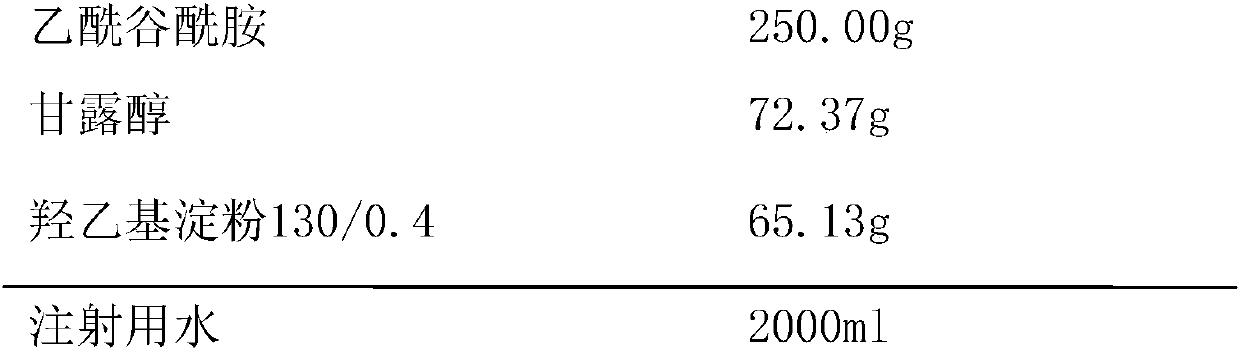Patents
Literature
51 results about "Aceglutamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
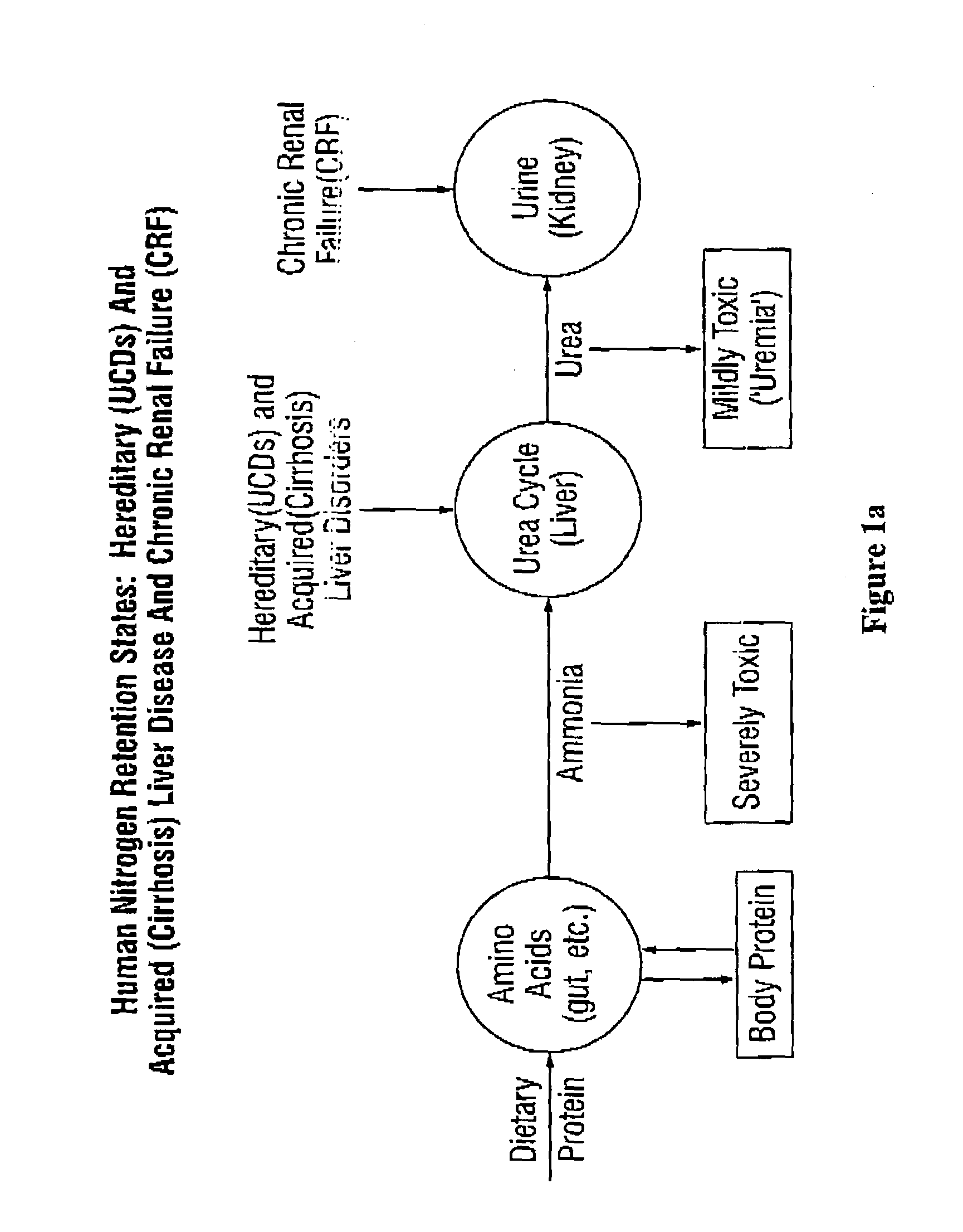
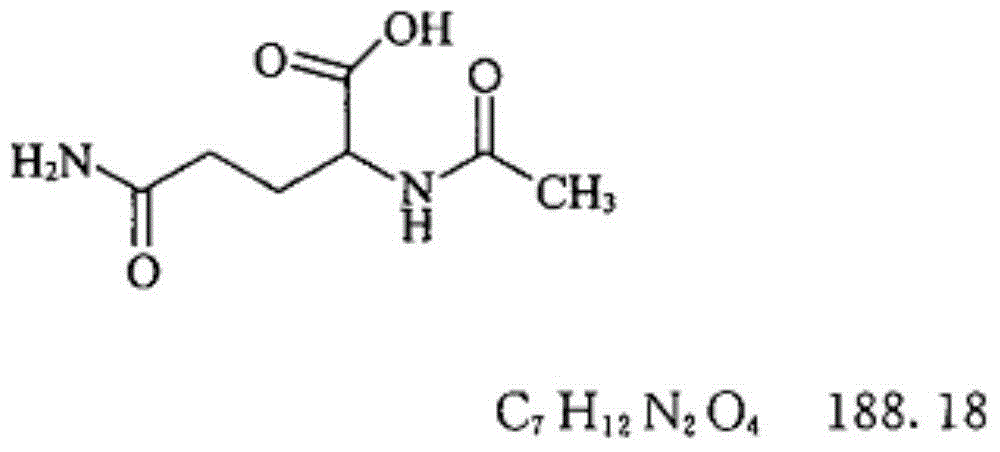
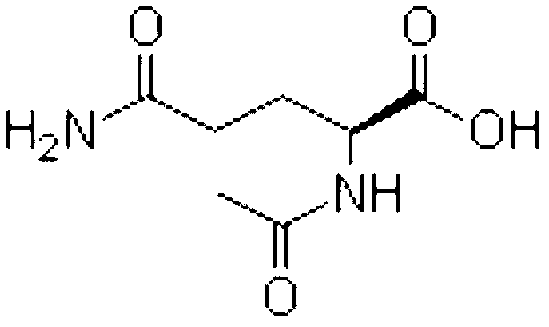
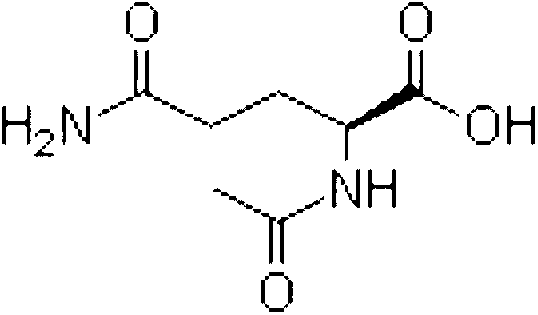
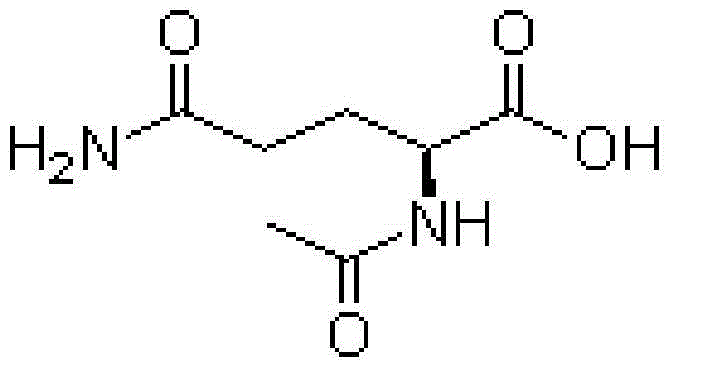
Aceglutamide (brand name Neuramina), or aceglutamide aluminum (brand name Glumal), also known as acetylglutamine, is a psychostimulant, nootropic, and antiulcer agent that is marketed in Spain and Japan. It is an acetylated form of the amino acid L-glutamine, the precursor of glutamate in the body and brain. Aceglutamide functions as a prodrug to glutamine with improved potency and stability.
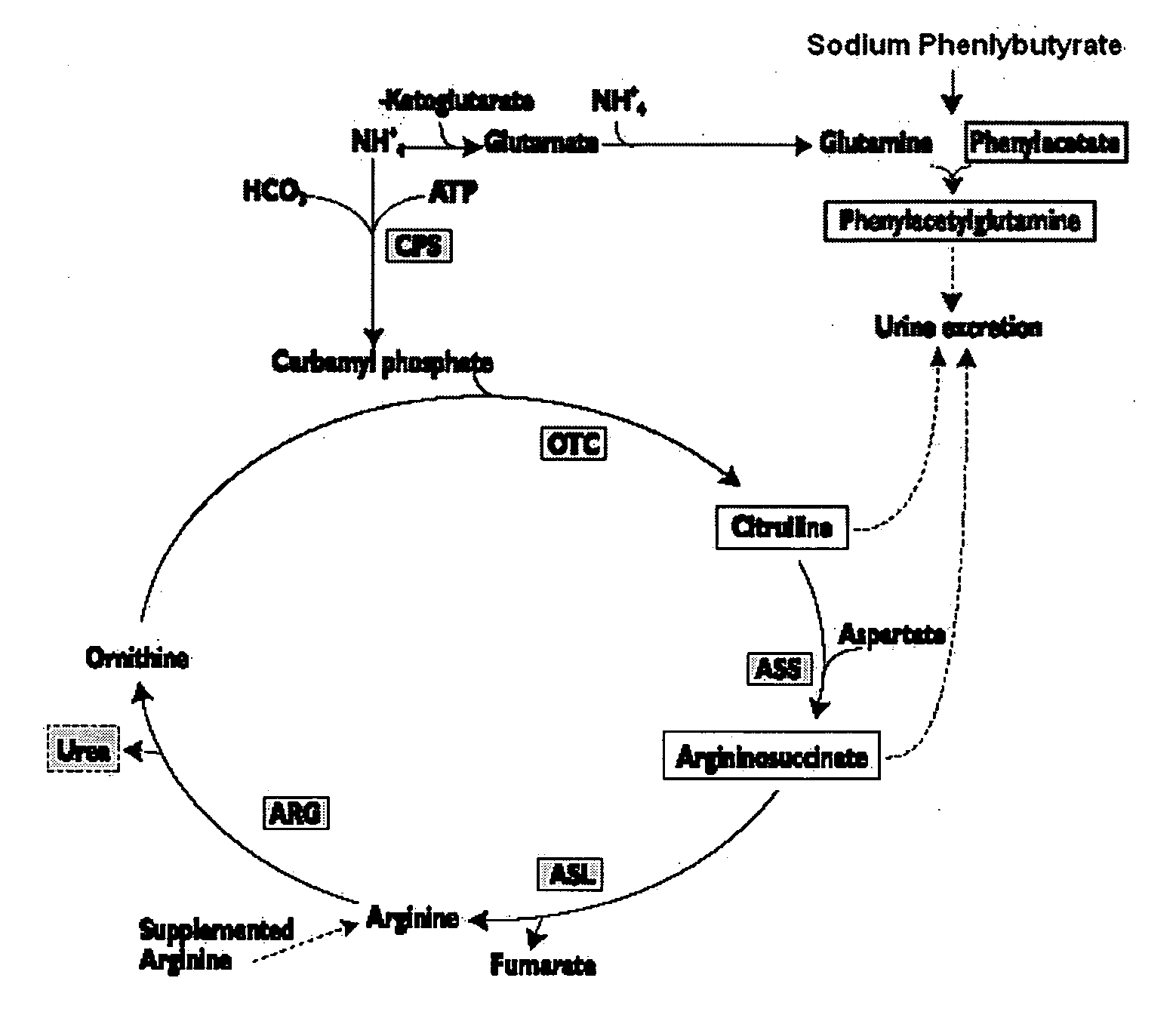
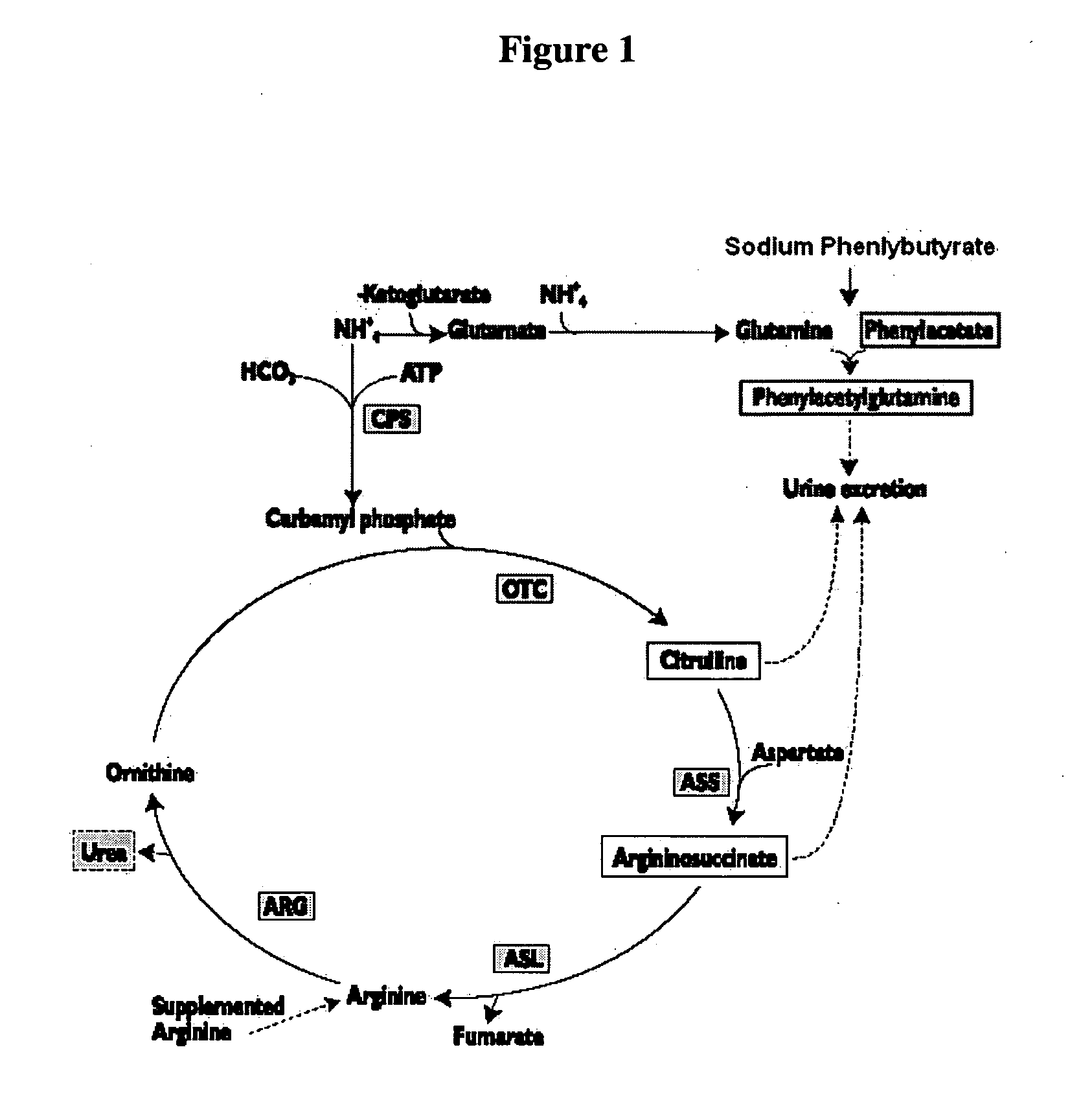
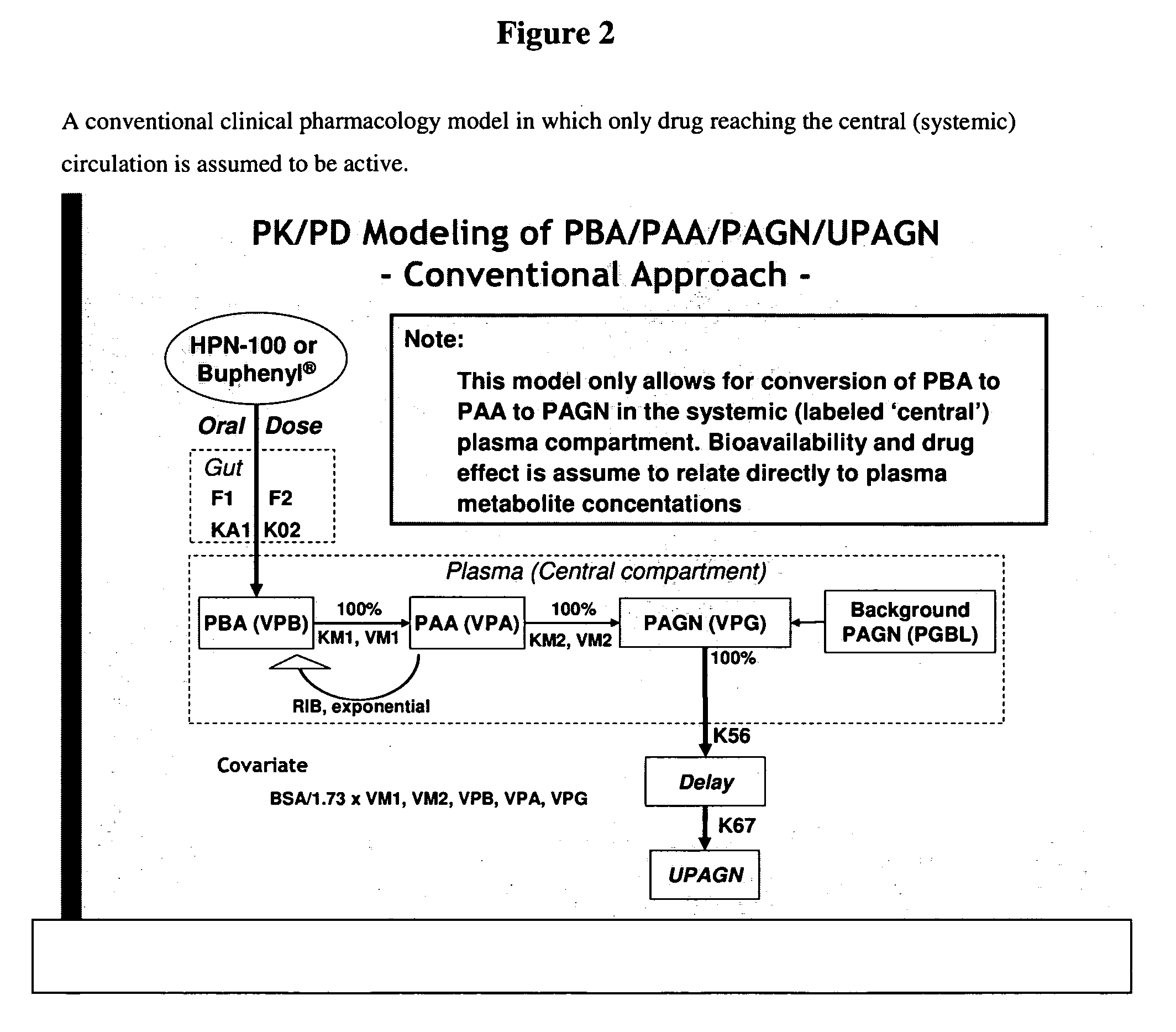
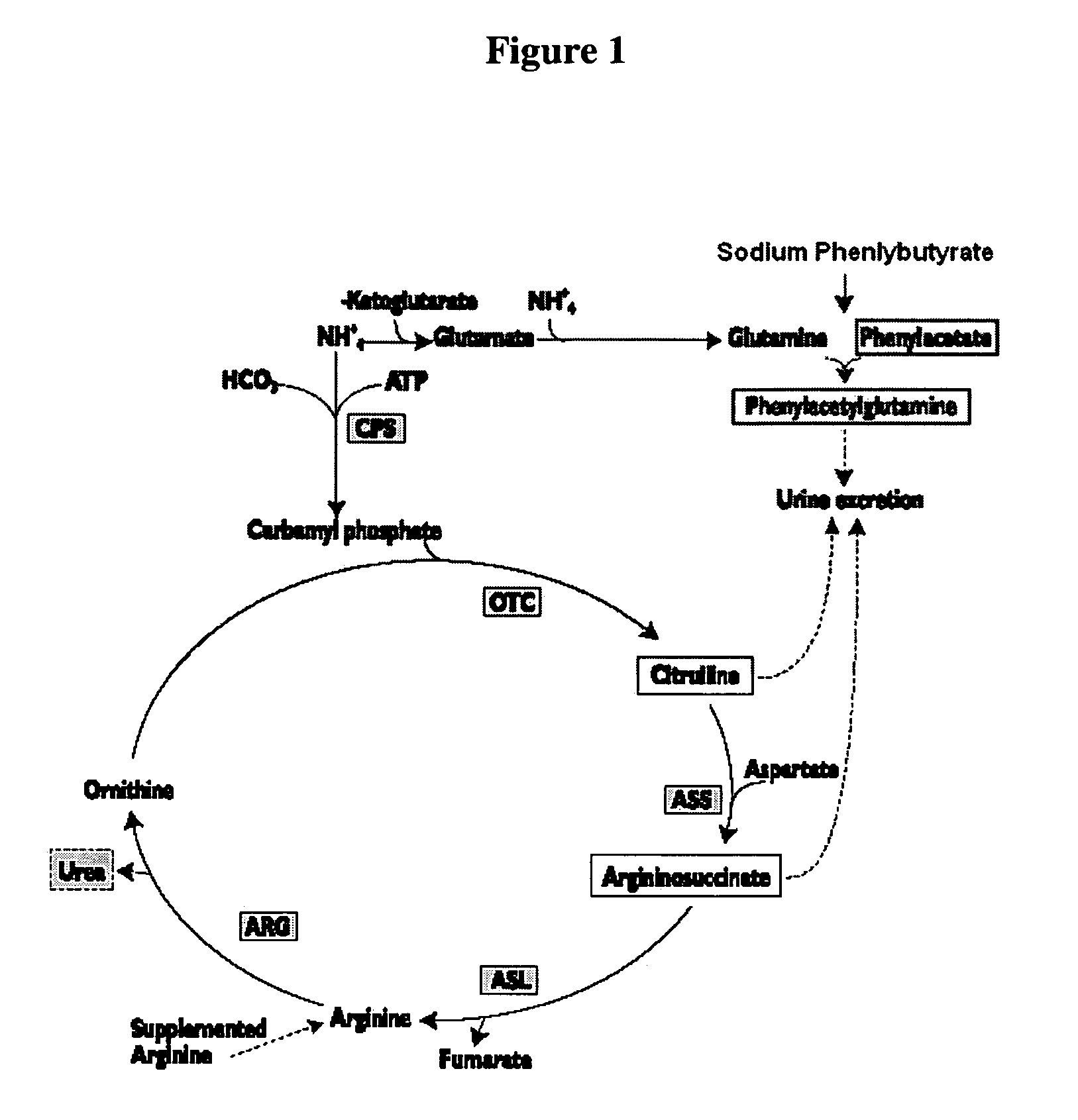
Methods of treatment using ammonia-scavenging drugs
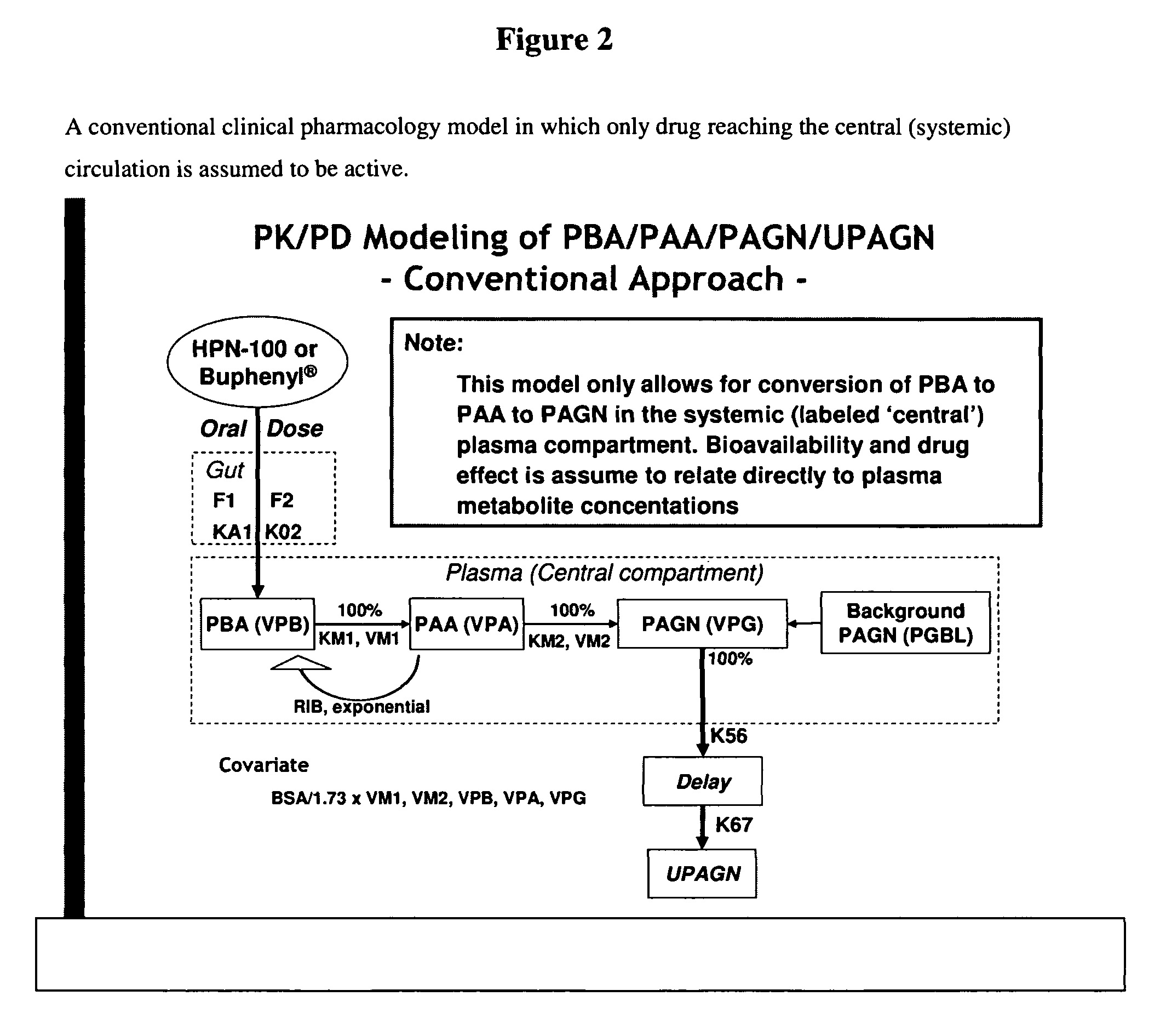
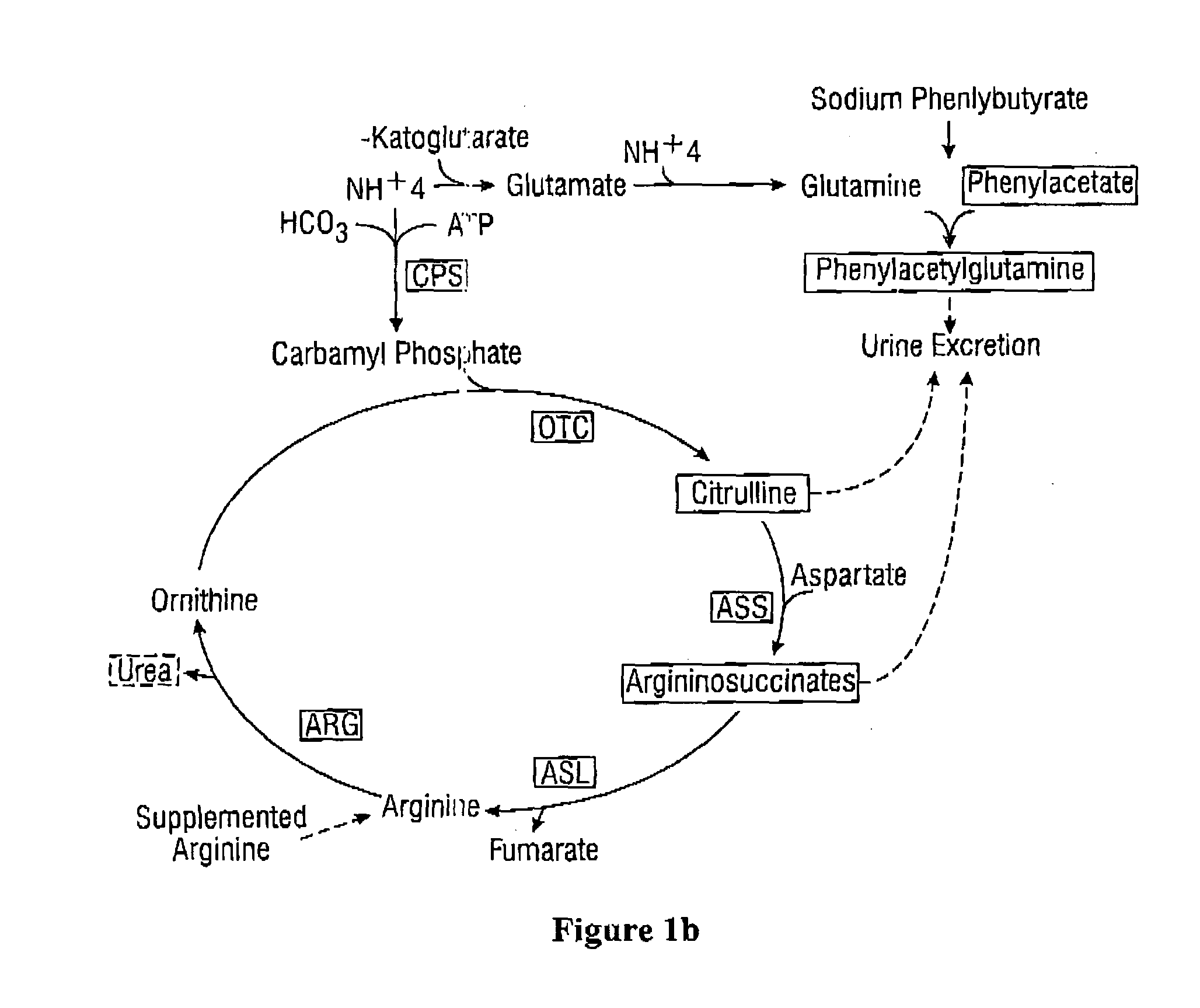
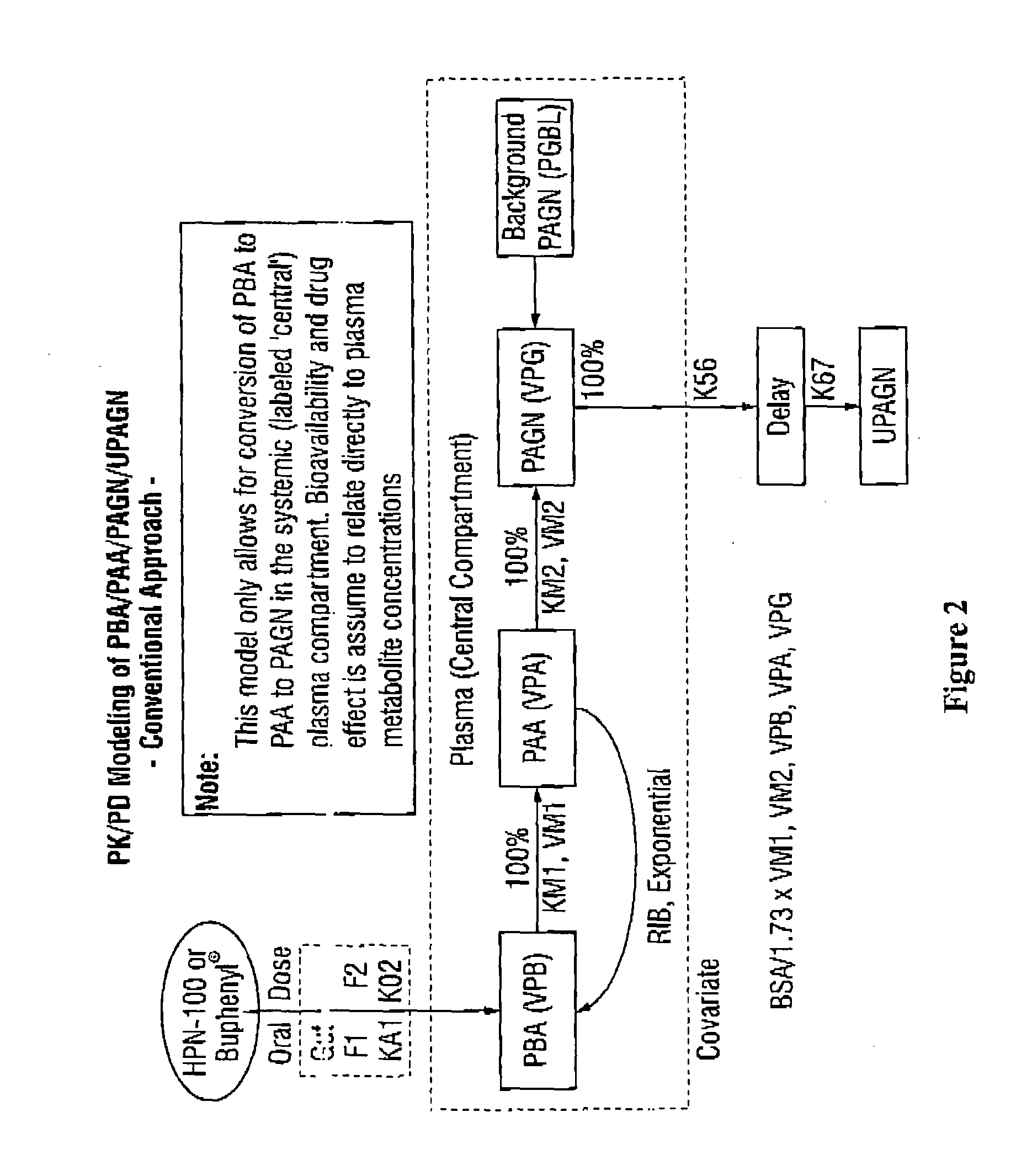
The invention provides a method for determining a dose and schedule and making dose adjustments of PBA prodrugs used to treat nitrogen retention states, or ammonia accumulation disorders, by measuring urinary excretion of phenylacetylglutamine and / or total urinary nitrogen. The invention provides methods to select an appropriate dosage of a PBA prodrug based on the patient's dietary protein intake, or based on previous treatments administered to the patient. The methods are applicable to selecting or modifying a dosing regimen for a subject receiving an orally administered ammonia scavenging drug.
Owner:HORIZON THERAPEUTICS LLC
Methods of treatment using ammonia-scavenging drugs
