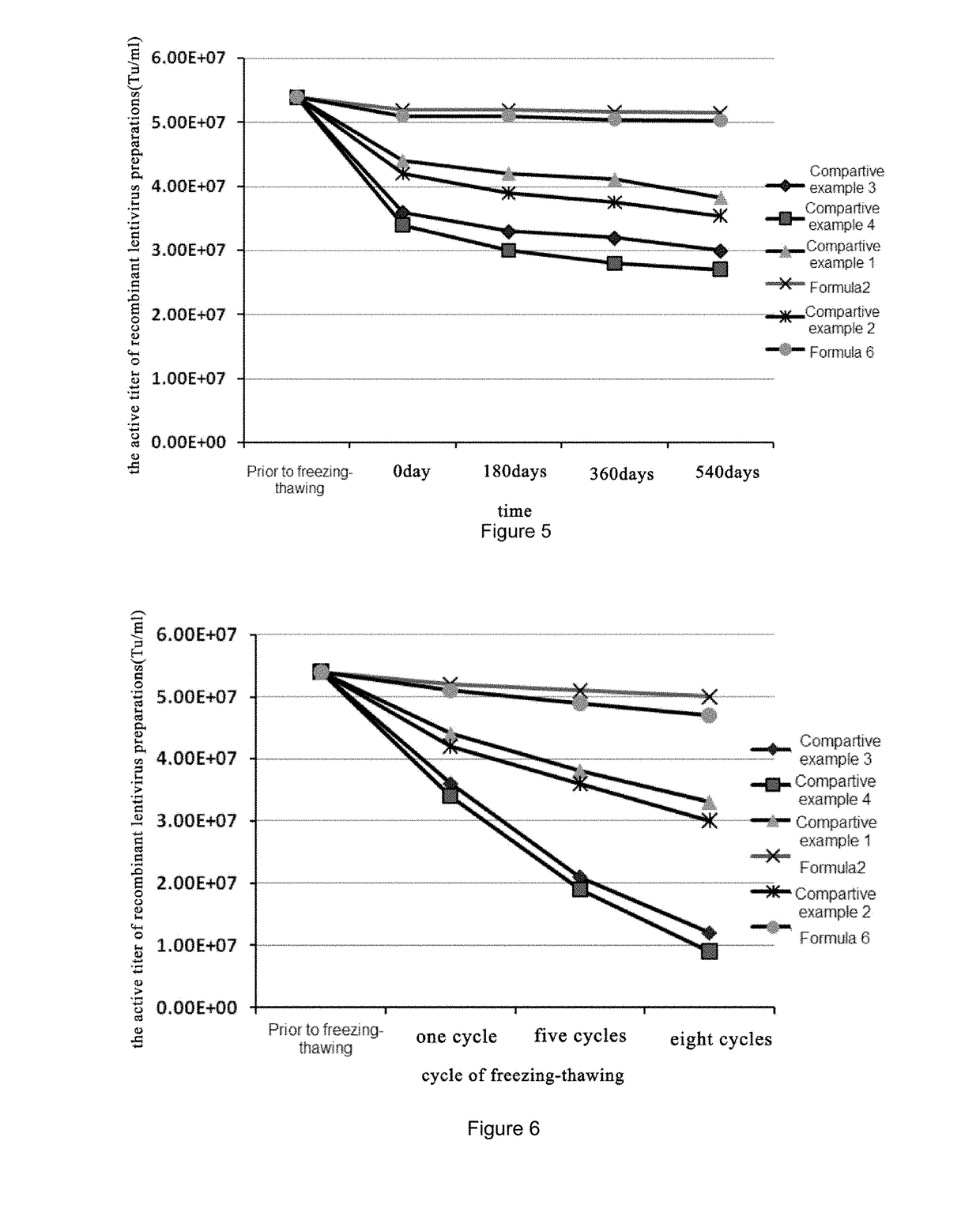
Recombinant lentiviral vector preparation
ActiveUS20150056696A1Improve stabilityMaintain long-term stabilityNervous disorderAntiviralsMedicineViral vector
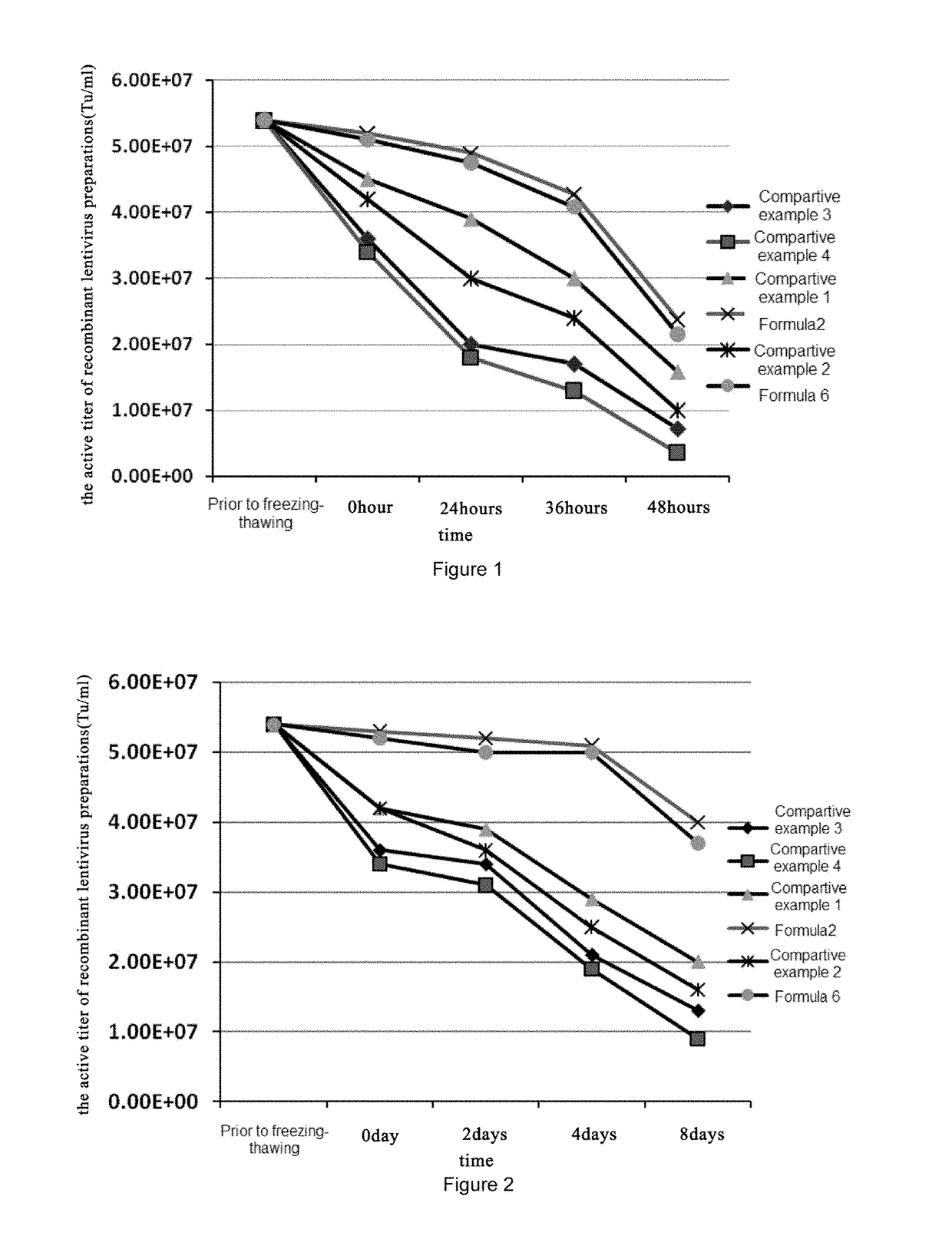
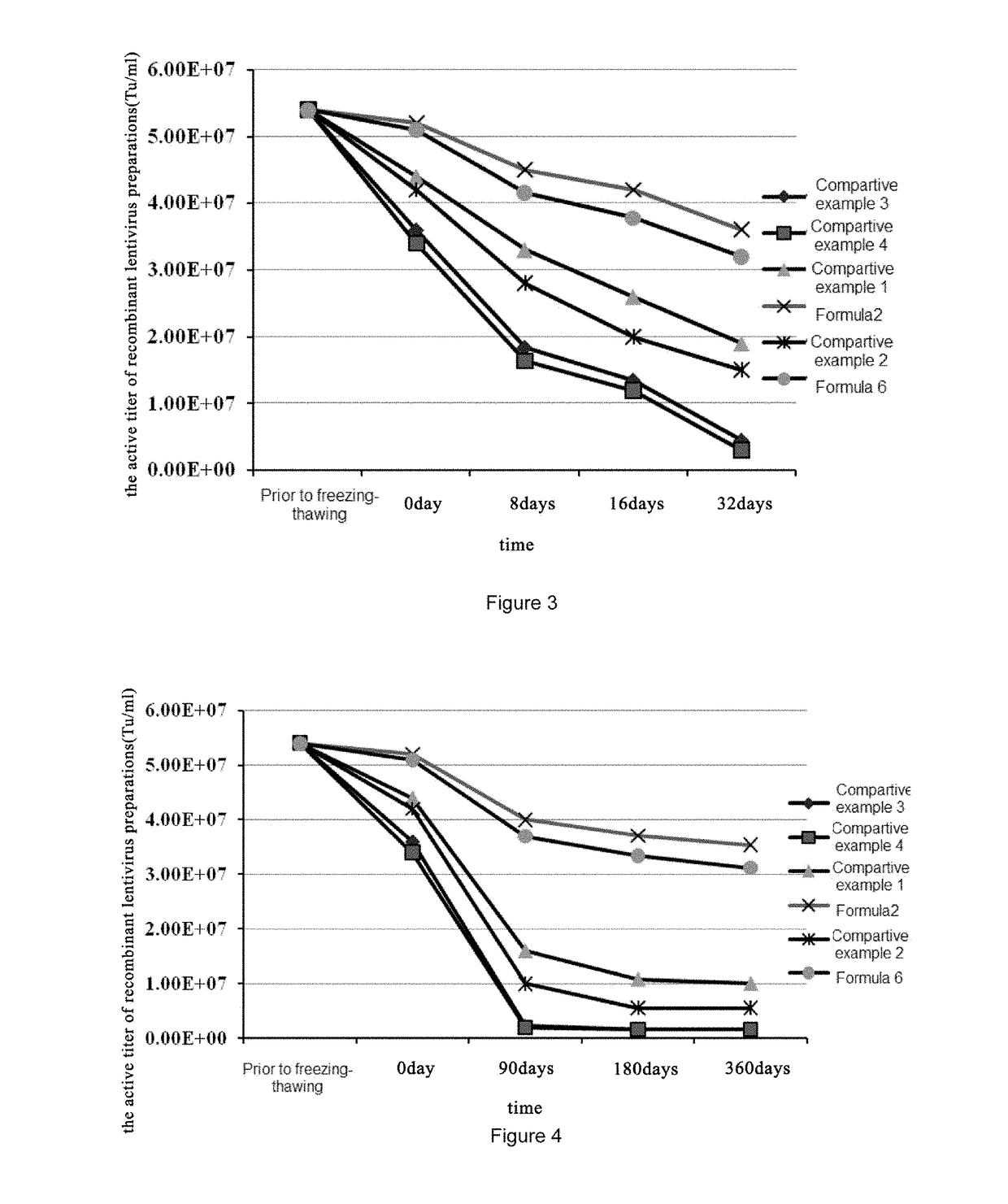
Herein is provided a recombinant lentiviral vector preparation, in which the preparation comprises: a) an effective dose of the recombinant lentiviral vector; b) a histidine hydrochloride buffer for keeping a pH value of the preparation in the range of 6.0-8.0; and c) a carbohydrate.
