Recombinant carnosine hydrolase mutant and application thereof
A hydrolase and mutant technology, applied in the field of bioengineering, can solve the problems of unsuitability for industrial production, low catalyst activity, and few varieties of carnosine hydrolase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
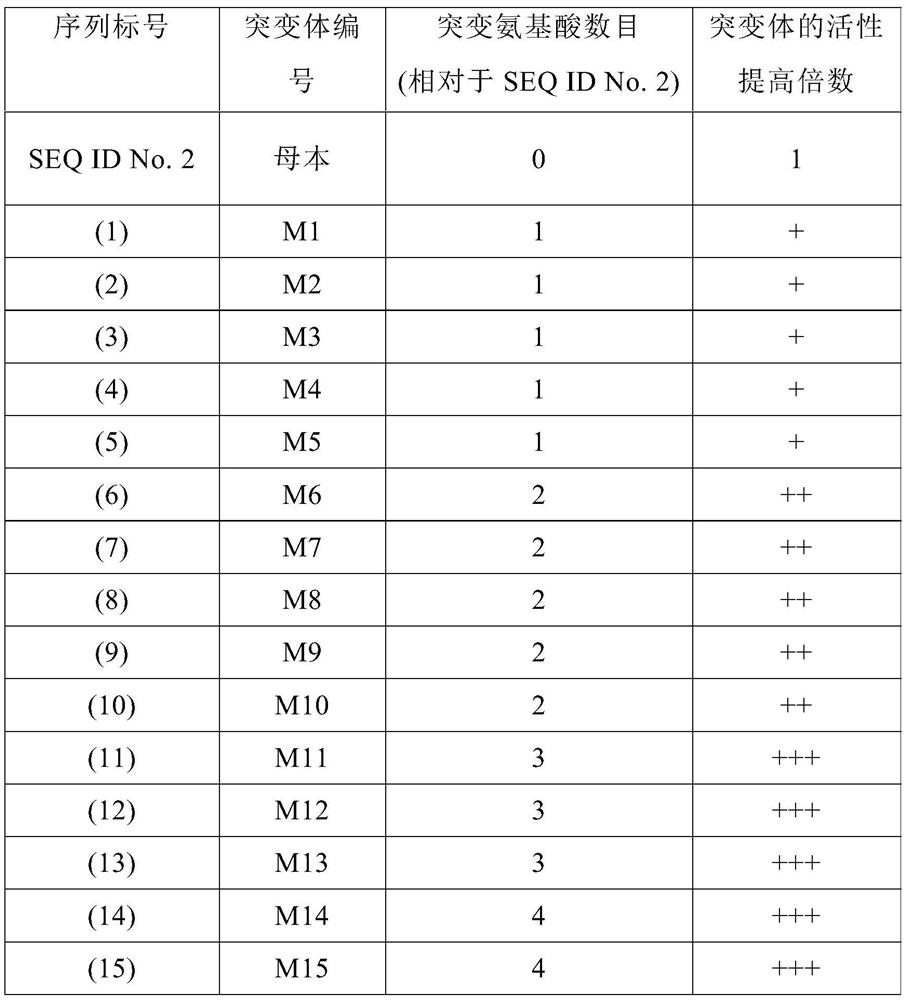
Examples
Embodiment 1
[0059] Embodiment 1. Molecular transformation of recombinant carnosine hydrolase
[0060] A random mutant library of carnosine hydrolase SmPepD was established by error-prone PCR technology, and high-throughput screening of the mutant library was performed using OPA derivatization to screen for mutants with increased activity in a low pH environment.
[0061] Design primers at both ends:
[0062] Forward primer 5'-CCG GAATTC GTGTCTGAATTGTCTCAGCTTT-3',
[0063] reverse primer 5
[0064] '-CT CGAGTG CGGCCGCAAGCTTACCGAGGCTCGAGATGAA-3'
[0065] The recombinant plasmid pET28a-SmPepD was used as a template for amplification. Polymerase chain reaction PCR system (50 μL): rTaq 0.25 μL, 10×Buffer 5 μL, dNTP Mix 4 μL, template plasmid about 100 ng, Primer F 2 μL, Primer R 2 μL, MnCl 2 (10mM) 0.5μL, diH 2 O to make up to 50 μL. The PCR program was: 98°C for 3min, 98°C for 30s, 55°C for 30s, 72°C for 10min, 30 cycles, 72°C for 10min. The PCR products were stored at 4°C for futur...