ActiveUS8642012B2Mitigate issueReduce dosageBiocideMetabolism disorderDosing regimenPhenylacetylglutamine
The invention provides a method for determining a dose and schedule and making dose adjustments of PBA prodrugs used to treat nitrogen retention states, or ammonia accumulation disorders, by measuring urinary excretion of phenylacetylglutamine and / or total urinary nitrogen. The invention provides methods to select an appropriate dosage of a PBA prodrug based on the patient's dietary protein intake, or based on previous treatments administered to the patient. The methods are applicable to selecting or modifying a dosing regimen for a subject receiving an orally administered ammonia scavenging drug.
Owner:HORIZON THERAPEUTICS LLC
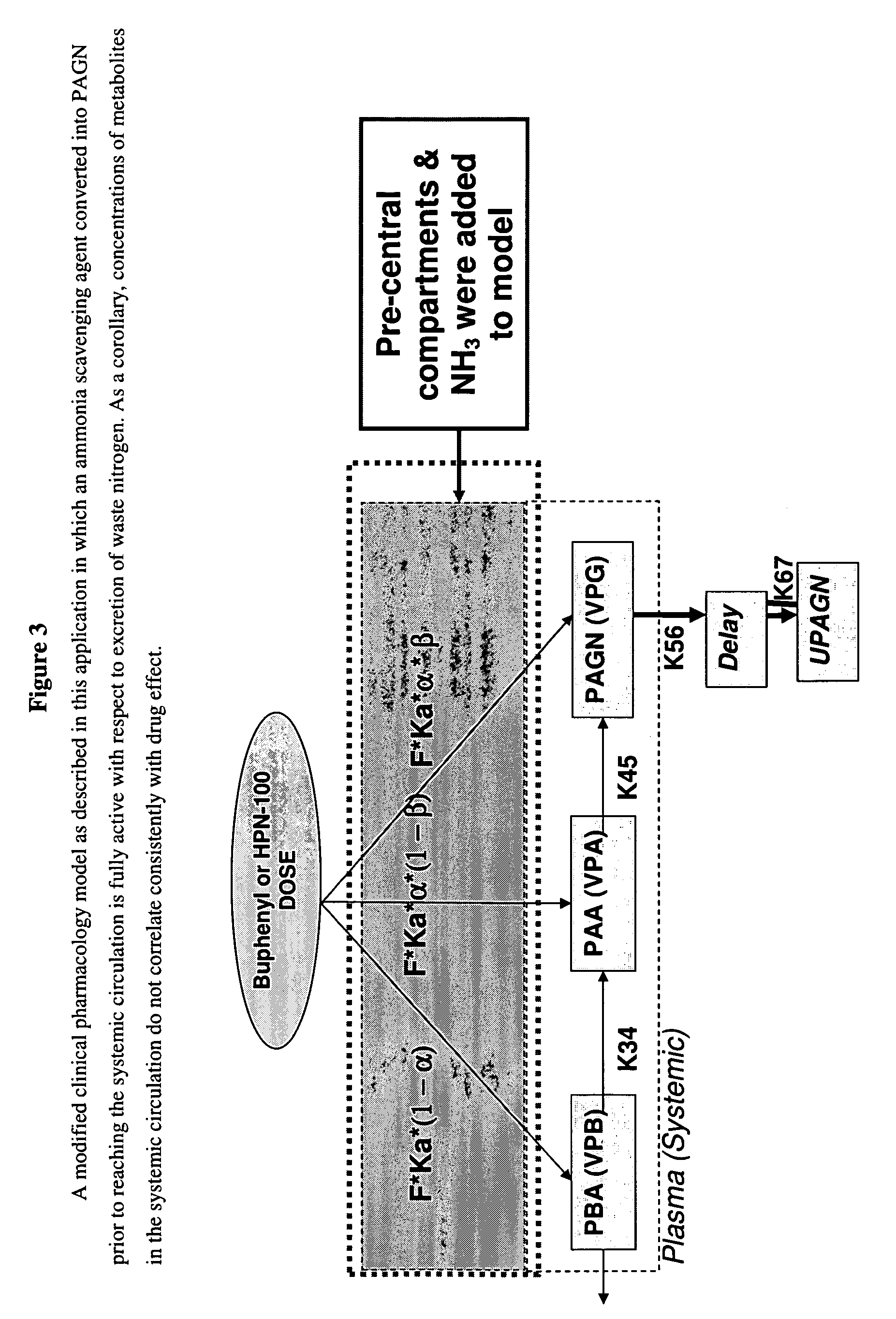
Dosing and monitoring patients on nitrogen-scavenging drugs
The invention provides a method for determining a dose and dosing schedule, and making dose adjustments of patients taking PBA prodrugs as nitrogen scavengers to treat nitrogen retention states, including ammonia accumulation disorders as well as chronic renal failure, by measuring urinary excretion of phenylacetylglutamine and / or total urinary nitrogen. The invention provides methods to select an appropriate dosage of a PBA prodrug based on the patient's dietary protein intake, or based on previous treatments administered to the patient. The methods are applicable to selecting or modifying a dosing regimen for a subject receiving an orally administered waste nitrogen scavenging drug, and to monitoring patients receiving such drugs.
Owner:HYPERION THERAPEUTICS
Treatment regimen for administration of phenylacetylglutamine, phenylacetylisoglutamine, and/or phenylacetate
InactiveUS6943192B2High plasma concentrationFull penetrationBiocidePeptide/protein ingredientsDiseasePhenylacetylglutamine