Owner:BEIJING SOLOBIO GENETECH
Compound amino acid injection and preparation method thereof
InactiveCN110507604AReduce degradationStable in natureOrganic active ingredientsMetabolism disorderTryptophanBottle
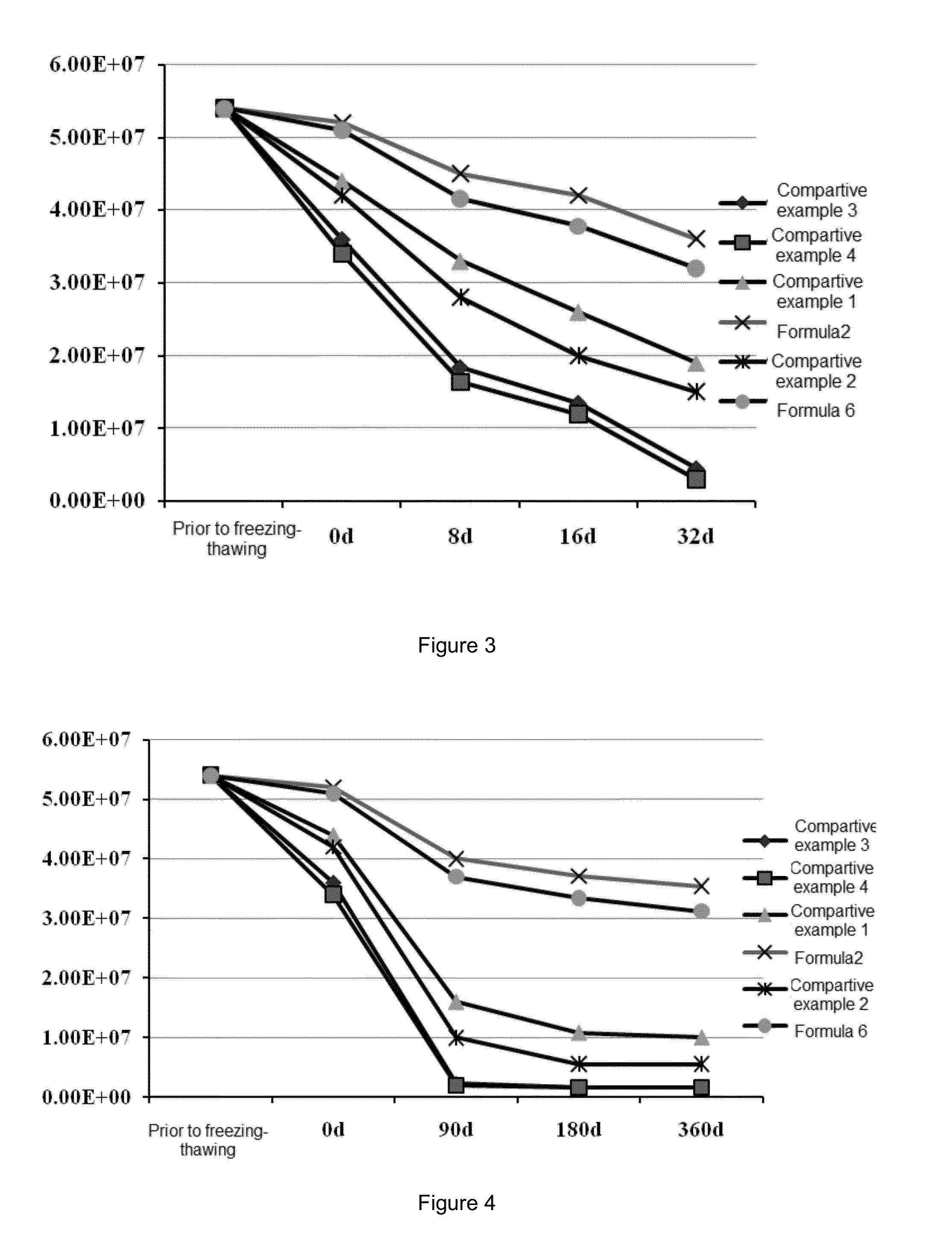
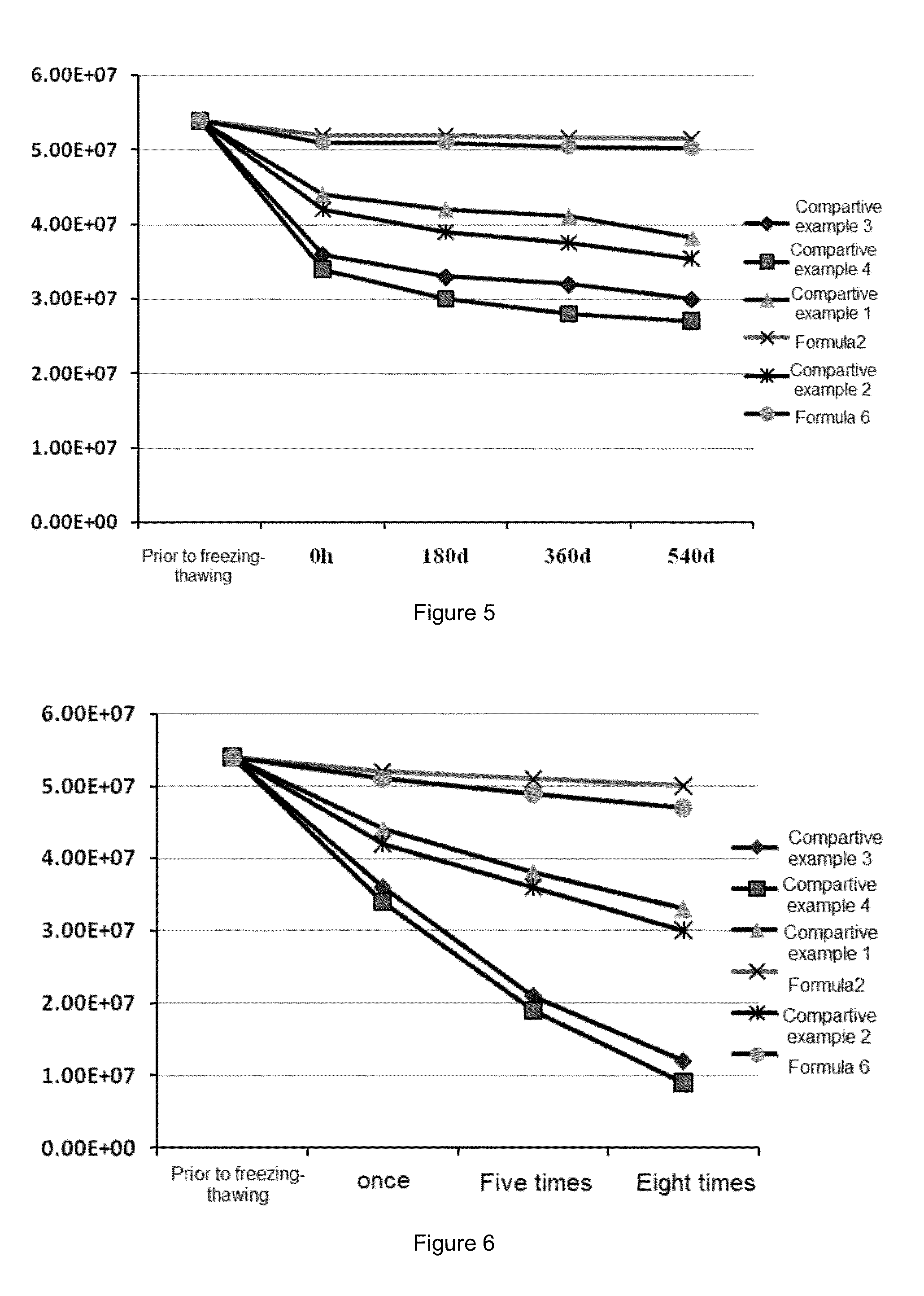
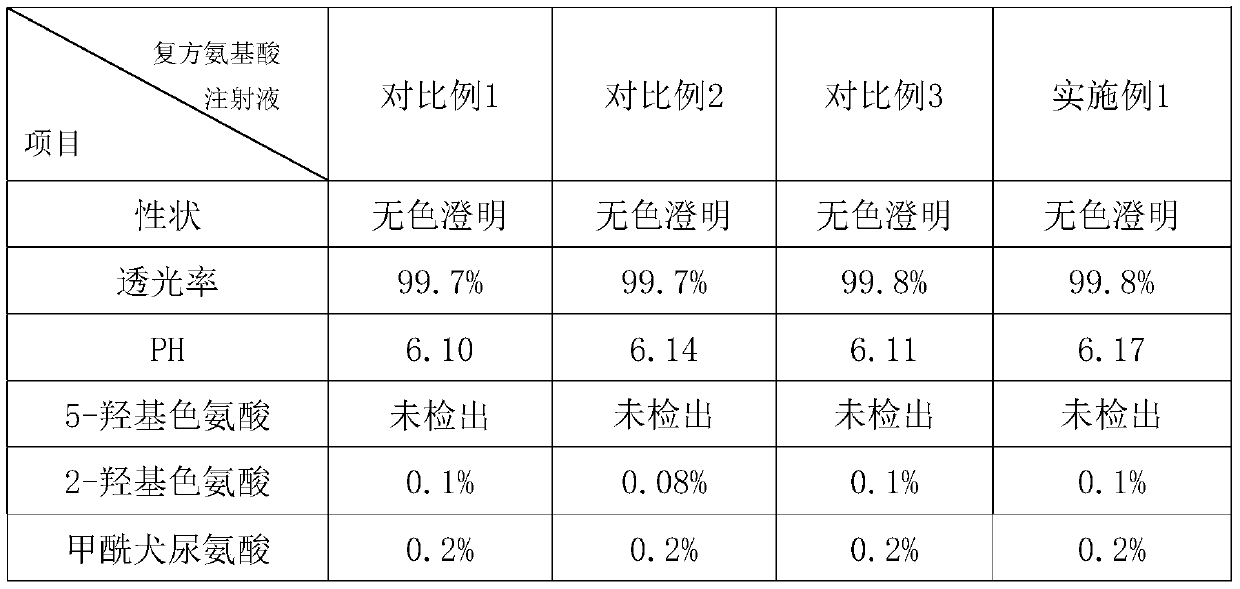
The invention provides a preparation method of a compound amino acid injection. The preparation method comprises the following steps: respectively washing a preparation container and a pipeline with alkaline water, a metal ion chelating agent solution and water for injection; putting the water for injection into a liquid preparation tank, adding sodium hydroxide, and stirring while adding until the sodium hydroxide is dissolved; introducing N2 into the liquid preparation tank, then sequentially adding tyrosine, isoleucine, leucine, lysine hydrochloride, methionine, threonine, valine, alanine,arginine hydrochloride, aspartic acid, glutamic acid, proline, serine, glycine, phenylalanine, histidine hydrochloride, xylitol and cysteine hydrochloride, and stirring and dissolving one by one; continuously introducing N2 into the liquid preparation tank, ensuring that the oxygen content is within 3%, cooling the materials, adding water for injection, adding tryptophan, stirring, dissolving, andsupplementing water to a full amount to obtain a mixed solution; and filtering and sterilizing the mixed solution, injecting the mixed solution into an infusion bottle, capping and sealing the bottle, and performing sterilizing and performing light inspection to obtain the compound amino acid injection.
