Secukinumab injection and preparation method thereof
A technology of secukinumab and injection, applied in the direction of antibodies, pharmaceutical formulas, antibody medical components, etc., can solve the problems of poor substitutability and few types of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
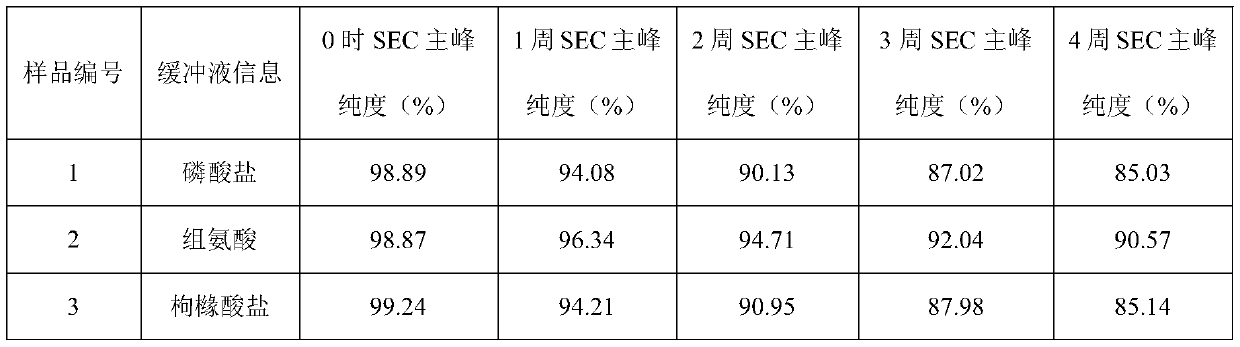
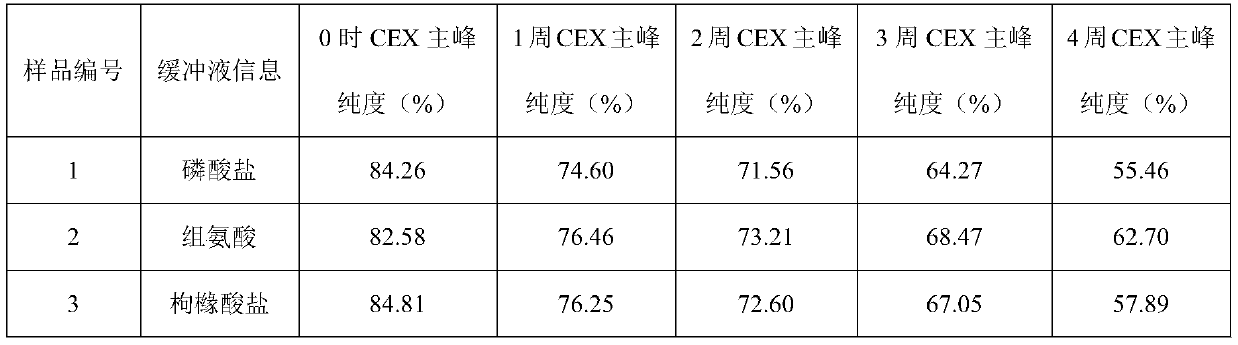
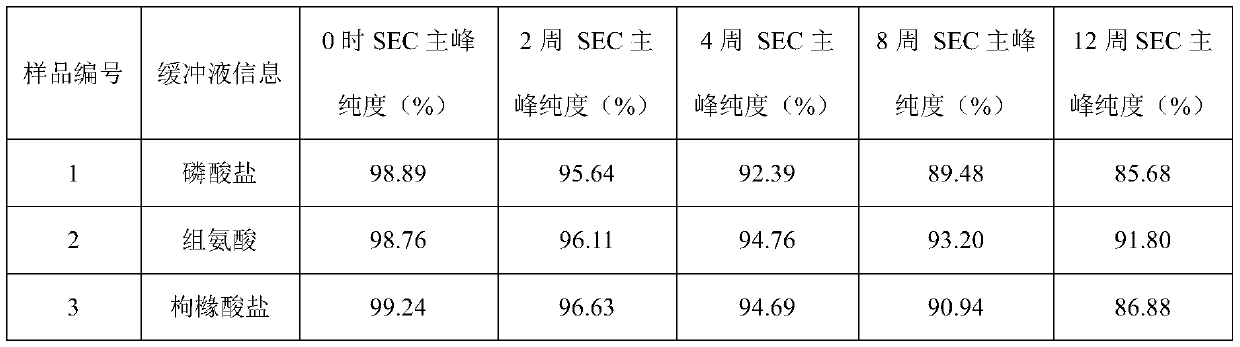
[0043] Example 1: Buffer
[0044] The effect of buffer type (phosphate, histidine, citrate) was evaluated in prefilled syringes.
[0045] Prescription 1: secukinumab 150mg / ml, sodium dihydrogen phosphate and disodium hydrogen phosphate 20mmol / L, methionine 5mmol / L, trehalose 200mmol / L, polysorbate 80 0.02%, pH5.8;
[0046]Prescription 2: secukinumab 150mg / ml, histidine and histidine hydrochloride 20mmol / L, methionine 5mmol / L, trehalose 200mmol / L, polysorbate 80 0.02%, pH5.8;
[0047] Prescription 3: secukinumab 150mg / ml, citric acid and sodium citrate 20mmol / L, methionine 5mmol / L, trehalose 200mmol / L, polysorbate 80 0.02%, pH5.8.
[0048] The above three groups of prescription injections were filled into prefilled syringes, and stability studies were carried out under long-term (4°C), accelerated (25°C) and high temperature (40°C) conditions to evaluate physical stability (SEC-HPLC (SEC Macromolecular protein: Chinese Pharmacopoeia 2015 general rule), visible particles measu...
experiment example 2
[0061] Experimental example 2: pH
[0062] Based on the prescription secukinumab 150mg / ml, histidine and histidine hydrochloride 20mmol / L, methionine 5mmol / L, trehalose 200mmol / L, polysorbate 80 0.02%, adjust the pH respectively To 4.5, 5.0, 5.5, 5.8, 6.0, 6.5, 7.0, to investigate the effect of different pH on the stability of secukinumab. The samples were stored under high temperature conditions for 4 weeks, and the stability of secukinumab was evaluated by SEC-HPLC and CEX-HPLC. It was determined from SEC-HPLC, CEX-HPLC that protein aggregation and hydrolysis were minimal around pH 6.0.
experiment example 3
[0063] Experimental Example 3: Stabilizer
[0064] Initial formulation development for an injectable dosage form aimed to evaluate the effect of different stabilizers on the formation of secukinumab soluble and insoluble aggregates (SEC - Effect of HPLC, DLS, Visible Particles and Insoluble Particles by Light Obscuration), Chemical Stability (CEX-HPLC). Stabilizers are selected from glycerin, propylene glycol, and trehalose, and the prescription is as follows:
[0065] Prescription 1: secukinumab 150mg / ml, histidine and histidine hydrochloride 20mmol / L, methionine 5mmol / L, glycerin 270mmol / L, polysorbate 80 0.02%, pH5.8;
[0066] Prescription 2: secukinumab 150mg / ml, histidine and histidine hydrochloride 20mmol / L, methionine 5mmol / L, propylene glycol 271mmol / L, polysorbate 80 0.02%, pH5.8;
[0067] Prescription 3: secukinumab 150mg / ml, histidine and histidine hydrochloride 20mmol / L, methionine 5mmol / L, trehalose 200mmol / L, polysorbate 80 0.02%, pH5.8.
[0068] Through the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com