Herein is disclosed a method of treating neoplastic disease, including cancer, comprising administering a pharmaceutical composition, the pharmaceutical composition comprising a highly concentrated aqueous solution of phenylacetylglutamine and phenylacetylisoglutamine in a 4:1 ratio, at an infusion rate of from 100 mL / hr to 400 mL / hr. In a further embodiment, herein is also disclosed a method of treating neoplastic disease, including cancer, comprising administering a pharmaceutical composition, the pharmaceutical composition comprising a highly concentrated aqueous solution of phenylacetate and (phenylacetylglutamine or phenylacetylisoglutamine) in a 4:1 ratio, at an infusion rate of from 100 mL / hr to 400 mL / hr. Herein are also disclosed the pharmaceutical compositions used in the above methods.
Owner:BURZYNSKI STANISLAW R
Metabolic marker combination for evaluating risks of cardiovascular disease of subject and application thereof
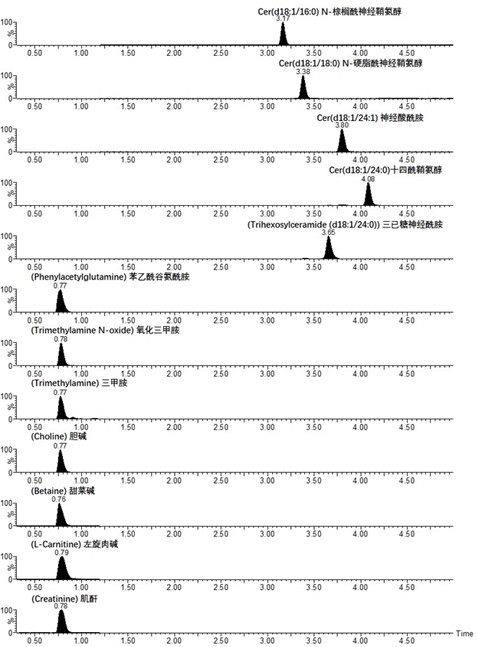
The invention discloses a metabolic marker combination for evaluating the risks of cardiovascular and cerebrovascular disease of a subject. The metabolic marker combination comprises ceramide Cer d18:1 / 16:0, ceramide Cer d18:1 / 18:0, ceramide Cer d18:1 / 24:0, phenylacetyl glutamine, trimethylamine, glycine betaine and choline. The cardiovascular and cerebrovascular diseases are selected from coronary heart disease, atherosclerosis, atrial fibrillation and heart failure. The metabolic marker combination disclosed by the invention has the advantages of high sensitivity, good specificity, quantification, high detection flux and the like in the aspect of evaluating or predicting the risks of cardiovascular and cerebrovascular diseases.