Owner:武汉久安药业有限公司
Complex nutritional flavorous enhancer
The invention belongs to the field of seasoning technology and particularly relates to a complex nutritional flavorous enhancer, which consists of the following raw materials by weight part: 10-25 parts of glycine, 35-65 parts of monosodium glutamate, 5-15 parts of L-aspartic acid, 3-10 parts of I+G, 1-5 parts of DL alanine, 0.5-1.5 parts of DL-serine, 1-5 parts of L-lysine hydrochloride, 0.5-5 parts of methionine, 2-10 parts of pyrophosphate sodium, 2-10 parts of L-histidine hydrochloride, 0.5-5 parts of disodium succinate, 4-10 parts of animal protein hydrolysates, 4-10 parts of plant protein hydrolysates and 2-8 parts of mushroom powder. The complex nutritional flavorous enhancer preserves the original flavor of raw materials and has strong and ample flavor. With the animal protein hydrolysates, plant protein hydrolysates and mushroom powder added to the complex nutritional flavorous enhancer, the complex nutritional flavorous enhancer has high nutritional value, and does not make one thirsty upon using it.
Owner:广东百味佳味业科技股份有限公司
Method for labeling escherichia coli proteome by using SILAC (Stable Isotope Labeling with Amino Acids in Cell Cultures) and special culture medium
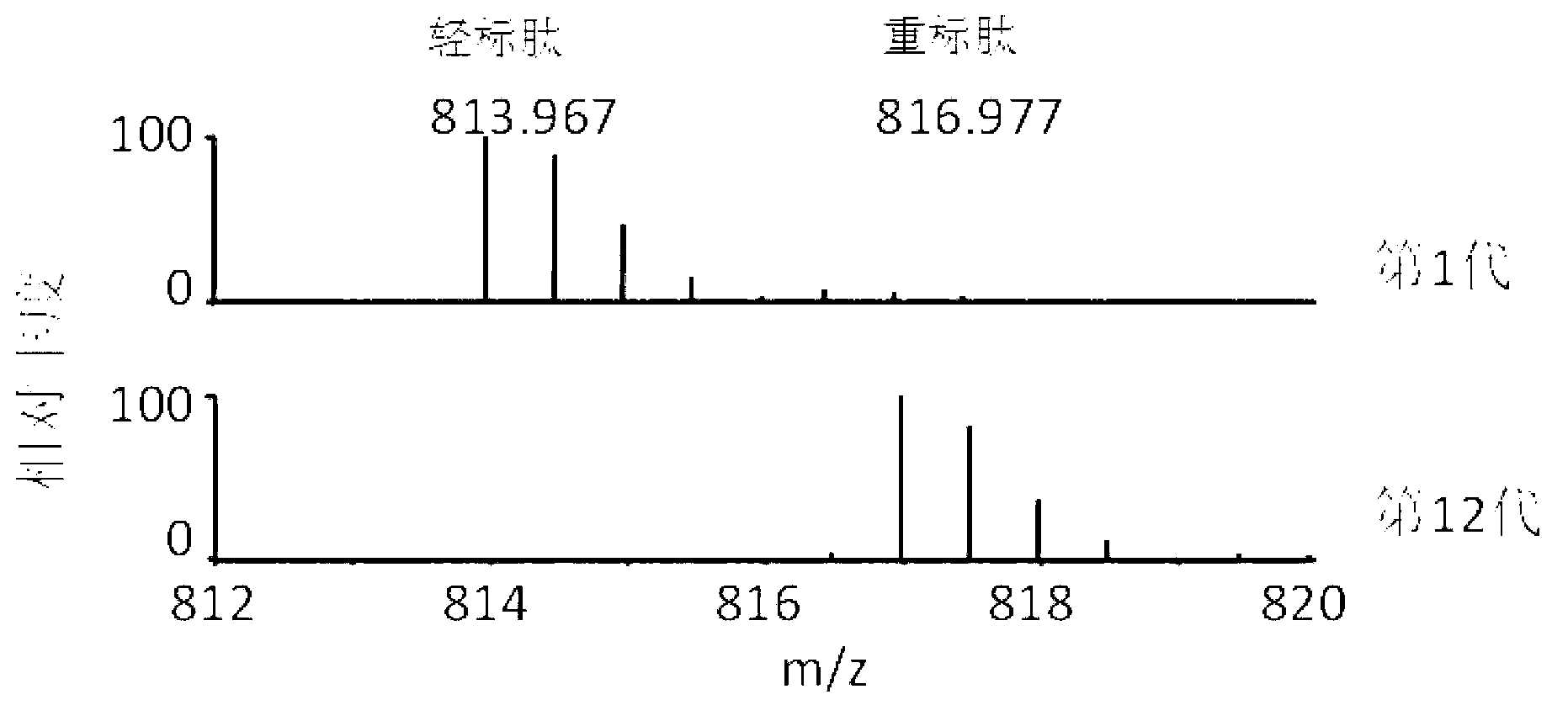
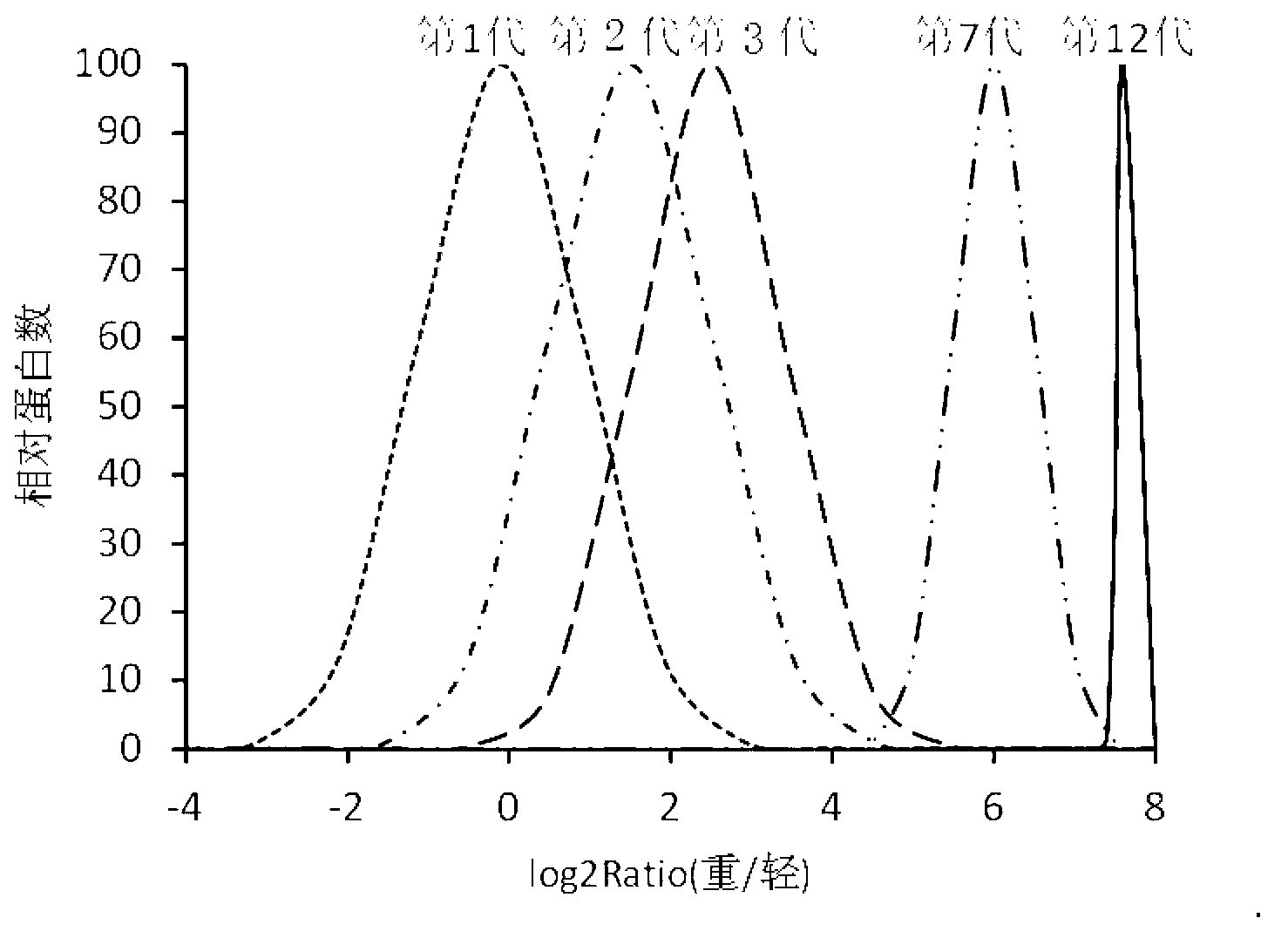
ActiveCN102796682ASave cumbersome stepsOmit conditionsBacteriaMicroorganism based processesEscherichia coliStable Isotope Labeling
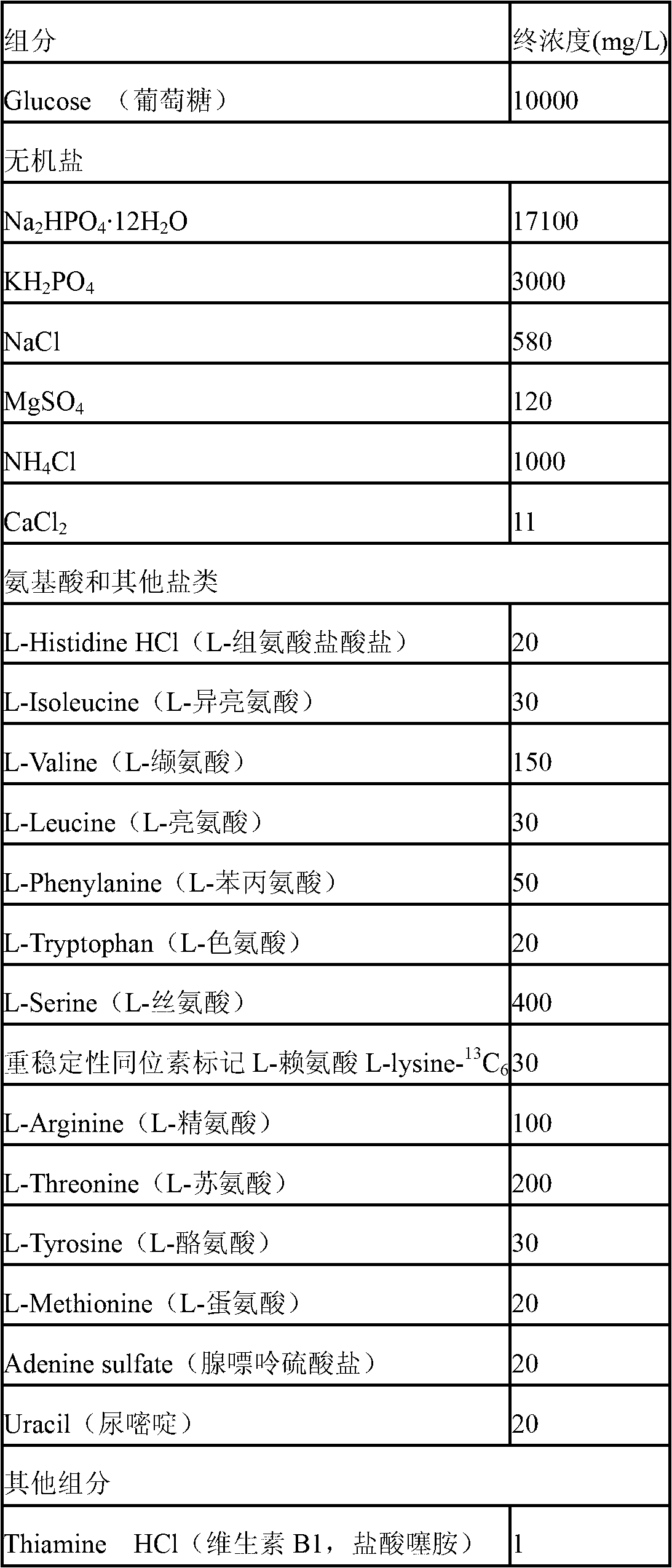
The invention discloses a method for labeling escherichia coli proteome by using SILAC (Stable Isotope Labeling with Amino Acids in Cell Cultures) and a special culture medium. The invention provides a special culture medium for the SILAC-labeled escherichia coli. The special culture medium comprises inorganic salt, histidine hydrochloride, isoleucine, valine, leucine, phenylalanine, tryptophan, serine, heavy stable isotope labeled lysine, arginine, threonine, tyrosine, methionine, adenine sulfate, uracil, vitamin B1 and glucose; and the inorganic salt is Na2HPO4.12H2O, KH2PO4, NaCl, MgSO4, NH4Cl and CaCl2. Experiments prove that, the special culture medium for the SILAC-labeled escherichia coli, disclosed by the invention, creates a condition for researches of labeling the escherichia coli proteome, preparing a quantitative internal label and highly-precisely quantifying the escherichia coli proteome.
Owner:ACADEMY OF MILITARY MEDICAL SCI
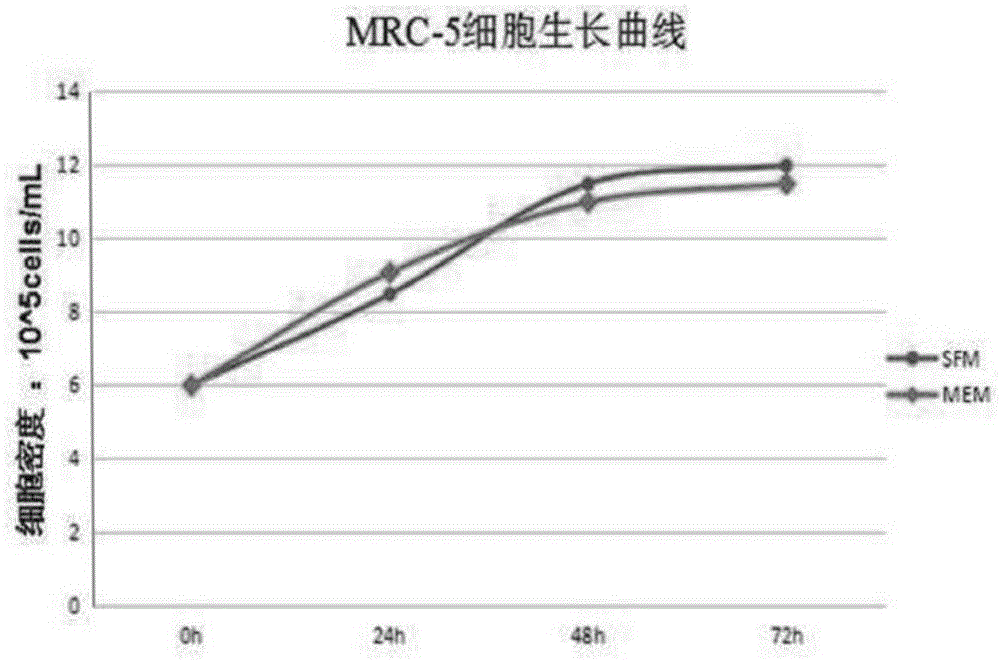
