Metabolic marker combination for evaluating risks of cardiovascular disease of subject and application thereof
A technology for metabolic markers, cardiovascular and cerebrovascular diseases, applied in instruments, measuring devices, scientific instruments, etc., can solve problems such as difficult to meet the medical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1, blood sample collection
[0062] Vacuum blood collection tubes with clinical EDTA anticoagulation were used to collect 3-5ml fasting venous blood intravenously from patients with coronary heart disease and healthy controls. Plasma was separated at 1500 g x 15 minutes within 1 hour after collection. Aliquot (150 microliters) into 200 microliter centrifuge tubes, quickly store at -80°C for future use and complete information registration. These include: (1) training set: 183 clinical plasma samples of patients with coronary heart disease and 94 samples of healthy control plasma; (2) validation set: 147 clinical plasma samples of coronary heart disease patients and 94 control plasma samples of healthy subjects.
Embodiment 2
[0063] Example 2. Preparation of Calibrator (Standard) Curve Working Solution, Internal Calibrator and Quality Control
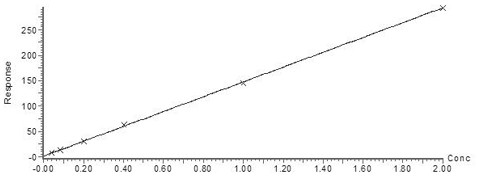
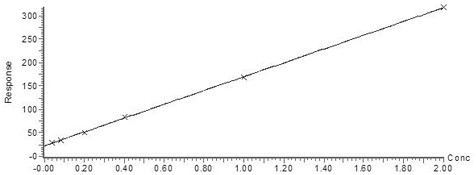
[0064] The standard substance of the marker to be tested was dissolved into a 0.1 mM stock solution in a solvent of isopropanol: acetonitrile ratio of 9:1. Then further dilute with 50 mg / mL bovine serum albumin (BSA, Aladdin) solution to form a mixed calibrator (standard) curve working solution.
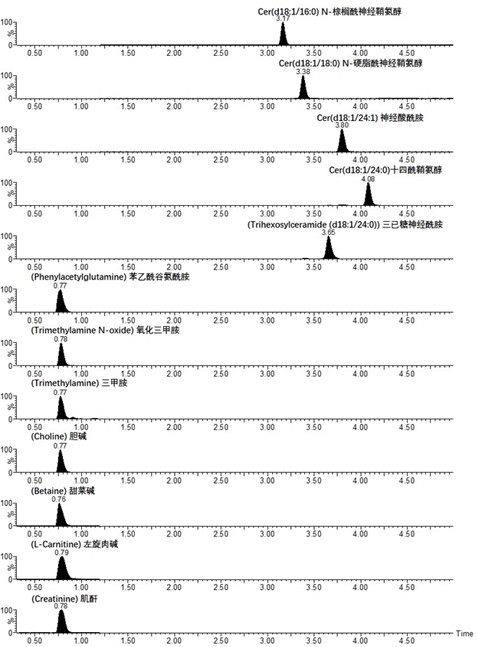
[0065] Exemplarily, ceramide Cer d18:1 / 16:0, ceramide Cerd18:1 / 18:0, ceramide GlcCer d18:1 / 12:0 and sphingosine-1-phosphate can be added to the mixed calibrator working solution Alcohol, the concentration points are 2μM, 1μM, 0.4μM, 0.2μM, 0.08μM and 0.04μM; ceramide Cer d18:1 / 24:0, ceramide Cerd18:1 / 24:1, trihexosylceramide d18:1 / 24:), and phenylacetylglutamine at individual concentration points of 10 μM, 5 μM, 2 μM, 1 μM, 0.4 μM μM, and 0.2 μM; also works in mixed calibrator Trimethylamine oxide, trimethylamine, choline, L-carnitine, betaine, and creatinine we...
Embodiment 3
[0068] Example 3. Pretreatment of plasma samples and extraction of diagnostic marker compositions
[0069] Take 10 microliters of the above-mentioned 518 cases of plasma samples, calibrators (standards) curve working solution and quality control products respectively into the V-bottom 96-well plate, then add 190 microliters of the internal calibrator working solution, and affix the aluminum seal Membrane, shake and mix at 650 rpm for 20 minutes. Then centrifuge at 4000×g for 20 minutes, and take 100 microliters of supernatant to be detected by high performance liquid chromatography-mass spectrometry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com