Owner:HUMAN METABOLOMICS INST INC
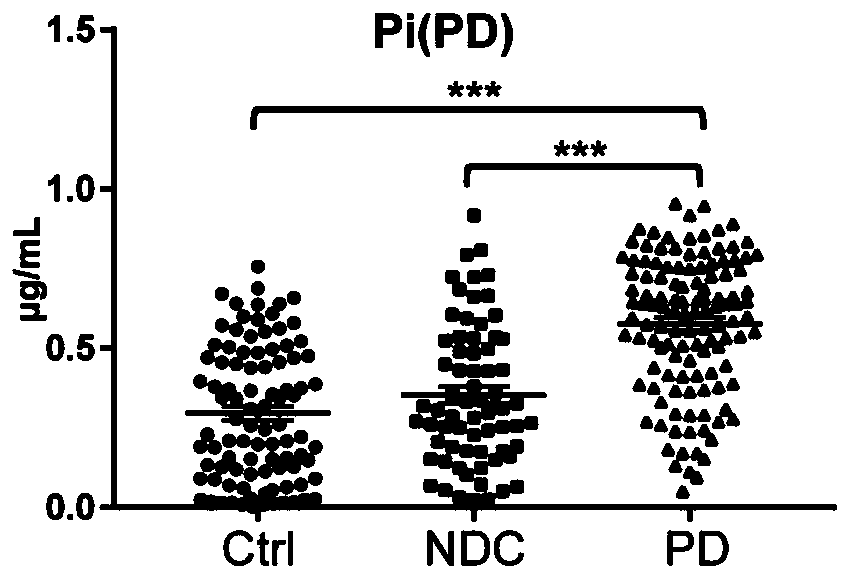
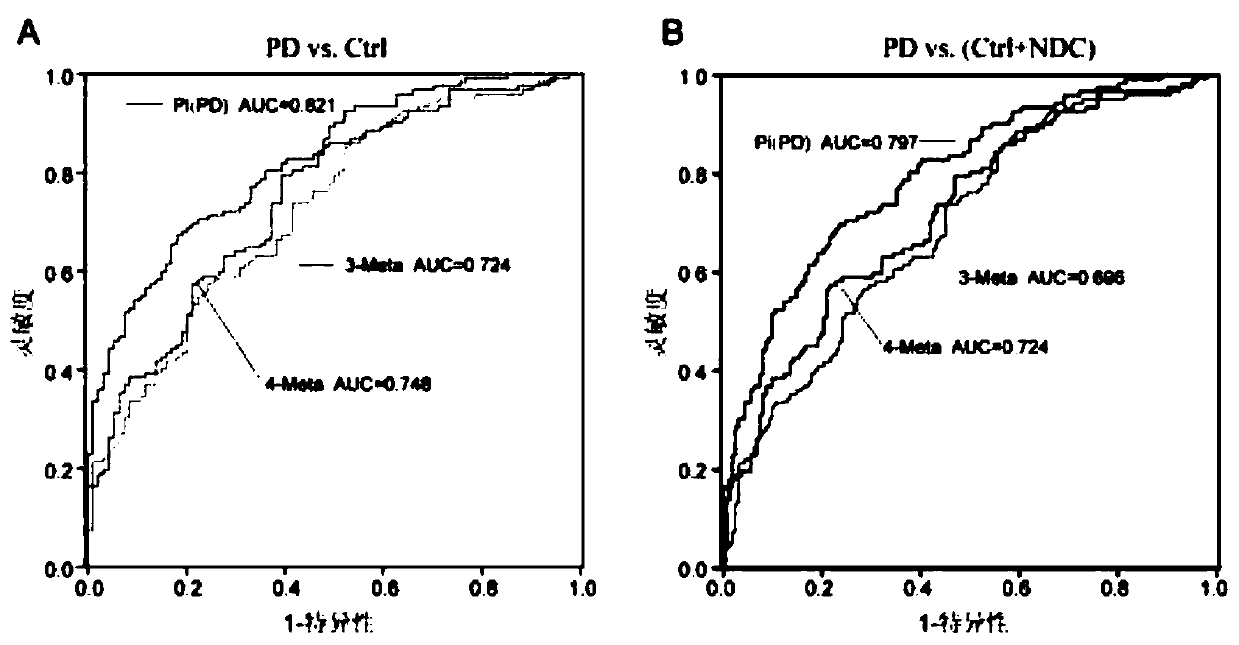
Combined marker and detection kit for diagnosing Parkinson's disease
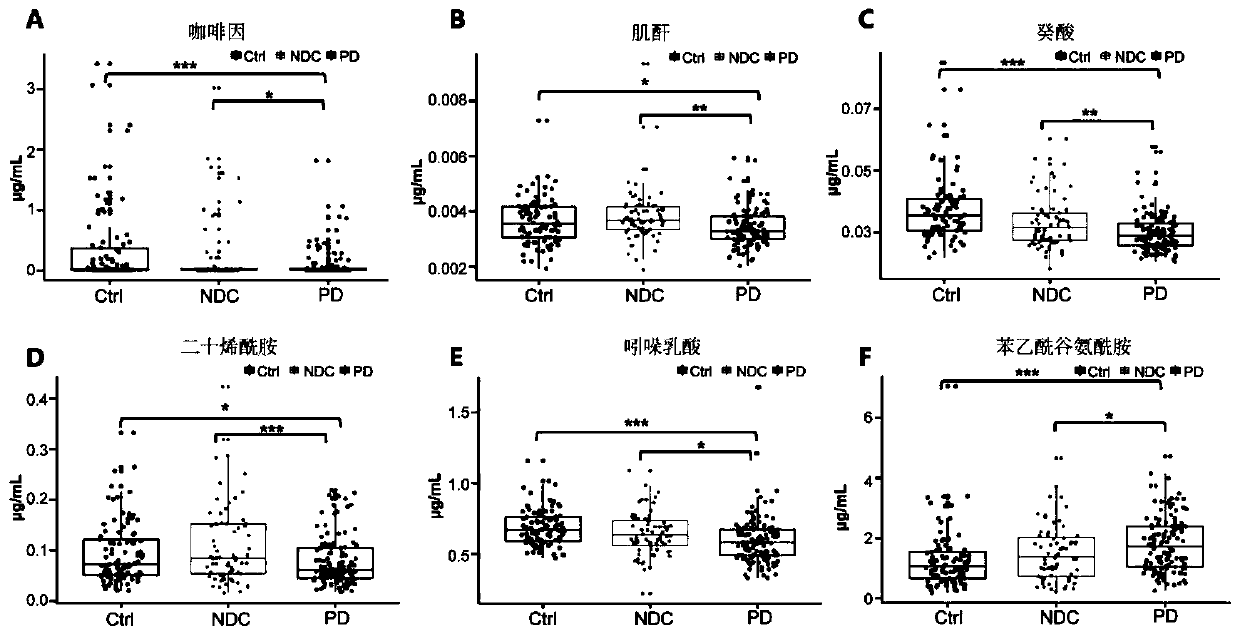
The invention relates to new application of small molecular metabolites caffeine, creatinine, eicosamide, phenylacetylglutamine, decanoic acid and indolelactic acid in a plasma sample as combined markers in preparation of a kit for diagnosing Parkinson's disease patients in subjects. The invention also relates to the kit for detecting the Parkinson's disease patients in the subjects, and the kit is used for calculating the variables of the combined markers based on a binary logistic regression equation by detecting the respective relative concentrations of the combined markers in the plasma from the subjects, and judging whether the subjects suffer from Parkinson's disease or not based on determined intercept values. The kit can realize high-sensitivity detection of several metabolites involved in the invention, and has the characteristics of low detection cost and good repeatability. The combined use of the small molecule metabolites can be used for assisting the clinical diagnosis ofthe Parkinson's disease, and has higher development and application values.
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
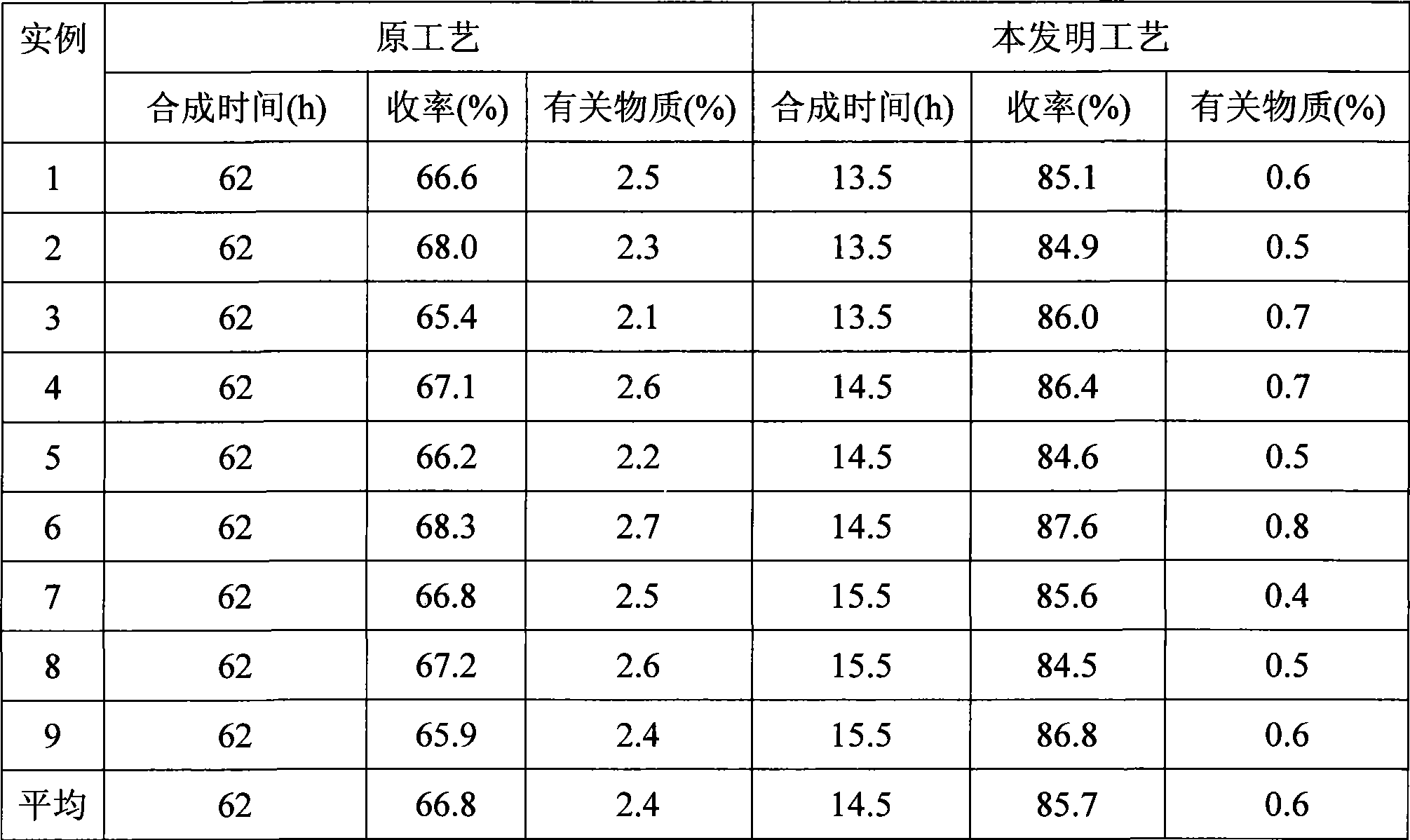
Preparation of aceglutamide
ActiveCN101434559AIncrease labor costIncrease material costOrganic compound preparationCarboxylic acid amides preparationAlcoholFiltration
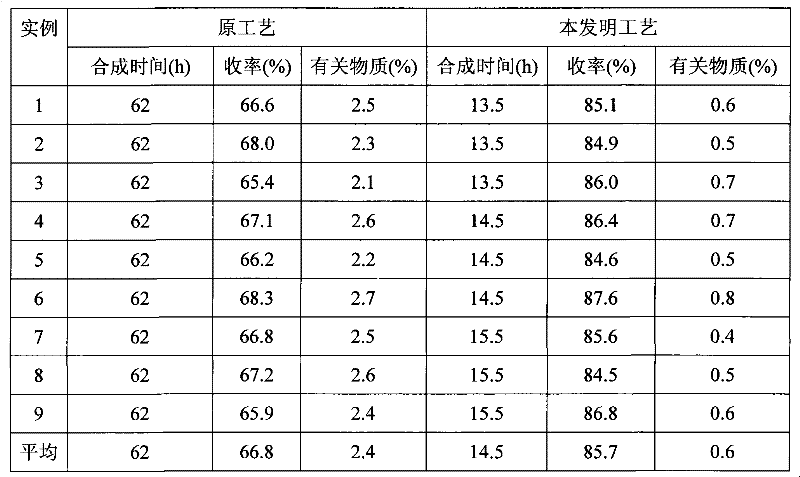
The invention provides a preparation method of acetylglutamine, which includes the steps of acylation neutralization reaction, refining, drying and the like. Based on the original technology, the preparation method saves the technological processes of drying, heating and dissolving crude products. After neutralization reaction, filtration and the preparation of the crude products, alcohol is directly used for dissolving crystals and an end product is obtained by drying. The preparation method is simple, and has low cost, high product yield and good quality. All indicators are fully consistent with the existing quality standards; therefore, the preparation method is applicable to industrialized production.