Protein-free serum-free medium suitable for diploid cell culture and application
The invention provides a protein-free serum-free medium suitable for diploid cell culture and a preparing method thereof. The medium contains calcium chloride, magnesium sulfate, potassium chloride, sodium dihydrogen phosphate, L-arginine hydrochloride, L-asparaginate, L-cystine dihydrochloride, L-histidine hydrochloride, L-leucine, L-isoleucine and the like. The medium can meet the requirement of the growth or maintenance stage of diploid cells and prevents contact with exogenous viruses possibly existing in protein and serum to reduce potential safety hazards; as the components are simple and definite, standard products can be purchased in the market, the difference between different product batches is small, and the step of purifying down-stream products is simplified; a scientific basis is provided for large-scale standard production. In addition, the protein-free serum-free medium can be applied in the growth or maintenance stage of the diploid cells.
Owner:SICHUAN BAINUOJI TECH CO LTD
Capsule containing ginseng saponin, radix notoginseng saponin and amino acids and preparation method thereof
ActiveCN101618044AQuality improvementEasy to transportOrganic active ingredientsMetabolism disorderDiseaseThreonine
The invention provides a compound capsule containing ginseng saponin Rb1, ginseng saponin Re, radix notoginseng saponin R1, tryptophan, threonine, leucine, valine, methionine, isoleucine, phenylalanine and lysine hydrochloride, wherein the compound capsule can also contain arginine hydrochloride and / or histidine hydrochloride. The preparation method of the capsule comprises the step of preparing various amino acids and saponin into coating micro-pills respectively. The capsule has stable quality, convenient transportation, storage and use and good absorbing effect. An advanced micro-pill coating preparation technique is adopted, the amino acids, the pseudo-ginseng saponin and the ginseng saponin are respectively prepared into the coating micro-pills with various colors, the quality is more stable by respective packaging, and the absorption of an organism is further promoted, thereby retaining the high potency of the capsule. The capsule has definite curative effect and wide adaptive disease, and clinical observation shows that the capsule can effectively enhance the immunity of the organism, can quicken the healing of wounds and has a very good curative effect on various leukopenia and gastrointestinal tract malabsorption diseases.