Embodiment 3
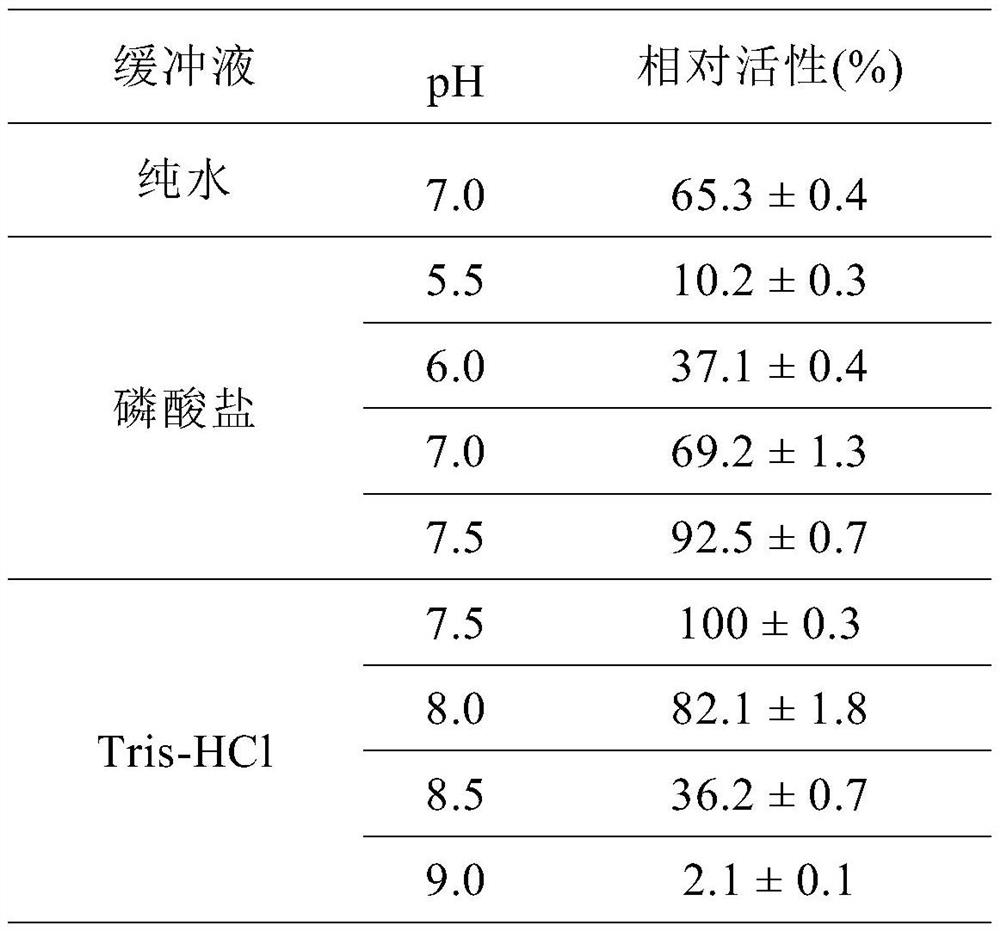
[0082] Example 3 Different pH to recombinant SmPepD M13 Effect on Synthetic Activity
[0083] 20 μL of SmPepD M13 The crude enzyme solution (freeze-dried enzyme powder concentration 5mg / mL) was added to 180μL of buffer solutions with different pHs containing 20mmol / LL-carnosine, and the reaction was shaken at 37°C for 5-20min to investigate the carnosine hydrolase SmPepD M13 Activity in different pH buffers. The buffer systems used were phosphate buffer (pH 5.5-7.5) and Tris-HCl (pH 7.5-9.0). The results are shown in Table 2. In the Tris-HCl buffer solution with a pH of 7.5, the relative activity of the enzyme is the highest, which is defined as 100%. Compared with the parent enzyme SmPepD, the mutant SmPepD M13 The optimum pH shifted from 8.0 to 7.5, and the activity at pH 6.0 was significantly improved.