Owner:SHANGHAI ZHAOHUI PHARMA +1
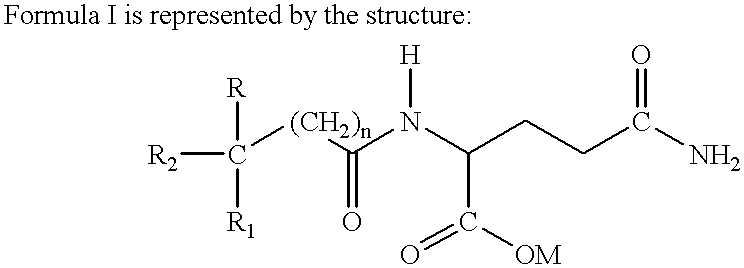
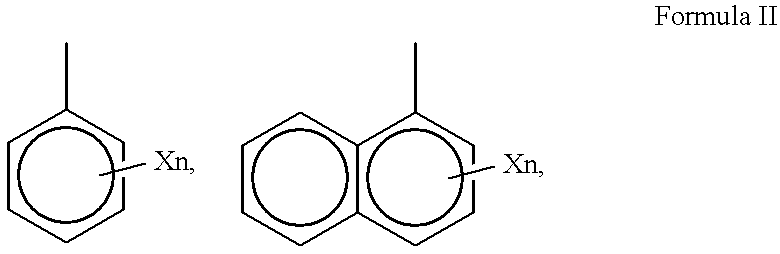
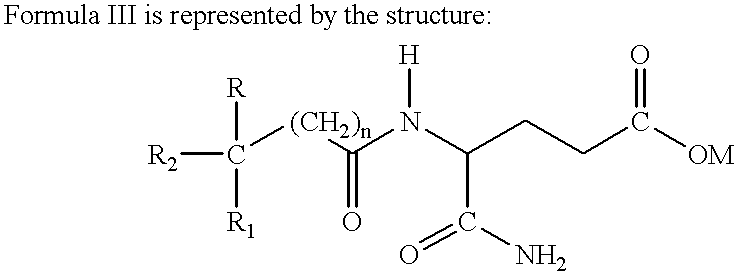
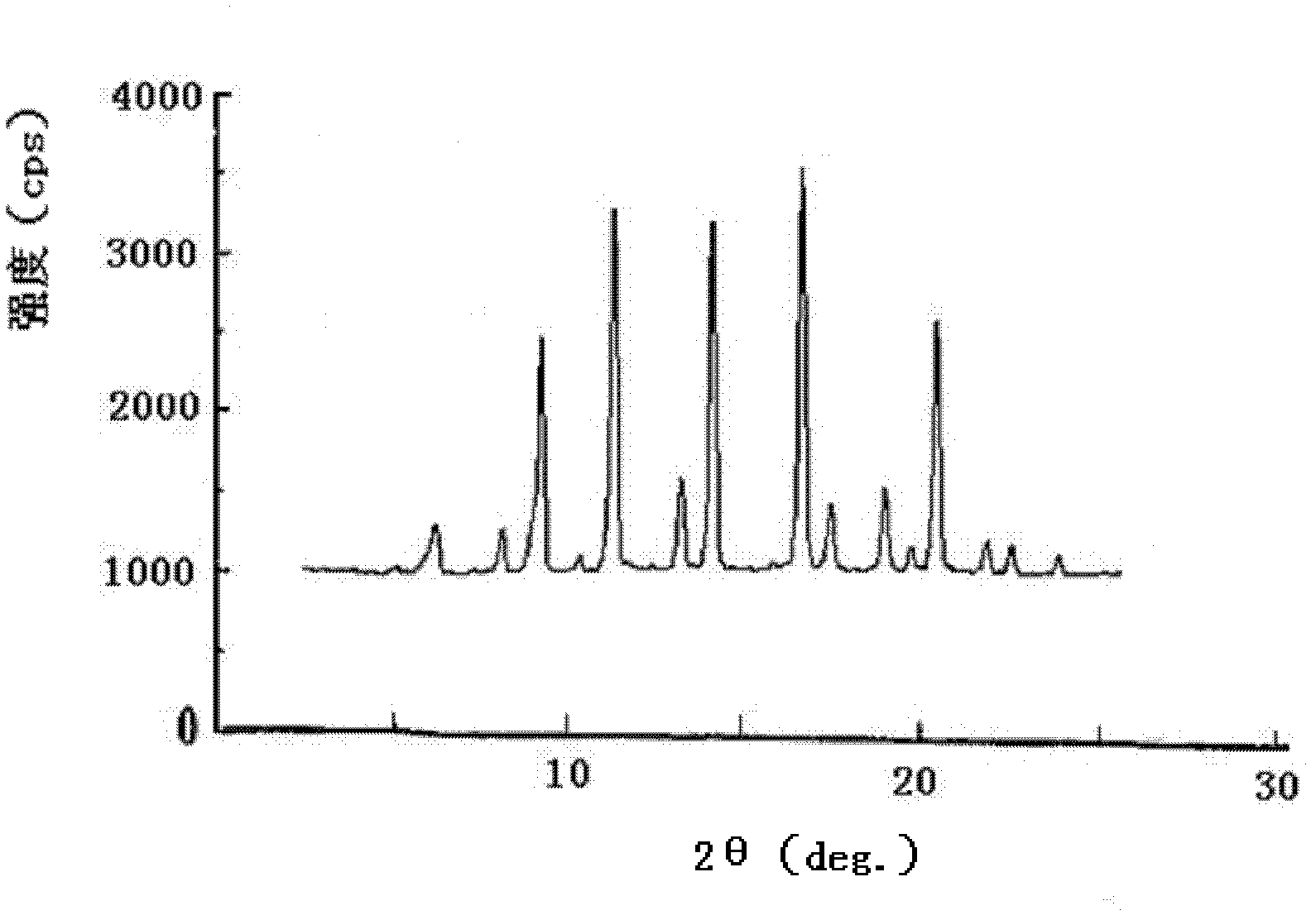
Acetylglutamine compound and pharmaceutical composition thereof
ActiveCN102276496AUniform particle sizeStable in natureNervous disorderCarboxylic acid amide separation/purificationFreeze-dryingAceglutamide
The invention discloses an aceglutamide compound. The aceglutamide compound is crystals; and characteristic peaks in an X-ray powder diffraction pattern measured by using Cu-K alpha rays are displayed as 9.3, 11.2, 14.6, 17.0 and 20.9 at 2 theta. The aceglutamide compound has the advantages of uniform particle size and steady characteristic, and cannot be hydrolyzed and oxidized easily, so that the aceglutamide compound can be used for preparing a medicinal composition. The invention also discloses the medicinal composition. The medicinal composition contains the aceglutamide compound and a pharmaceutically acceptable carrier, an excipient or a diluent; and the optimal formulation is freeze-dried powder injection or injection. The medicinal composition has the advantages of stable qualityand high yield, and the preparation process is simple, so that the period of validity of the medicine is prolonged, and the quality of the product is guaranteed.
Owner:周晓东
Preparation method of aceglutamide
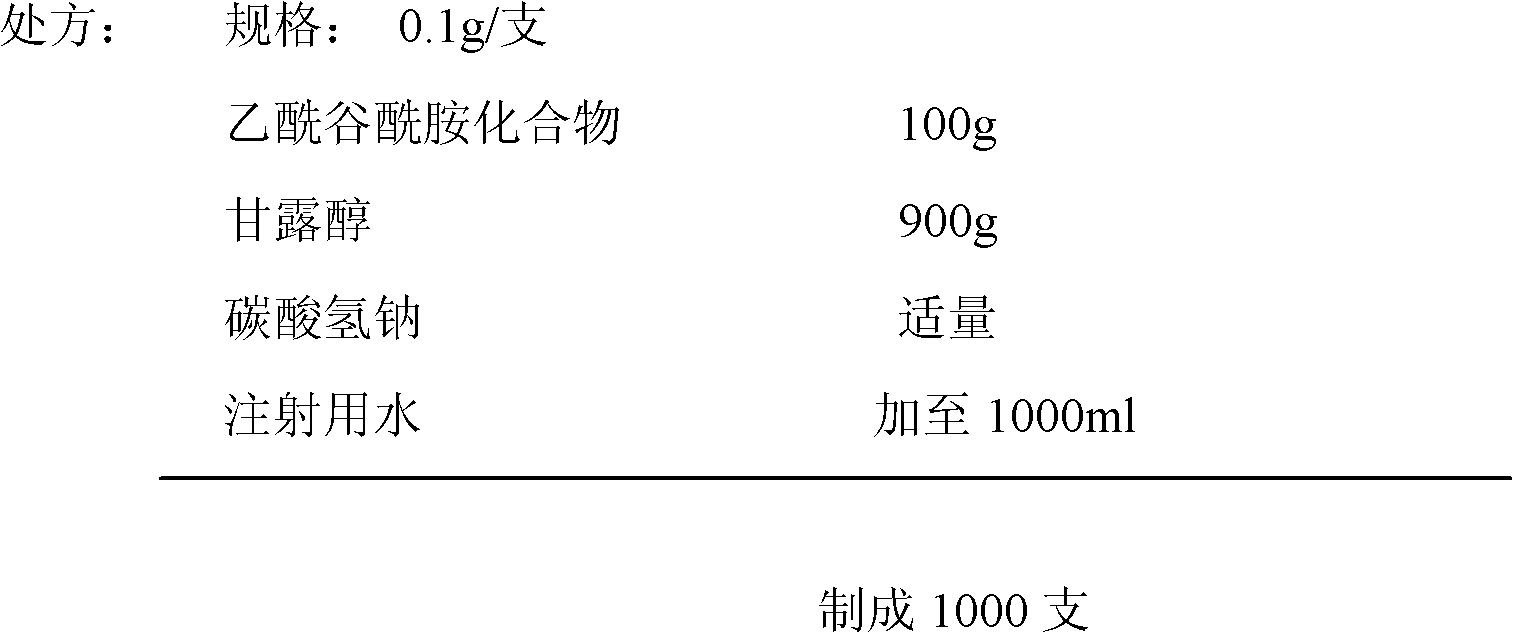
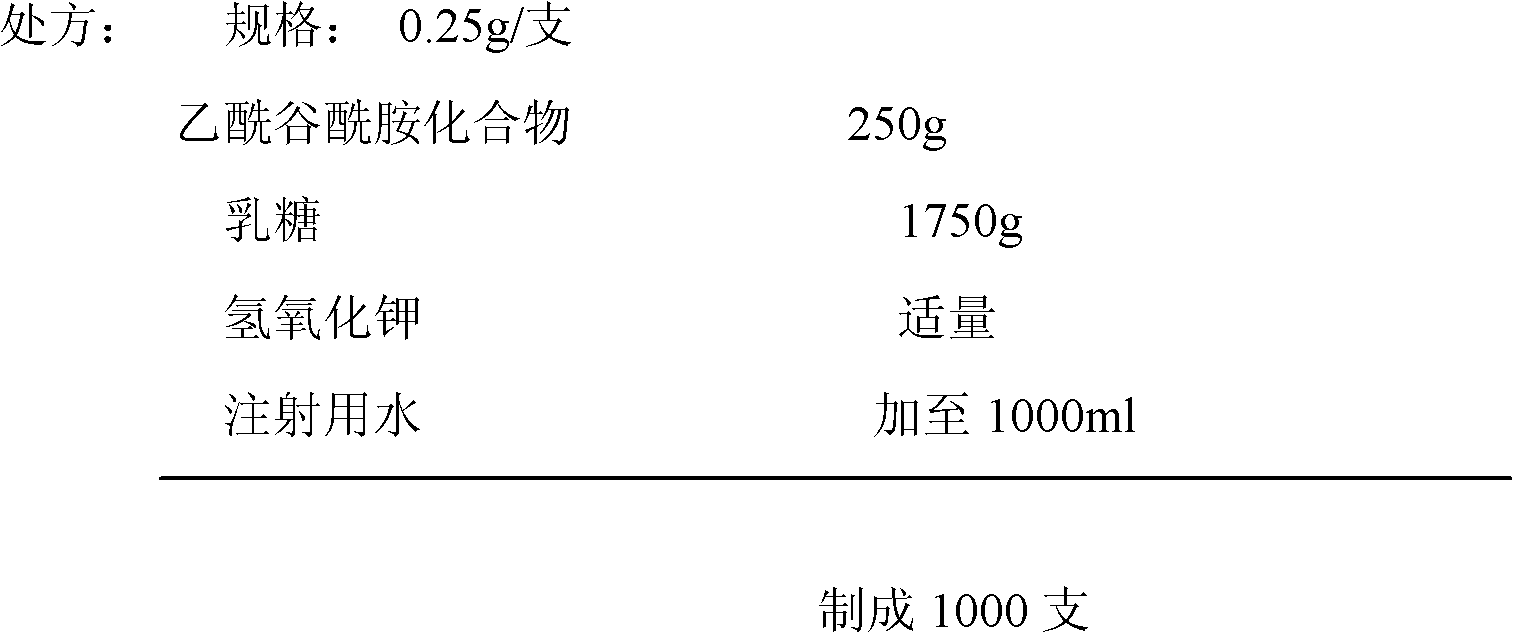
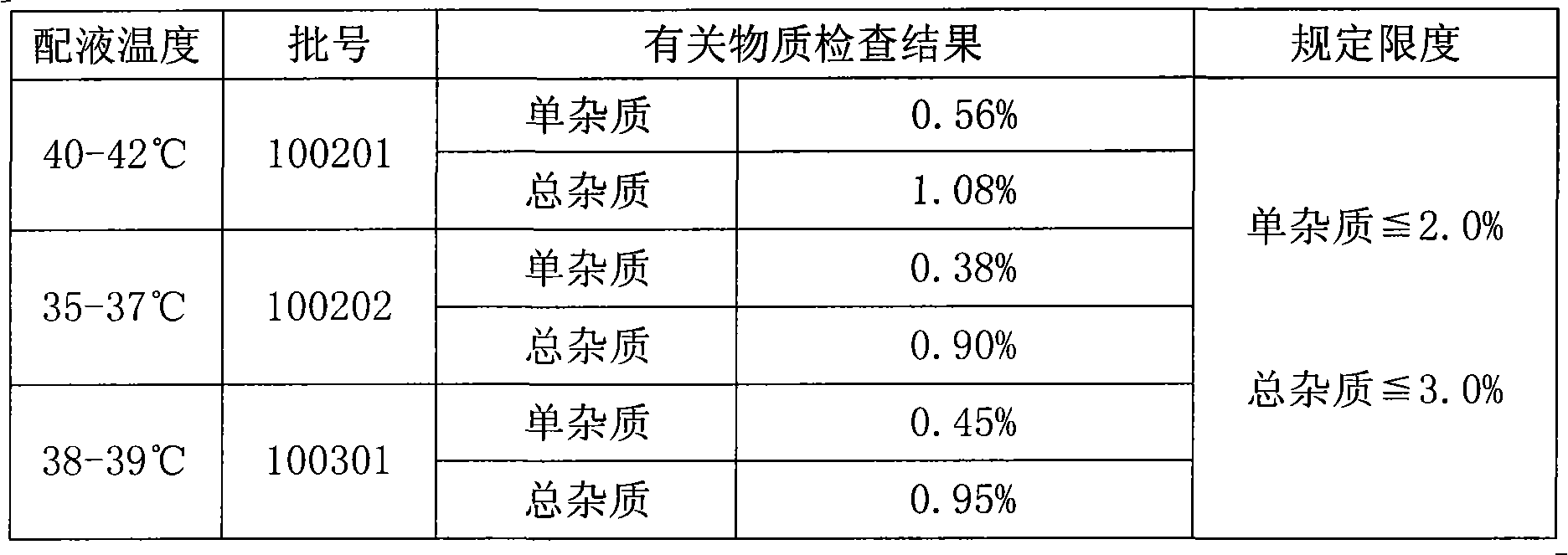
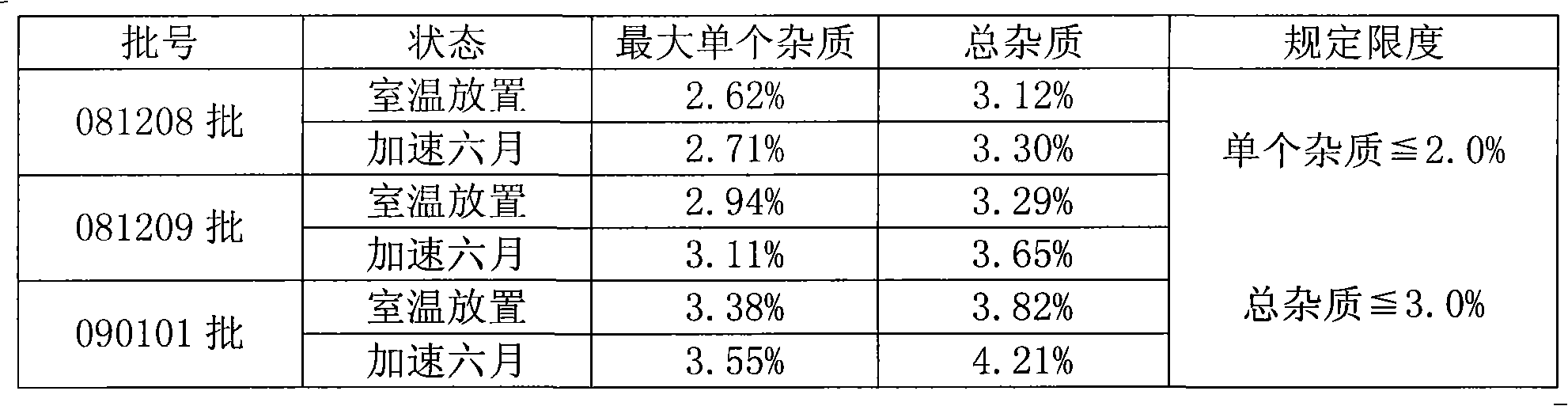
ActiveCN102018668AReduce riskSimple methodOrganic active ingredientsNervous disorderAceglutamideWater temperature
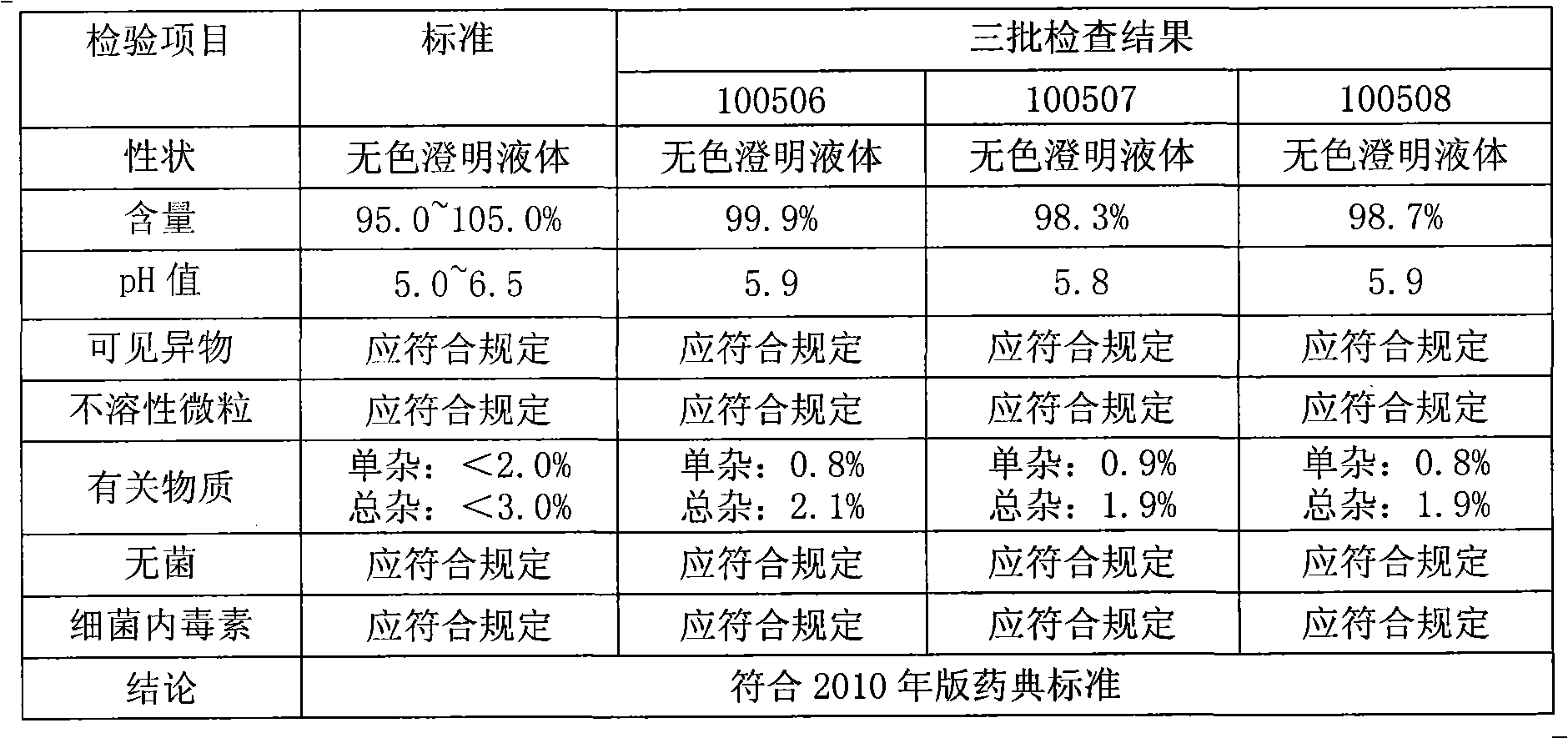
The invention provides a preparation method of aceglutamide. By utilizing the new method provided by the invention, the defect of the relevant substances of the aceglutamide due to the existing method can be overcome. In the method, the temperature of 70-80 DEG C of the prepared water for injection is improved into the water temperature of 35-42 DEG C; and after the product is subjected to detection, an accelerated test, a long term stability test and the like, a data result shows that all indexes of the relevant substance, content and the like of the aceglutamide can reach the standard of the China Pharmacopeia 2010 edition after the new method provided by the invention is adopted. The method provided by the invention is simple and practicable and is suitable for industrial production on a large scale.