Owner:LIVZON PHARM GRP INC
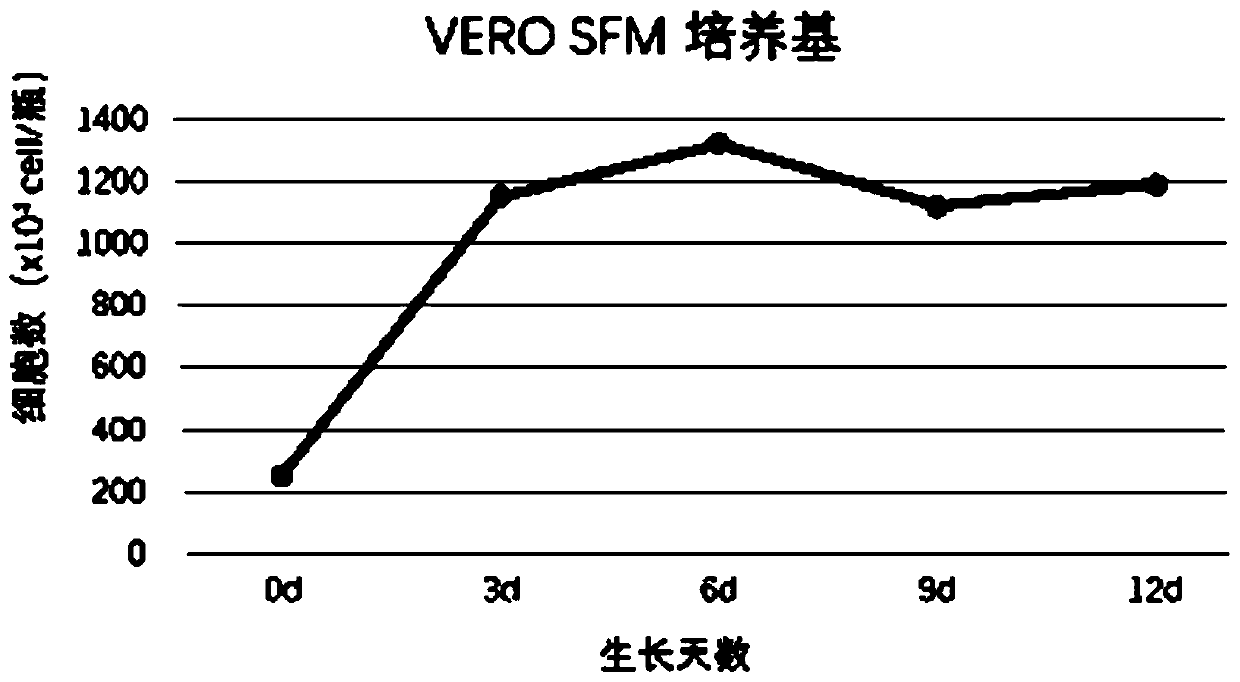
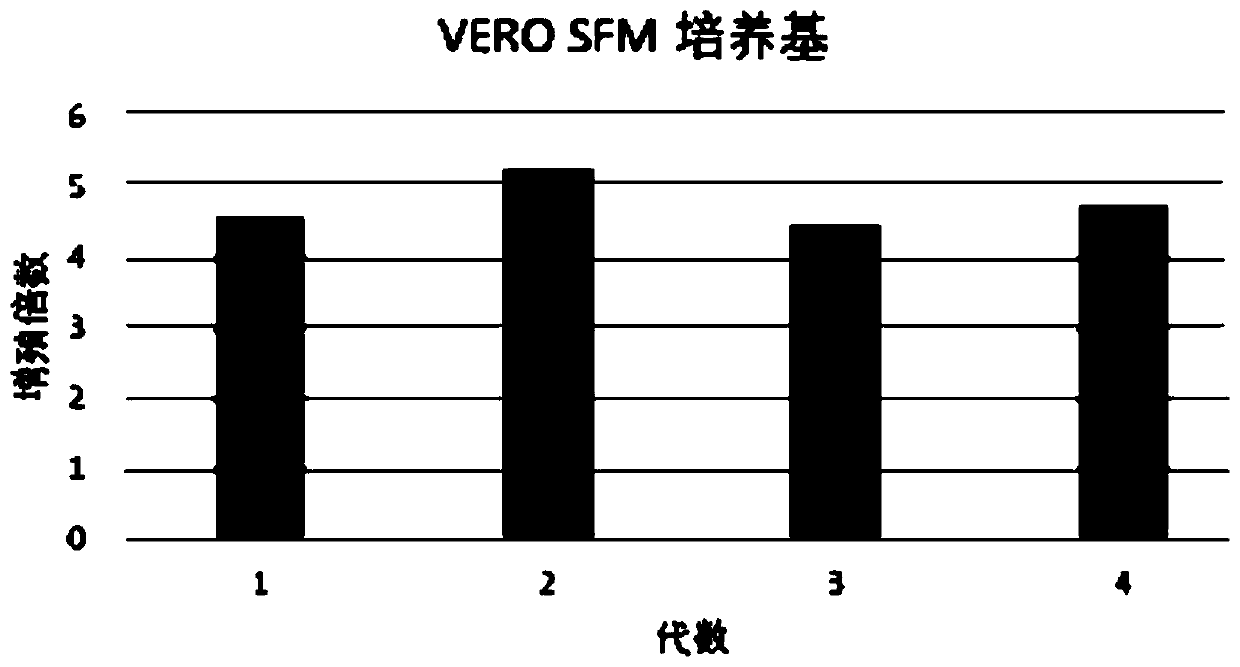
Serum-free culture medium for VERO serum-free cell culture and corresponding virus production
InactiveCN110628697ADoes not increase the percentage of apoptoticReduce manufacturing costCulture processMicroorganism based processesApoptosisTryptophan
The invention discloses a serum-free culture medium for VERO serum-free cell culture and corresponding virus production. The culture medium comprises the following components: amino acids, vitamins, inorganic salts, auxiliary components and proteins. The amino acids comprise glycine, alanine, arginine hydrochloride, cystine dihydrochloride, glutamic acid, glutamine, histidine hydrochloride monohydrate, isoleucine, leucine, lysine hydrochloride, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine disodium salt dihydrate, valine, hydroxyproline, ornithine and taurine. Theproteins comprise human transferrin and recombinant insulin. When being used for VERO cell culture, the culture medium does not depend on the use of animal serum, does not introduce animal-derived protein, greatly lowers the production cost, sufficiently ensures the stable product quality, and can implement long-time culture and passage without increasing the apoptosis ratio.
Owner:山东巨山能源科技有限公司
Composite amino acid freeze dried powder injection and its preparation process
InactiveCN1839813AImprove bioavailabilityImprove malabsorptionPowder deliveryOrganic active ingredientsActivated carbon filtrationTryptophan
The invention relaets to a compound amino acid freeze-dried powder injection, which comprises L-arginine hydrochloride, L-histidine hydrochloride, L-leucine, L-isoleucine, L-lysine hydrochloride, L-phenylalanine, L-threonine, L-valine, L-methionine, L-tryptophan, glycine, L-alanine, L-proline, L-tyrosine, L-serine, L-cysteine hydrochloride, L- aspartic acid, L-glutamic acid and sodium bisulphate. Its preparing process is also disclosed in the invention.
Owner:武汉同源药业有限公司
A kind of bactericidal pollution-free pesticide
The invention discloses a bactericidal pollution-free pesticide, which is prepared from leucine, valine, proline, methionine, glycocoll, threonine, tyrosine, serine, acetylcysteine, lysine hydrochloride, arginine hydrochloride, histidine monohydrochloride, tryptophan, alanine, glutamic acid, isoleucine, phenylalanine, sorbierite, sodium hydrogensulfite and water. The bactericidal pollution-free pesticide is used for preventing and controlling diseases such as gold diseases, black gold diseases, red gold diseases and the like of cereals, fruit trees and vegetables, does not pollute crops and environment, does not have residual toxicity, is long in lasting time of pesticidal potency, has the effects of prevention and control, and can prevent and control the diseases of the cereals, the fruit trees and the vegetables; and when the bactericidal pollution-free pesticide is applied, the application time is reduced, labors are saved, the drought tolerance and waterlogging tolerance of the cereals, the fruit trees and the vegetables are enhanced, so that root systems are developed, branches and leaves are flourish, healthy and strong, fruits are quick in coloring and early in maturity, the crops grow perfectly, excessive growth is inhibited, and the quality and yield can be improved.
Owner:张文吉
Cell culture medium without animal component
The invention provides an animal-source-free cell culture medium, which is prepared by proportionally mixing the components including calcium chloride, potassium chloride, anhydrous magnesium sulfate, sodium chloride, anhydrous sodium dihydrogen phosphate, L-arginine hydrochloride, L-cystine hydrochloride, L-glutamine, L-Leucine, L-isoleucine, L-histidine hydrochloride, L-tyrosine, D glucose, choline chloride, folic acid, nicotinamide, pyridoxal hydrochloride, riboflavin, thiamine hydrochloride and the like. Cells can be directly cultivated on the cell culture medium provided by the invention, rather than gradual domestication and cultivation, so that the density and the harvesting frequency of the cultured cells are increased and the expression quantity is increased so as to increase the vaccine yield in the field of medicine, thereby effectively avoiding the interference of animal-originated characteristics to the cultivated animal or human cells. The animal-source-free cell culture medium solves the problems that the cells cultivated by the prior serum-free medium have premature deaths and unsaturated forms.