[0084] Table 2. Carnosine hydrolase SmPepD M13 Activity in different pH buffers
[0085]
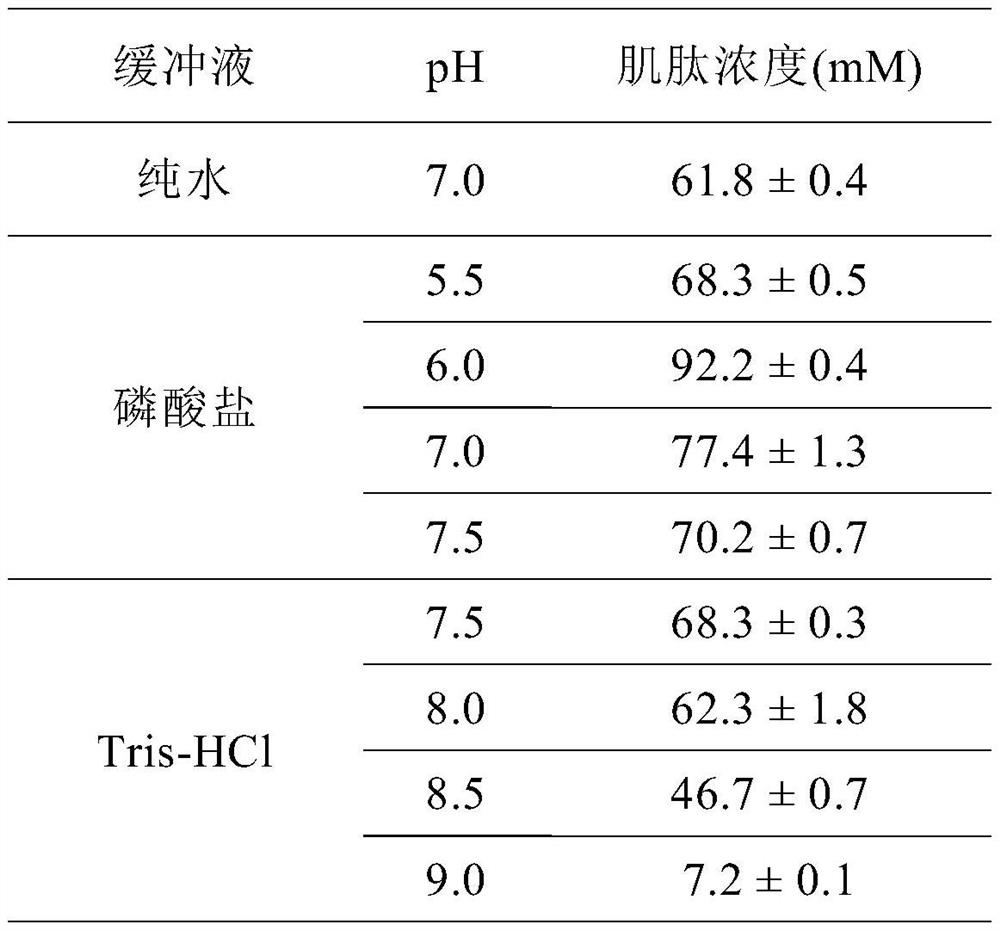
Embodiment 4
[0086] Example 4 Different pH to recombinant SmPepD M13 Effect of concentration on catalyzed synthesis of carnosine
[0087] (12) Replace the 134th proline with alanine in the amino acid sequence shown in SEQ ID No.2 in the sequence listing, replace the 309th alanine with isoleucine, and replace the 360th leucine is asparagine;
[0088] (13) Replace the 97th alanine with isoleucine in the amino acid sequence shown in SEQ ID No.2 in the sequence listing, replace the 275th alanine with tyrosine; replace the 301st valine is tryptophan;
[0089] (14) The 134th proline of the amino acid sequence shown in SEQ ID No.2 in the sequence listing is replaced with alanine, the 183rd alanine is replaced with valine, and the 281st arginine is replaced with half Cystine; asparagine at position 479 is replaced by alanine;
[0090] (15) Replace the 217th alanine with glutamic acid in the amino acid sequence shown in SEQ ID No.2 in the sequence listing, replace the 250th glycine with asparti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com