Owner:SHANGHAI ZHAOHUI PHARMA +1
Application of anti-hypoxia and anti-fatigue oral composition in health product
InactiveCN103211141ARevitalizationEffectively exert the health care function of anti-hypoxia and anti-fatigueFood preparationLife activityVitamin C
The invention relates to a health food field, particularly a health food containing the following components of: D-ribose, L-carnitine, vitamin C, arginine, taurine, aceglutamide, nicotinamide and corrigent. An effective combination proportion of a reasonable prescription is screened by experiments, to make the prescription efficacy generate synergism, thereby effectively performing anti-hypoxia and anti-fatigue health care effects, and helping sub-healthy patients and persons enter a plateau to regain sufficient energy needed by life activities and to recover body vitality.
Owner:莫海仪 +2
Aceglutamide injection
InactiveCN1444931AImprove the level of clinical applicationImprove securityNervous disorderPeptide/protein ingredientsAceglutamideMedicine
Owner:张嵩
Aceglutamide powder injection medicine composition for injection and preparation method of medicine composition
The invention relates to an aceglutamide powder injection medicine composition for injection and a preparation method of the medicine composition, in particular belongs to the technical field of medicines, relates to a medicine for treating brain traumatic coma, coma caused by neurosurgery, hepatic coma, hemiplegia, high paraplegia, infantile paralysis, nerve headache and lumbago, in particular relates to a medicine composition such as a freeze-dried powder injection prepared by taking aceglutamide as an active component and also relates to a preparation method of the medicine composition. In one embodiment of the invention, the aceglutamide powder injection medicine composition for injection comprises aceglutamide, a freeze-drying excipient and an acid-base regulation agent, wherein the freeze-drying excipient is selected from mannitol, glycine, lactose, saccharose, glucose and the like or the combination thereof. The aceglutamide powder injection for injection has excellent medicinal properties.
Owner:成都天台山制药股份有限公司
Phenylacetylglutamine as biomarker for healthy ageing
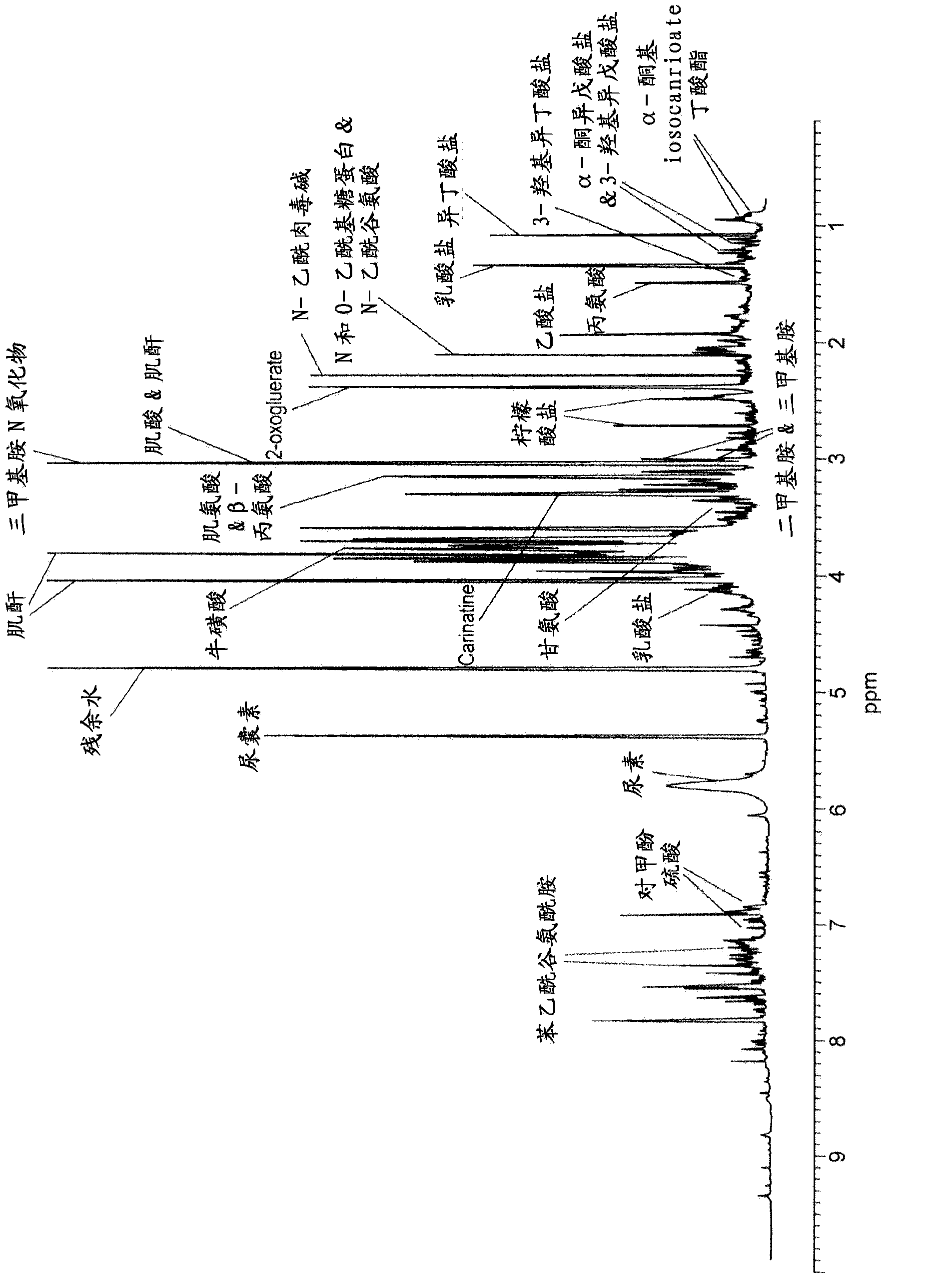
Using NMR / MS based metabonomics and targeted lipidomics approaches the inventors have explored the metabolic phenotypes of aging and longevity in a cohort compromising centenarians, elderly and young adults. The inventors have identified biomarkers for a reduced risk of developing ageing related chronic inflammatory disorders and propose an in vitro method of diagnosing a lifestyle that allows delaying and / or avoiding ageing related chronic inflammatory disorders using phenylacetylglutamine (PAG) as biomarker.
Owner:NESTEC SA
Detection method for hydroxysafflor yellow A metabolic product
ActiveCN106370743AData processing is simpleThe positive effect is clearComponent separationChemical structureMetabolite
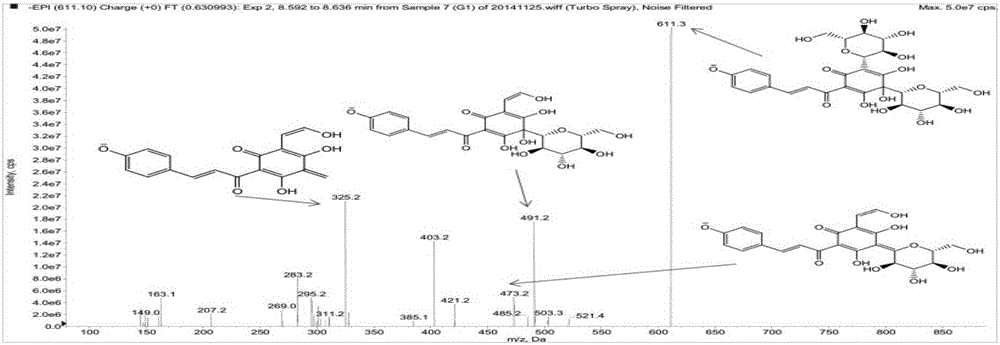
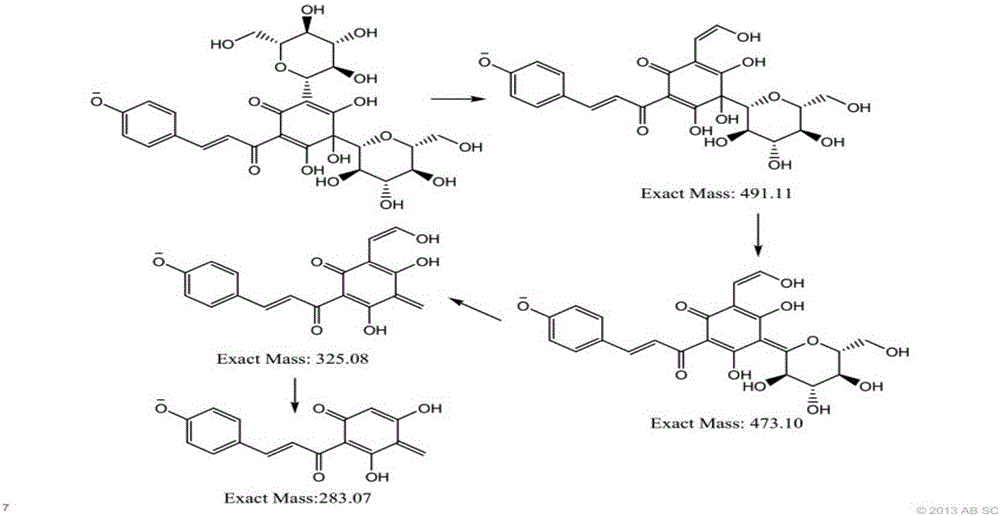
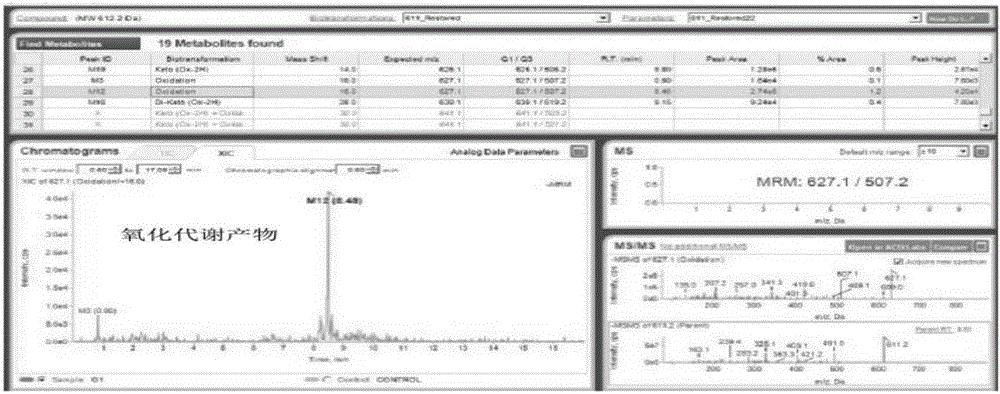
The invention discloses a detection method for hydroxysafflor yellow A metabolic products. The method includes the steps of: 1) performing intravenous injection to an SD rat with a safflower extract and aceglutamide injection, performing orbid blood sampling, and centrifugally separating the blood sample to obtain plasma; 2) sucking the plasma with a micro sample injector, adding acetonitrile with volume ratio of plasma to acetonitrile being 1:3-3.5, uniformly mixing the substances with vortex and centrifuging the mixture to obtain a supernatant, blow-drying the supernatant with nitrogen gas, dissolving the dried substance in acetonitrile, and centrifuging the solution to obtain a supernatant as a detection sample; and 3) detecting and analyzing the sample through high performance liquid chromatography-triple quadrupoles linear ion trap tandem mass spectrometry, and analyzing the metabolic products in a p-MRM-IDA-EPI mode. In the invention, a p-MRM method is employed in the mass spectrum with combination of Light Sight software to quickly identify and analyze the sample for tracing metabolic change of component change and structure change in the plasma; 11 hydroxysafflor yellow A metabolic products are found in the result. The method can be used for obtaining a time change curve of the hydroxysafflor yellow A and the metabolic product thereof in plasma, and successfully deduces and identifies the molecular structures of seven metabolic products and isomers of the hydroxysafflor yellow A. The method, compared with a chemical structure analytic method, is more quick and sensitive.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
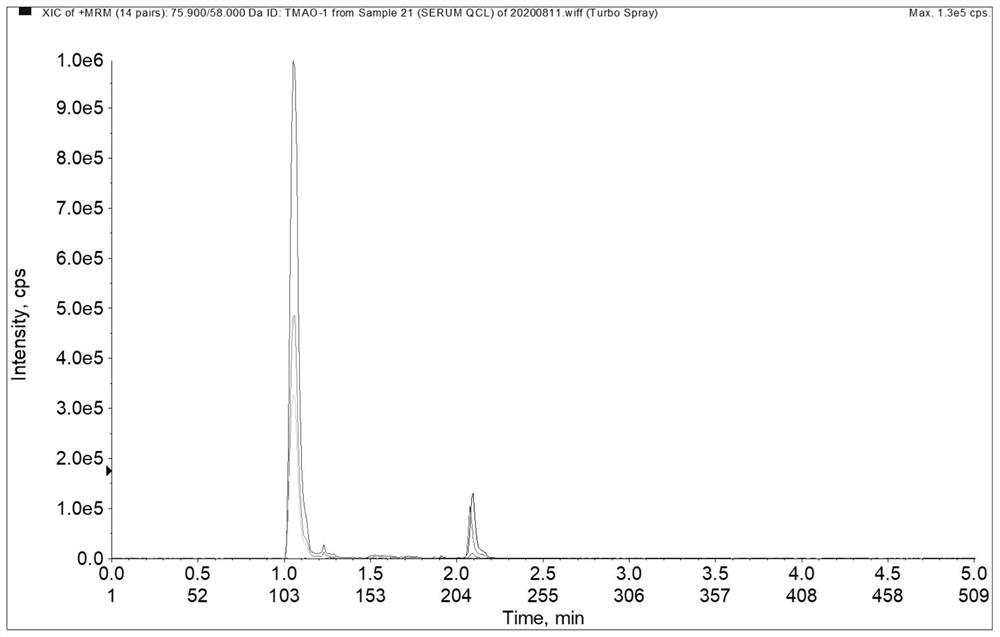
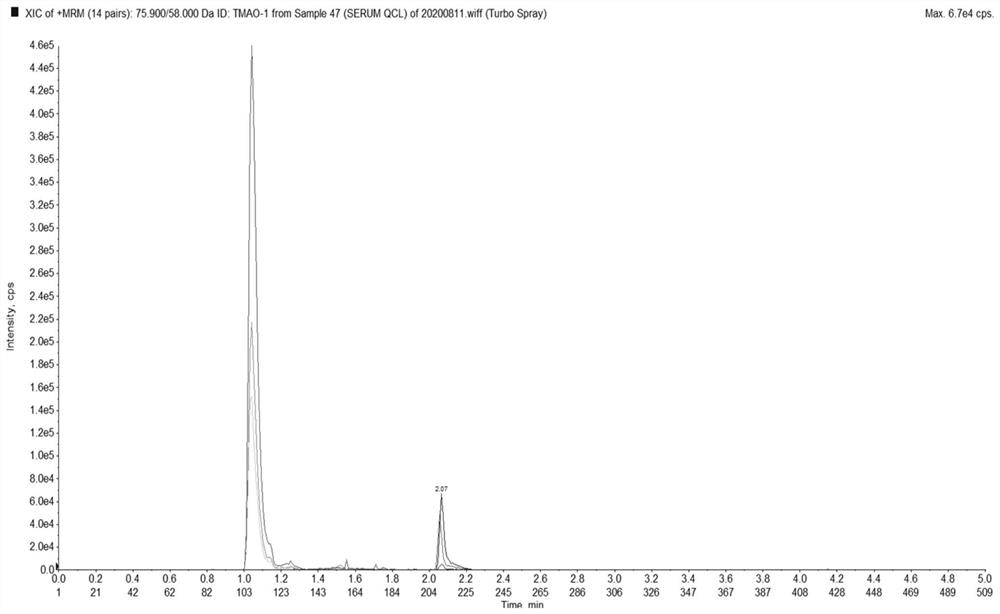
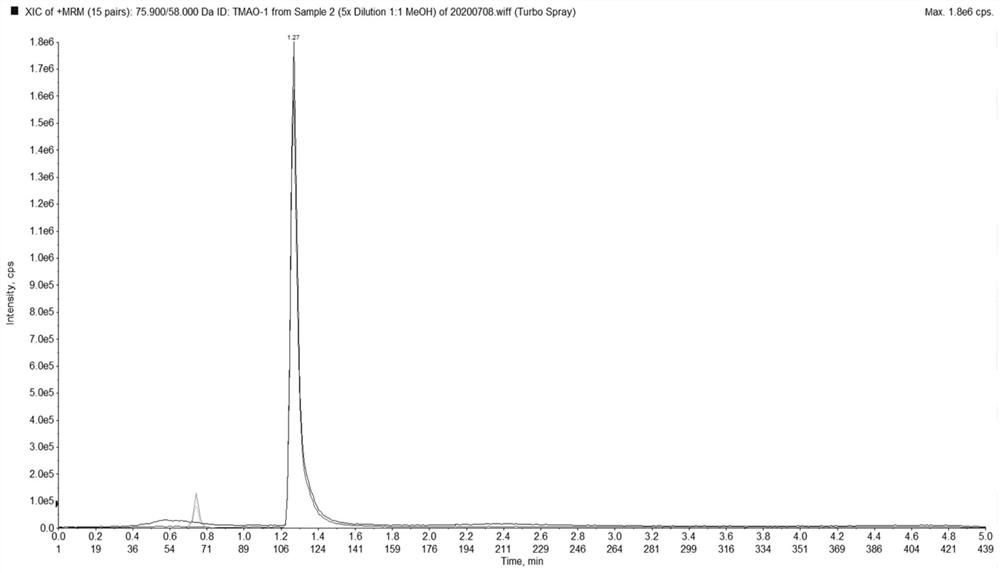
Method for simultaneously detecting trimethylamine oxide and phenylacetyl glutamine as well as detection kit and application thereof
PendingCN113176364AImprove accuracyEasy to handleComponent separationPhenylacetylglutamineSmall sample