Owner:CHANGCHUN MEINENG BIOLOGICAL ENG
Recombinant carnosine hydrolase mutant and application thereof
The invention relates to a recombinant carnosine hydrolase mutant, a recombinant expression vector and a recombinant expression transformant containing the gene of the enzyme mutant, a preparation method of the recombinase, and a method for preparing L-carnosine through reverse hydrolysis of the recombinase. Compared with the prior art, the recombinant carnosine hydrolase mutant provided by the invention has good thermal stability and low optimal pH, relatively cheap L-histidine hydrochloride can be directly used as a raw material for catalytic preparation of L-carnosine, higher product concentration can be reached, and the recombinant carnosine hydrolase mutant has good industrial application prospects in production of the L-carnosine.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Compound amino acid injection for livestock and preparation method thereof
InactiveCN111358753AImprove solubilityIncrease contentOrganic active ingredientsMetabolism disorderCrop livestockAnimal science
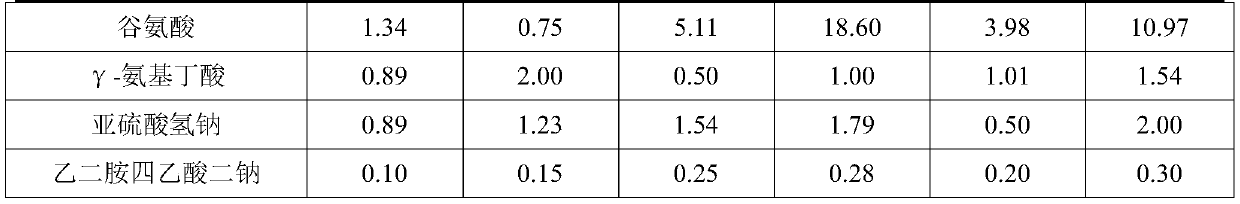
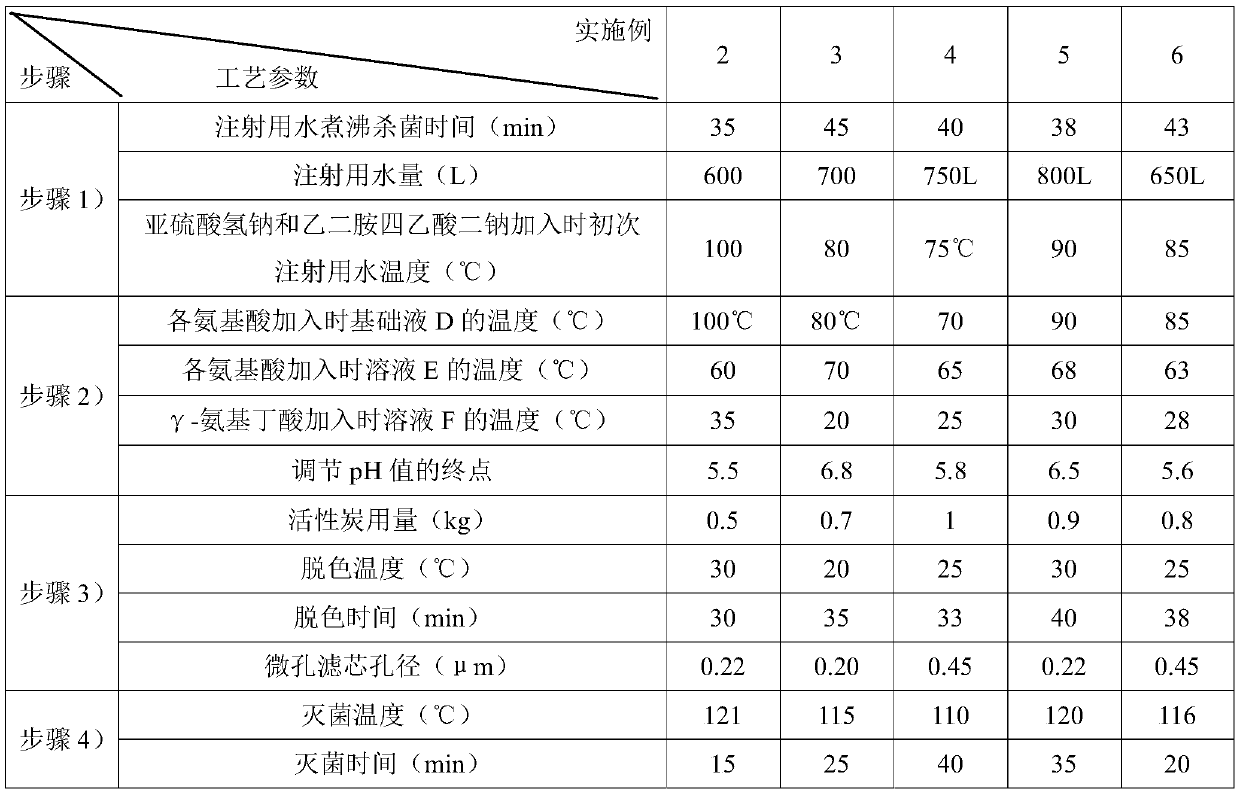
The present invention discloses a compound amino acid injection for livestock, and belongs to the technical field of animal husbandry and veterinary. Effective ingredients of the injection include 18kinds of amino acids and gamma-aminobutyric acid required by the animal body; by adjusting the amount of leucine and using arginine hydrochloride, histidine hydrochloride, lysine hydrochloride and cysteine hydrochloride, the content of the amino acids in the compound amino acid injection for the livestock is increased, so that the injection is more stable, the production cost is reduced, the injection has the better curative effect, and the problem of relatively low amino acid content of an existing compound amino acid injection for the livestock is solved. The invention also discloses a preparation method of the compound amino acid injection for the livestock. The compound amino acid injection for the livestock is prepared by multistep feeding, so that the stability and solubility of tryptophan, tyrosine and the gamma-aminobutyric acid are improved, oxidation is prevented, and the problem that the existing compound amino acid injection for the livestock is low in stability is solved;and the compound amino acid injection for the livestock is suitable for body repair of the livestock in the disease treatment period and the recovery-after-illness period.
Owner:HEBEI KEXING PHARMA
Preparation process of L-carnosine synthetase
PendingCN112592942AImprove efficiencyHigh product concentrationFermentationHydrolysisEnzyme catalyzed
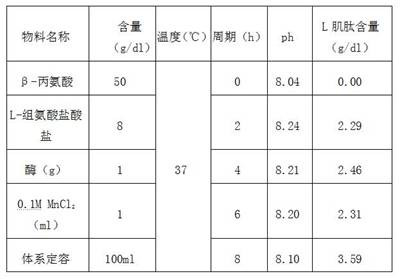
The invention discloses a preparation process of L-carnosine synthetase. The preparation process comprises the following steps: by using beta-alanine and L-histidine hydrochloride as raw materials andMnCl2 as a catalyst, adding L-carnosine hydrolase, and performing enzymatic reaction catalysis at 35-40 DEG C under the pH value of 8.0-8.5 to synthesize L-carnosine. Aiming at the defects of the prior art in the enzymatic reverse hydrolysis synthesis of the L-carnosine catalyzed by the carnosine hydrolase, the conversion process conditions are optimized, the efficiency and the product concentration of the peptide hydrolase for synthesizing the L-carnosine are improved, and the preparation process has the advantages of low raw material price, short enzymatic conversion time, simplicity and convenience in operation, high product concentration, high conversion efficiency, low production cost, and the like.
Owner:NANTONG ZILANG BIOPHARMA TECH CO LTD
Edible salt substitute for recuperating 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight)
InactiveCN110679900AReduce intake"Five high" chronic diseases conditioning or reliefFood ingredient as mouthfeel improving agentAceglutamideBlood sugar
The invention relates to edible salt substitute for recuperating 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight). The edible salt substitute is prepared from the components in parts by weight: 0.5 to 1.5 parts of rose salt, 0.5 to 1.5 parts of Himalaya salt, 0.2 to 0.8 part of food grade histidine hydrochloride, 0.05 to 0.15 part of flos caryophyllata, 0.05 to 0.15 part of radix aucklandiae, 0.05 to 0.15 part of fructus foeniculi, 0.05 to 0.15 part of radix curcumae longae, 0.05 to 0.15 part of galanga, 0.05 to 0.15 part of rhizoma zingiberis, 0.05 to 0.15 part of netmeg, 0.05 to 0.15 part of cortex cinnamomi, 0.05 to 0.15 part of fructus crataegi, 0.05 to 0.15 part of sodium tartaric acid, and 0.05 to 0.15 part of aceglutamide. The edible salt substitute for recuperating the 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight) provided by the invention adopts the combination of multiple low sodium salt with different components and multiple condiments, has the efficacies of the edible salt, and substitutes the edible salt so as to reduce the edible salt intake of a human body and recuperate or alleviate the 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight).
Secukinumab injection and preparation method thereof
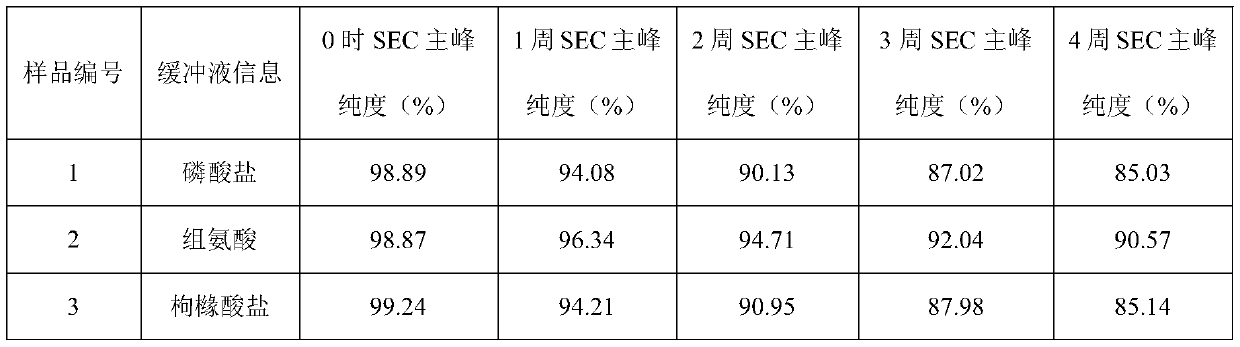
The invention provides a secukinumab injection. The secukinumab injection is composed of 50 mg / ml-300 mg / ml of secukinumab, 5-50 mmol / L of histidine and histidine hydrochloride, 5-50 mol / L of methionine, 150 mmol / L-400 mmol / L of polyols, 0.01%-0.02% of polysorbate and water for injection as balance and has a pH of 5.0-7.0. Through stability test, the secukinumab injection is proved to be stable inquality and higher in stability than commercial products on the market and meets the relevant specifications of Chinese Pharmacopoeia on various indexes, thereby having a good application prospect.
Owner:TONGHUA DONGBAO PHARMA
Cardioplegic solution
InactiveCN103933540AReduce the incidence of postoperative cardiac insufficiencyTripeptide ingredientsHeterocyclic compound active ingredientsMedicineAdenosine
The invention discloses acardioplegic solution. Per 1000 mL of a bicarbonate buffer solution comprises 15 mmol of NaCl, 10 mmol of KCl, 4 mmol of MgCl2.6H2O, 180 mmol of histidine, 18 mmol of histidine hydrochloride, 2 mmol of tryptophan, 1 mmol of alpha-ketoglutaric acid, 30 mmol of raffinose, 3 mmol of glutathione, 0.2 mmol of adenosine and 0.6-0.8 mmol of a sodium channel blocker. The cardioplegic solution effectively protects heart and helps to reduce occurrence rate of postoperation cardiac insufficiency.
Owner:王寿世
Compound amino acid solution for tree nutrient solution and preparation process thereof
The invention provides a compound amino acid solution for a tree nutrient solution and a preparation process thereof. Specifically, the compound amino acid solution for the tree nutrient solution comprises the following components by mass: 1000 parts of deionized water, 0.20-0.30 part of tyrosine, 4.80-5.00 parts of leucine, 3.48-3.56 parts of isoleucine, 3.50-3.70 parts of valine, 2.21-2.29 parts of methionine, 1.92-2.08 parts of alanine, 5.23-5.43 parts of phenylalanine, 0.71-0.79 part of glutamic acid, 2.42-2.58 parts of aspartic acid, 4.22-4.36 parts of lysine acetate, 2.42-2.58 parts of threonine, 7.54-7.66 parts of glycine, 0.90-1.10 parts of proline, 0.90-1.10 parts of serine, 4.82-5.12 parts of arginine hydrochloride, 2.41-2.62 parts of histidine hydrochloride, 0.82-0.98 part of tryptophan and 0.007-0.012 part of cystine. The compound amino acid provided by the invention is accord with the growth requirements of malnourished trees, and by setting the preparation process of the compound amino acid solution, a compound amino acid solution sample with high stability can be obtained, thus avoiding great loss of the raw and auxiliary materials in the making process of the compound amino acid solution.