The invention relates to the technical field of analysis and detection, in particular to a method for simultaneously detecting trimethylamine oxide and phenylacetylglutamine as well as a detection kit and application thereof. According to the detection method provided by the invention, a to-be-detected sample is subjected to sample pretreatment, then detection is performed by adopting a liquid chromatography-tandem mass spectrometry method, the sample pretreatment comprises the following steps: the to-be-detected sample is subjected to precipitation treatment by adopting a precipitant to obtain separated supernate, and the precipitant is an acetonitrile and methanol mixed solution containing 10-100mM ammonium acetate. The method has the advantages of simple sample reservation, simple sample treatment, small sample use amount and fast detection speed, can reduce the problems of two-time sample reservation, time consumption, cost increase and the like due to respective determination, and is easy to popularize and apply; the detection method has high precision and low matrix effect, and can significantly improve the accuracy of trimethylamine oxide and phenylacetyl glutamine detection.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Medicinal composition containing carthamin yellow and its preparation process and usage
InactiveCN1768740ASignificant clinical effectTo promote metabolismOrganic active ingredientsPowder deliveryHepatic comaDisease
The invention provides a medicinal composition containing carthamus tinctorius yellow color, which comprises aceglutamide and carthamus tinctorius yellow color by a predetermined proportion, and can be used for preparing medicament for the treatment of cerebrovascular diseases, hepatic coma, hemiplegia, paralysis, memory obstacle, coronary disease and angiitis.
Owner:阿尔贝拉医药控股(通化)有限公司
Toothpaste containing anticancer agents
InactiveUS20060246016A1Effective treatmentPreventive effectCosmetic preparationsToilet preparationsOral diseaseDisease
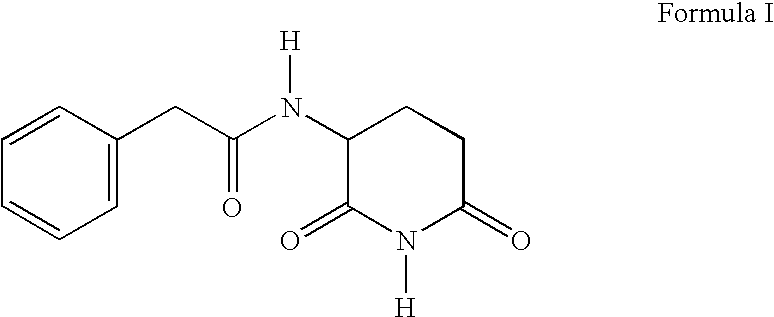
A novel dentifrice composition is provided for prevention or treatment of carcinoma of the oral cavity, caries and periodontal diseases of the oral cavity. The dentifrice composition contains a silica abrasive and medicinal agents useful in the treatment of human neoplastic disease. The medicinal agent is selected from the group consisting of 3-N-phenylacetylamino-2,6-piperidinedione, phenylacetylglutamine, phenylacetylisoglutamine, phenylbutyrate, phenylacetate, combinations thereof and pharmaceutically acceptable salts thereof. The components of the dentifrice composition act advantageously to allow the composition to remove plaque, tartar, and oral disease-causing bacteria.
Owner:BURZYNSKI STANISLAW
Novel aceglutamide compound and pharmaceutical composition thereof
ActiveCN103012192AImprove stabilityLower blood ammoniaOrganic active ingredientsPowder deliveryBlood ammoniaHigh humidity
The invention discloses a novel aceglutamide compound. The purity of the compound is greater than 99.6%. At the same time, the invention further discloses a pharmaceutical composition of the novel aceglutamide compound. The composition comprises the following components in parts by weight: 90-600 parts of aceglutamide, 6-40 parts of alliin, 1-8 parts of mannitol, 0.8-5 parts of disodium hydrogen phosphate and 0.5-3 parts of sodium dihydrogen phosphate. In the pharmaceutical composition of the novel aceglutamide compound disclosed by the invention, the synergistic effect is generated among the mannitol, the disodium hydrogen phosphate and the sodium dihydrogen phosphate, so that the stability of the composition under high temperature, high humidity and bright light is enhanced; and the aceglutamide and the alliin mutually take synergistic effect and can play a role of observably reducing blood ammonia.
Owner:东台海滨科技创业园管理有限公司
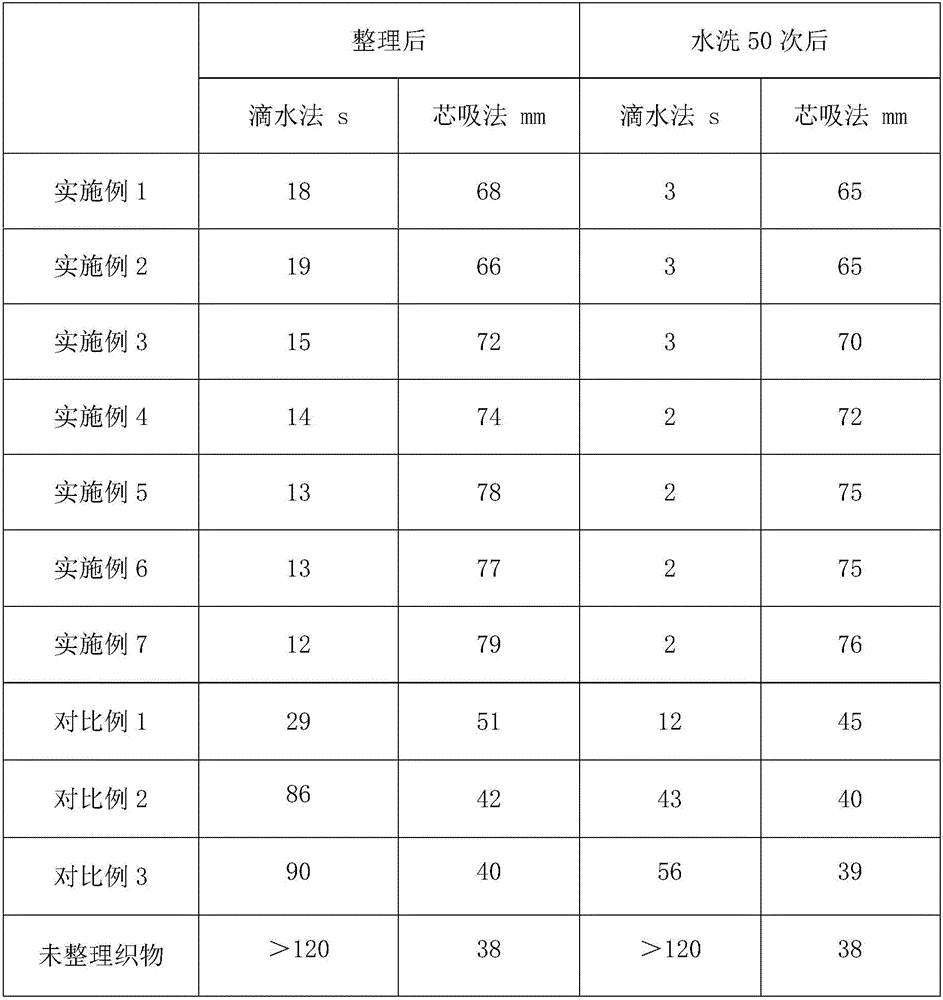
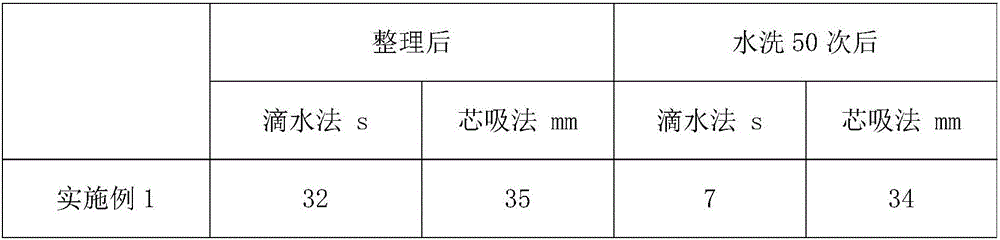
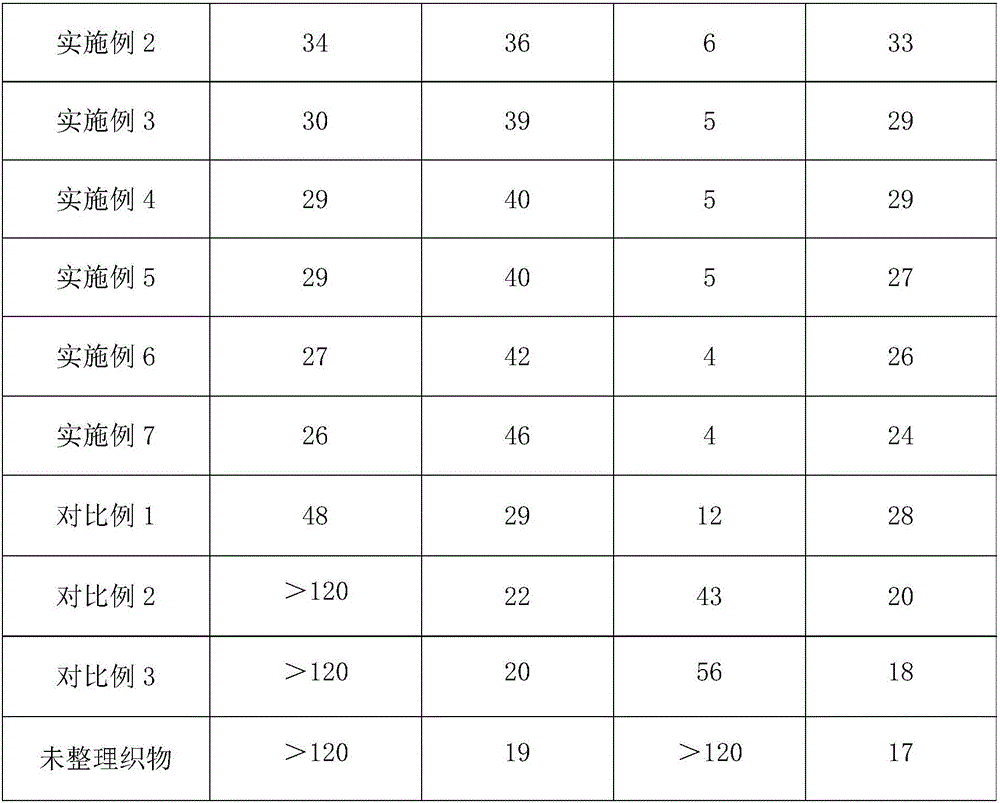
Water-soluble thermal-reactive polyurethane hydrophilic, flame-retardant and antibacterial finishing agent and application thereof
InactiveCN106498749AIncrease the degree of cross-linkingImprove hydrophilicityBiochemical fibre treatmentHeat resistant fibresAceglutamideGlycerol
The invention relates to the technical field of textile auxiliary agents, and discloses a water-soluble thermal-reactive polyurethane hydrophilic, flame-retardant and antibacterial finishing agent. The water-soluble thermal-reactive polyurethane hydrophilic, flame-retardant and antibacterial finishing agent comprises components by following weight part: 15-20 parts of water-soluble thermal-reactive polyurethane, 0.1-10 parts of aceglutamide and 2-12 parts of glycerol. The aceglutamide is added as an additive in the finishing agent, and the water-soluble thermal-reactive polyurethane hydrophilic, flame-retardant and antibacterial finishing agent can catalyze proceeding of reaction and significantly improve crosslinking rate of the polyurethane and synthetic fibers, the lower concentration polyurethane can endow the synthetic fibers and wool fibers with lasting hydrophilicity, and simultaneously significantly enhances reactivity and softness, and is high in durability, and a fabric after being tidied not only has good hydrophilicity, but also has fire resistance and antibacterial performance.