Owner:湖北长联杜勒制药有限公司
Cryoprotectant for mesenchymal stem cells, and preparation method of cryoprotectant
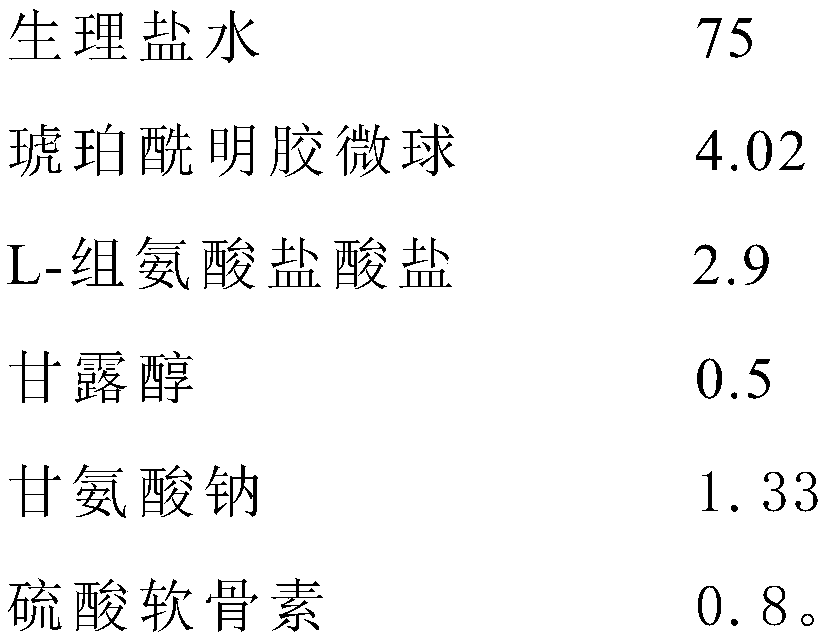
ActiveCN110292038AImprove viabilityAvoid stress injuryDead animal preservationMicrosphereMesenchymal stem cell
The invention provides a cryoprotectant for mesenchymal stem cells. The cryoprotectant is mainly prepared from the following components in parts by weight: 60-80 parts of physiological saline, 2.2-5.87 parts of succinylated gelatin microspheres, 1.40-3.11 parts of L-histidine hydrochloride and 0.3-0.8 part of mannitol. The cryoprotectant can protect the mesenchymal stem cells for a long time, andcan maintain cell viability.
Owner:CENTURY BIOSTRENGTH BEIJING PTY LTD
Compound amino acid injection solution 18AA-V composition and preparation method thereof
ActiveCN103800323AInhibit or alleviate degradationGood effectOrganic active ingredientsPeptide/protein ingredientsAntioxidantTryptophan
The invention belongs to the technical field of medicines and particularly discloses a compound amino acid injection solution 18AA-V composition. The composition is composed of following components: arginine hydrochloride, histidine hydrochloride, leucine, isoleucine, lysine hydrochloride, phenylalanine, threonine, valine, methionine, tryptophan, glycine, alanine, proline, tyrosine, serine, cysteine hydrochloride, aspartate, glutamic acid, xylitol, malate and injection water; the composition does not contain a sulfite antioxidant; the malate is used as a pH (Potential Hydrogen) value adjusting agent, a metal ion chelating agent or a stabilizing agent; the bioavailability of various amino acids can also be improved; the cysteine hydrochloride is used as the antioxidant; nitrogen is filled in the whole production process to reduce the residual oxygen in the product, so that the quality of the product is better than that of similar compound amino acid injection solution products available on the market and the clinical utilization is safer.
Owner:湖北一半天制药有限公司
Storage stable recombinant lentiviral vector preparation
ActiveUS9969984B2Improve stabilityMaintain long-term stabilityNervous disorderAntiviralsViral vectorBuffering agent
Herein is provided a recombinant lentiviral vector preparation, in which the preparation comprises: a) an effective dose of the recombinant lentiviral vector; b) a histidine hydrochloride buffer for keeping a pH value of the preparation in the range of 6.0-8.0; and c) a carbohydrate.
Owner:BEIJING SOLOBIO GENETECH
Histidine hydrochloride production method
InactiveCN104744373ASimplify the manufacturing processReduce manufacturing costOrganic chemistryIon exchangeIon-exchange resin
The invention relates to a histidine hydrochloride production method. The method concretely comprises the following steps: hydrolyzing a raw material ferrohemoglobin by hydrochloric acid, carrying out column chromatography separation by adopting ion exchange resin, and re-crystallizing by using hydrochloric acid to obtain histidine hydrochloride. The histidine hydrochloride production method has the advantages of simple preparation process and low production cost.
Owner:蒋学京
In-vitro energy supply substance suitable for skin collagen synthesis, raw material and preparation method
PendingCN113509403AEasy to synthesizeReduce saggingCosmetic preparationsToilet preparationsTryptophanPhenylalanine
The invention discloses an in-vitro energy supply substance suitable for skin collagen synthesis, a raw material and a preparation method. The in-vitro energy supply substance comprises proline or L-proline; a serine or an L-serine; an alanine or an L-alanine; an isoleucine or an L-isoleucine; a leucine or an L-leucine; aspartic acid or L-aspartic acid; tyrosine or L-tyrosine; glutamic acid or L-glutamic acid; phenylalanine, or L-phenylalanine; arginine hydrochloride or L-arginine hydrochloride; lysine hydrochloride or L-lysine hydrochloride; a valine or an L-valine; threonine, or L-threonine; histidine hydrochloride or L-histidine hydrochloride; tryptophan, or L-tryptophan; a methionine or an L-methionine; cystine or L-cystine; glycine; hyaluronic acid; tranexamic acid; glucose; sodium hydrogen sulfite or rosmarinic acid; dimethylaminoethanol; and the balance of water for injection. The traditional Chinese medicine composition can supplement water, preserve moisture, tighten and lift the skin, relieve skin sagging and improve fine lines, wrinkles, aging and the overall texture and color of the skin.
Owner:杭州拾珍医疗器械有限公司
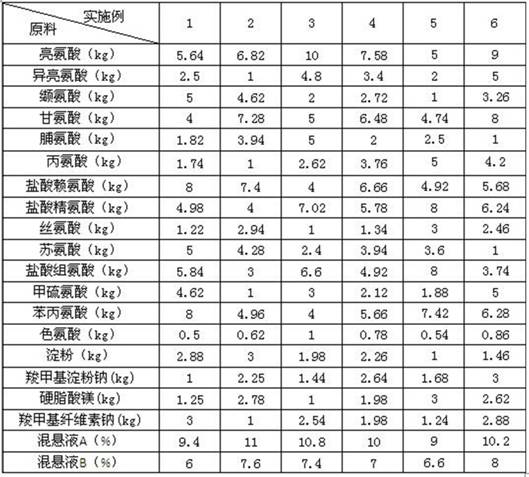
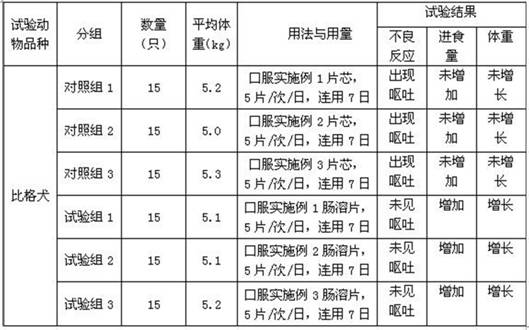
Compound amino acid enteric-coated tablet for dog and preparation method thereof
InactiveCN111821272AReduce adverse reactionsActive ingredients are stableOrganic active ingredientsMetabolism disorderTryptophanPhenylalanine
The invention belongs to the field of pharmaceutical preparations, and particularly discloses a compound amino acid enteric-coated tablet for dogs and a preparation method thereof. The compound aminoacid enteric-coated tablet is prepared from raw materials of effective components including leucine, isoleucine, valine, glycine, proline, alanine, lysine hydrochloride, arginine hydrochloride, serine, threonine, histidine hydrochloride, methionine, phenylalanine, tryptophan, starch, sodium carboxymethyl starch, magnesium stearate, sodium carboxymethyl cellulose, suspension A and suspension B. Thepreparation method comprises the steps of preparing a tablet core, preparing a separation layer and preparing an enteric coating layer. The compound amino acid enteric-coated tablets reduce adverse reactions caused by irritation to the stomach, supplements the dog's demand for amino acids, and has a simple preparation method. The preparation method is suitable for preparing the compound amino acid enteric-coated tablets for dogs, and the prepared compound amino acid enteric-coated tablets are suitable for dogs.
Owner:HEBEI KEXING PHARMA
Optimized anti-oxidative accellular protective solution
The invention relates to an optimized anti-oxidative accelular protective solution, which has a critical effect on protection of structural completeness and biological nature of acellular tissues in the whole course of the decellularizing process. The protective solution is prepared from DMEM cellular culture medium L-histidine hydrochloride, allopurinol, chondroitin sulfate, low molecular dextran, hydroxypropyl methyl cellulose, HEPES buffer solution, dexamethason hydrochloride, reduced glutathione and levofloxacin. The protective solution is a light-red liquid with specific pH value, specific crystal and colloid osmotic pressure. The optimized anti-oxidative accelular protective solution is easily available in raw materials and economical in price, has strong applicability, wide application range and excellent anti-oxidation performance, and has a protective effect in the process of decellularizing biological tissues.