Owner:FOSHAN XUNTUOAO TECH CO LTD
Aceglutamide composition freeze-dried powder injection for injection
InactiveCN103550175AImprove repair effectImprove bioavailabilityOrganic active ingredientsPowder deliveryChitosan nanoparticlesAceglutamide
The invention provides an aceglutamide composition freeze-dried powder injection for injection, and relates to the technical field of medicines and medicine preparation. The aceglutamide composition freeze-dried powder injection for injection comprises the following raw material components in parts by weight: 2.35-6.35 parts of aceglutamide, 6.70-10.70 parts of chitosan nanoparticles and 84.96-88.96 parts of water for injection. The aceglutamide composition freeze-dried powder injection for injection has the advantages that 1) the composition can remarkably enhance the repairing effect of aceglutamide, the use level of aceglutamide can be clinically reduced, and the adverse reaction of aceglutamide can be alleviated; 2) the chitosan nanoparticles have the effect of targeted therapy and are taken as a medicine carrier to improve the bioavailability of aceglutamide, thereby facilitating clinical application; 3) the chitosan nanoparticles can replace mannitol as a freeze-dried skeleton agent of the freeze-dried powder injection, so that the activity of mannitol on human body is eliminated.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Pharmaceutical composition containing aceglutamide and safflower effective ingredients and formulation thereof
InactiveCN1965878AAddressing Medication Safety IssuesQuick resultsOrganic active ingredientsPharmaceutical delivery mechanismDiseaseAdditive ingredient
The invention discloses a pharmaceutical composition and preparation comprising active constituents of aceglutamide and safflower, which can be used for preparing medicaments for treating cardiovascular and cerebrovascular diseases, and can be prepared into tablets, capsules, drop pills, injections, pills, syrups, granules, oral liquids, orally administered turbid liquors or oral emulsions.
Owner:北京天新园医药科技开发有限公司
Safflor yellow A-containing pharmaceutical composition, its preparation method and usage
InactiveCN1762342ATo promote metabolismImprove stabilityOrganic active ingredientsPowder deliveryVascular diseaseDisease
The invention discloses a pharmaceutical composition which is a compound preparation prepared from aceglutamide and Safflomin A. The pharmaceutical composition can be used for treating cerebrovascular diseases, liver stupor, memory obstacle, coronary disease and vascular diseases.
Owner:阿尔贝拉医药控股(通化)有限公司
Aceglutamide composition for injection and preparing method thereof
ActiveCN105796511AImprove stabilityGood anti-fatigue effectOrganic active ingredientsPowder deliveryFreeze-dryingAceglutamide
The invention provides aceglutamide freeze-dried powder for injection.The powder is prepared from 1-10 parts by weight of aceglutamide, 0.5-20 parts by weight of freeze-drying protective additive and a proper amount of pH modifier.Preferably, the freeze-drying protective additive is the composition of mannitol, dextran and saccharose in the proportion of 1:1:1.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Edible salt substitute for recuperating 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight)
InactiveCN110679900AReduce intake"Five high" chronic diseases conditioning or reliefFood ingredient as mouthfeel improving agentAceglutamideBlood sugar
The invention relates to edible salt substitute for recuperating 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight). The edible salt substitute is prepared from the components in parts by weight: 0.5 to 1.5 parts of rose salt, 0.5 to 1.5 parts of Himalaya salt, 0.2 to 0.8 part of food grade histidine hydrochloride, 0.05 to 0.15 part of flos caryophyllata, 0.05 to 0.15 part of radix aucklandiae, 0.05 to 0.15 part of fructus foeniculi, 0.05 to 0.15 part of radix curcumae longae, 0.05 to 0.15 part of galanga, 0.05 to 0.15 part of rhizoma zingiberis, 0.05 to 0.15 part of netmeg, 0.05 to 0.15 part of cortex cinnamomi, 0.05 to 0.15 part of fructus crataegi, 0.05 to 0.15 part of sodium tartaric acid, and 0.05 to 0.15 part of aceglutamide. The edible salt substitute for recuperating the 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight) provided by the invention adopts the combination of multiple low sodium salt with different components and multiple condiments, has the efficacies of the edible salt, and substitutes the edible salt so as to reduce the edible salt intake of a human body and recuperate or alleviate the 'Five Highs' chronic diseases (High blood pressure, High blood sugar, High blood fat, High uric acid, High body weight).
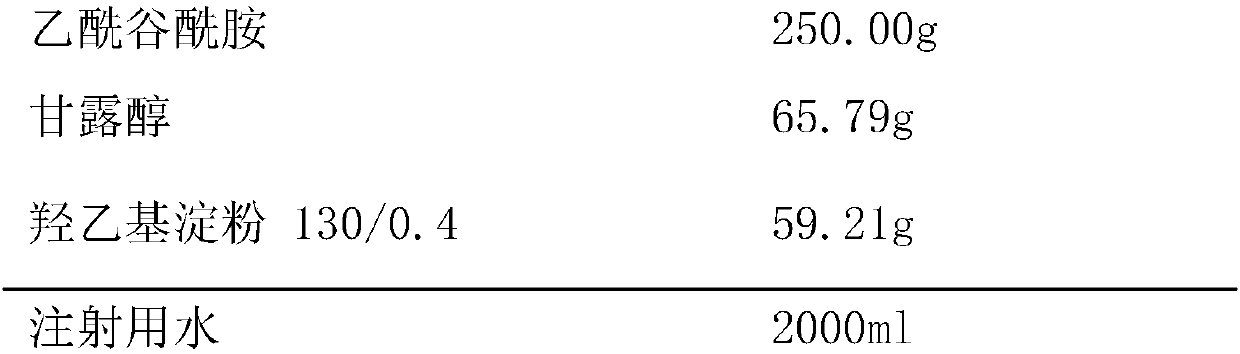
Aceglutamide freeze-drying powder injection preparation with double auxiliary materials for injection
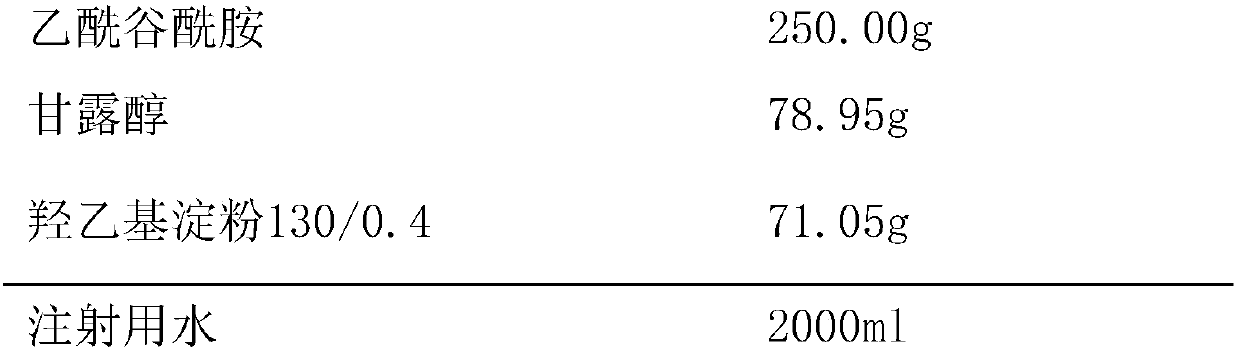
InactiveCN107811977ASimple preparation processImprove product qualityOrganic active ingredientsPowder deliveryHydroxyethyl starchMANNITOL/SORBITOL
The invention provides a acetylglutamine freeze-dried powder injection for injection with double auxiliary materials, relates to the field of pharmaceutical preparations and preparation methods, and mainly solves the problem of freeze-drying of the auxiliary materials in the production formula of the acetylglutamine freeze-dried powder injection for injection in the prior art during production. The disadvantages of long drying time, high drying temperature and high energy consumption. The acetylglutamine freeze-dried powder for injection uses double excipients. The excipients include mannitol and hydroxyethyl starch 130 / 0.4. The weight ratio of alcohol and hydroxyethyl starch 130 / 0.4 is 100:80‑100:100. The acetylglutamine freeze-dried powder injection for injection is prepared by using the above-mentioned main drug and auxiliary materials. The acetylglutamine freeze-dried powder for injection provided by the invention is stable in quality, safe and effective, simple in production process, short in freeze-drying time during production, low in drying temperature and low in energy consumption.
Owner:刘兴付
Acetyglutamide aluminium oral tablet, capsule and its preparing method
InactiveCN1813703ASolve the problem of bitter and unbearable tasteOvercome the lack of choicePeptide/protein ingredientsDigestive systemAdhesiveAceglutamide
The present invention belongs to a medicine preparation containing organic effective component, in particular, it relates to an acetyl-glutamine aluminium oral tablet or capsule preparation. Its composition contains (by weight portion) 200-400 portions of acetyl-glutamine, 0-30 portions of filling agent, 1-10 portions of moistening agent, 0-10 portions of adhesive, 0-10 portions of disintegrating agent and 0-10 portions of lubricating agent. Said invention also provides its preparation method and concrete steps.
Owner:王兰周
Heat-insulating thermal-insulating 3D printing material and preparation method thereof
InactiveCN108752882ALow thermal conductivityImprove insulation effectAdditive manufacturing apparatusAceglutamideMechanical property
The invention discloses a heat-insulating thermal-insulating 3D printing material and a preparation method thereof. The 3D printing material comprises the following raw materials in parts by weight: 55-80 parts of polylactic acid, 18-30 parts of melamine resin micro powder, 13-16 parts of triphenylphosphine, 5-11 parts of aceglutamide, 6-11 parts of multi-amino polyether methylene phosphonic acid,5-10 parts of water-soluble thiosulfate and 2-5 parts of nickel nitrate. The heat-insulating thermal-insulating 3D printing material disclosed by the invention is not only small in heat conduction coefficient and good in heat-preservation effect, but also has the advantages of being good in fire and flame retardancy, good in mechanical property and long in service life, and in addition, the heat-insulating thermal-insulating 3D printing material has a good antibacterial effect, safe to use and high in market popularization value.
Owner:鄂俊鸣
Use of pharmaceutical composition containing aceglutamide and safflower
ActiveCN1843393APromote brain metabolismFunction increaseOrganic active ingredientsNervous disorderSurgical operationAceglutamide
The invention discloses the use of pharmaceutical composition containing aceglutamide and safflower extract in preparing medicament for tissue damage assimilation and recovery, wherein animal and human body clinical experiments show that the composition can be used for preparing medicament for promoting assimilation and recovery of bone fracture injury, surgical operation wound and neurotrauma.
Owner:通化谷红制药有限公司
Amino-acid-containing aceglutamide pharmaceutical composition for injection and application thereof
ActiveCN112168789AReduce adsorptionGuaranteed contentPowder deliveryOrganic active ingredientsSucroseAceglutamide
The invention provides application of amino acid in improving the stability of aceglutamide for injection. It is found that in a freeze-drying protective agent containing the amino acid, when the aceglutamide composition is used as an injection product for use, the stability to light, heat and temperature can be improved. The invention further provides the aceglutamide composition containing the amino acid, mannitol and cane sugar. The aceglutamide composition comprises the following components of, in parts by weight, 50-100 parts of aceglutamide, 60-100 parts of amino acid, 80-160 parts of mannitol, 80-160 parts of cane sugar and a proper amount of pH regulator, wherein the amino acid is one or more of glutamic acid or aspartic acid. The aceglutamide for injection is high in stability, and can exert the original effect and ensure the curative effect when being subsequently used as a powder injection or an injection.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Preparation of aceglutamide
ActiveCN101434559BIncrease labor costIncrease material costOrganic compound preparationCarboxylic acid amides preparationAlcoholAceglutamide
Owner:SHANGHAI ZHAOHUI PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com