Owner:拜欧迪赛尔成都生物科技有限公司
Serum-free protein-free culture medium
InactiveCN109536435AEffective display of pH statusReduce manufacturing costCulture processCell culture mediaSodium bicarbonateArginine
The invention discloses a serum-free protein-free culture medium. The serum-free protein-free culture medium comprises amino acids, vitamins, inorganic salts, a buffering agent, phenolsulfonphthalein,dextrose, sodium pyruvate and sodium bicarbonate, wherein the amino acids include at least one of L-alanine, L-arginine monohydrochloride, L-asparagine, L-glutamic acid, L-aspartic acid, L-cystine dihydrochloride, L-glutamine, L-glycine, L-isoleucine, L-histidine hydrochloride, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-serine, L-threonine, L-tryptophan, L-tyrosine disodiumsalt and L-valine. The serum-free protein-free culture medium disclosed by the invention has the advantages that the structure is stable, the stable pH value range is maintained, and besides, the phenolsulfonphthalein can effectively display the pH value state of the culture medium; and the culture medium does not contain protein or serum completely, is low in production cost, is definite in chemical components, facilitates separation and purification of products, and can improve the quality of the products.
Owner:镇江桥美科技有限公司
Health food capable of replenishing amino acids and enhancing immunity and preparation method thereof
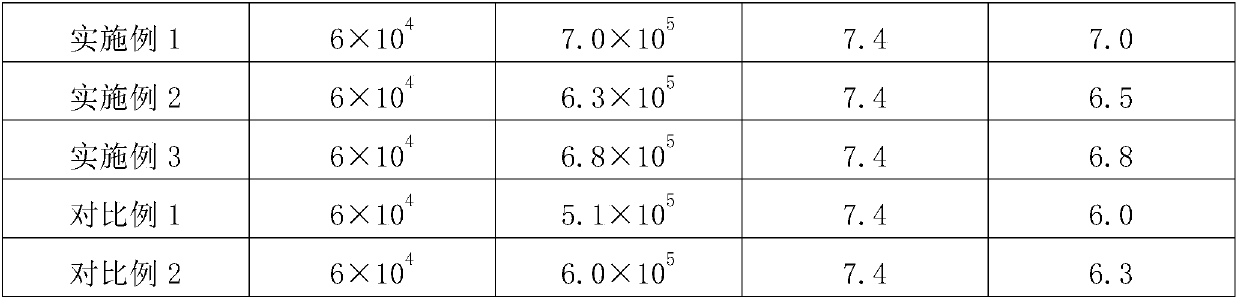
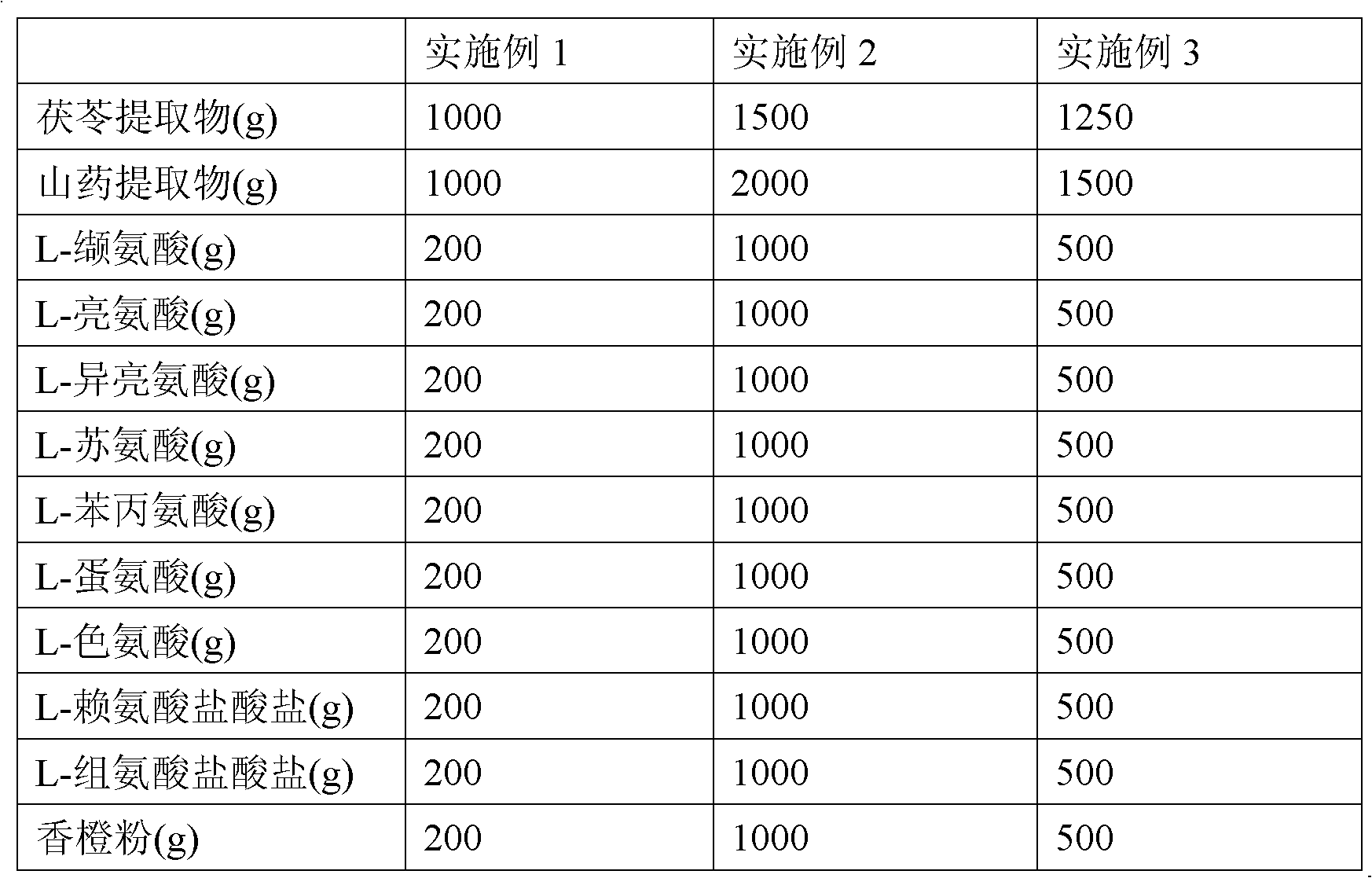
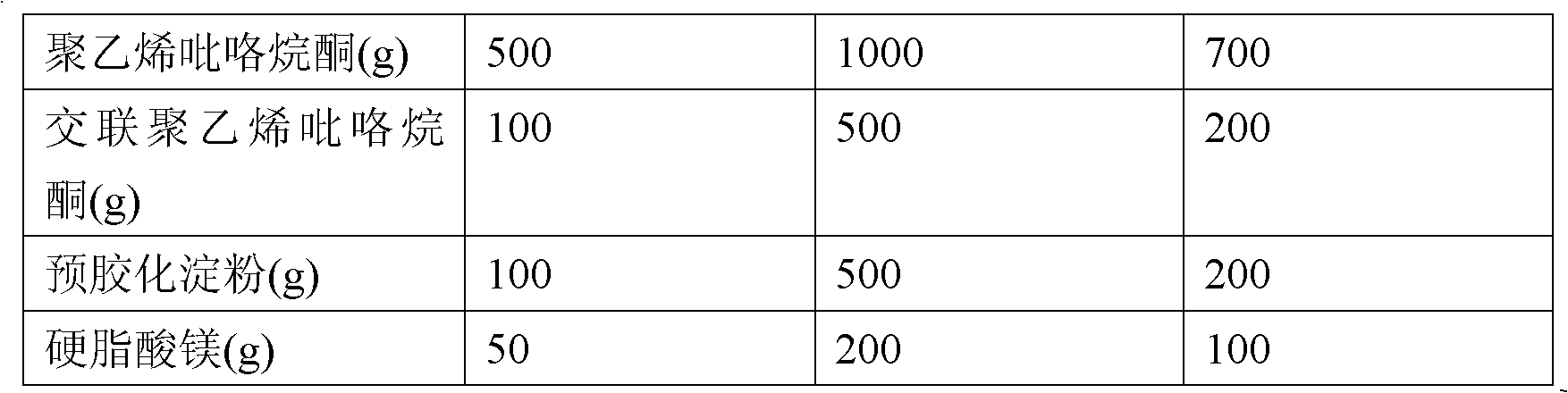
The invention discloses a health food capable of replenishing amino acids and enhancing immunity and a preparation method thereof. The health food includes the following components, by weight, 10 to 15 parts of poria cocos extracts, 10 to 20 parts of yam rhizome extracts, 2 to 10 parts of L-valine, 2 to 10 parts of L-leucine, 2 to 10 parts of L-isoleucine, 2 to 10 parts of L-threonine, 2 to 10 parts of L- phenylalanine, 2 to 10 parts of L-methionine, 2 to 10 parts of L- tryptophan, 2 to 10 parts of L-lysine monohydrochloride, 2 to 10 parts of L-histidine hydrochloride and 2 to 10 parts of orange powder. The health food has the characteristics of high absorbability, high utilization, high biological activity and high safety, and is low in cost, easy to digest and of no toxic and side effects. The health food specially aims at meeting the requirement of children, the traditional Chinese medicine theory and the modern nutriology are combined and comprehensiveness, science, economy, safety and the like are taken into considerations so that the health function of enhancing immunity can be well performed.
Owner:TIANJIN TIANSHI BIOLOGICAL DEV +2
Rash preventing and itching relieving liquid
InactiveCN108143805ANo pollutionEasy to useAnthropod material medical ingredientsHydroxy compound active ingredientsIrritationTert-leucine
The invention discloses rash preventing and itching relieving liquid, and belongs to the technical field of itching relieving preparations for external use. The rash preventing and itching relieving liquid is prepared from the following materials: 5-13 parts of black fungus, 8-14 parts of cortex phellodendri, 78-83 parts of common cockroach, 10-15 parts of histidine hydrochloride, 200-300 parts ofsorbitol, 10-15 parts of methionine, 20-25 parts of leucine, 10-15 parts of aspartic acid, 5-15 parts of alanine and 400-600 parts of distilled water. The rash preventing and itching relieving liquidprovided by the invention is simple in using method, and the finished product (the rash preventing and itching relieving liquid) is non-toxic and free from irritation and foreign smell, good in itching relieving effect, capable of effectively preventing rash, free from greasy feeling to skin and free from pollution to clothing; the rash preventing and itching relieving liquid is applicable to adult people and is also quite suitable for babies. The rash preventing and itching relieving liquid provided by the invention is applicable to intolerant itching caused by mosquito bites, and meanwhile,the rash preventing and itching relieving liquid is suitable for itching and pain caused by bites of bees, wasps, lice and the like.
Owner:QINGDAO RONGHE TIANZE CULTURE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com