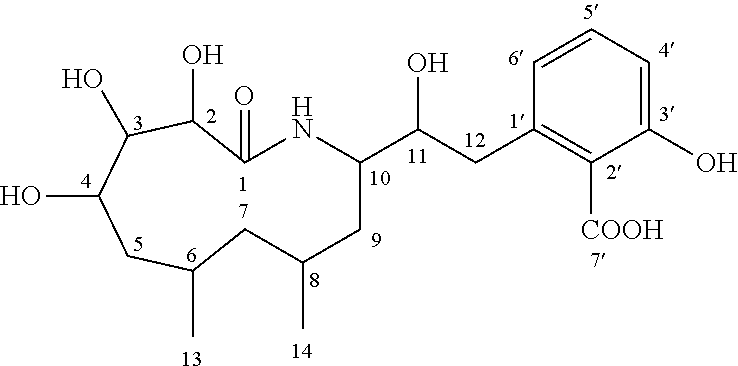
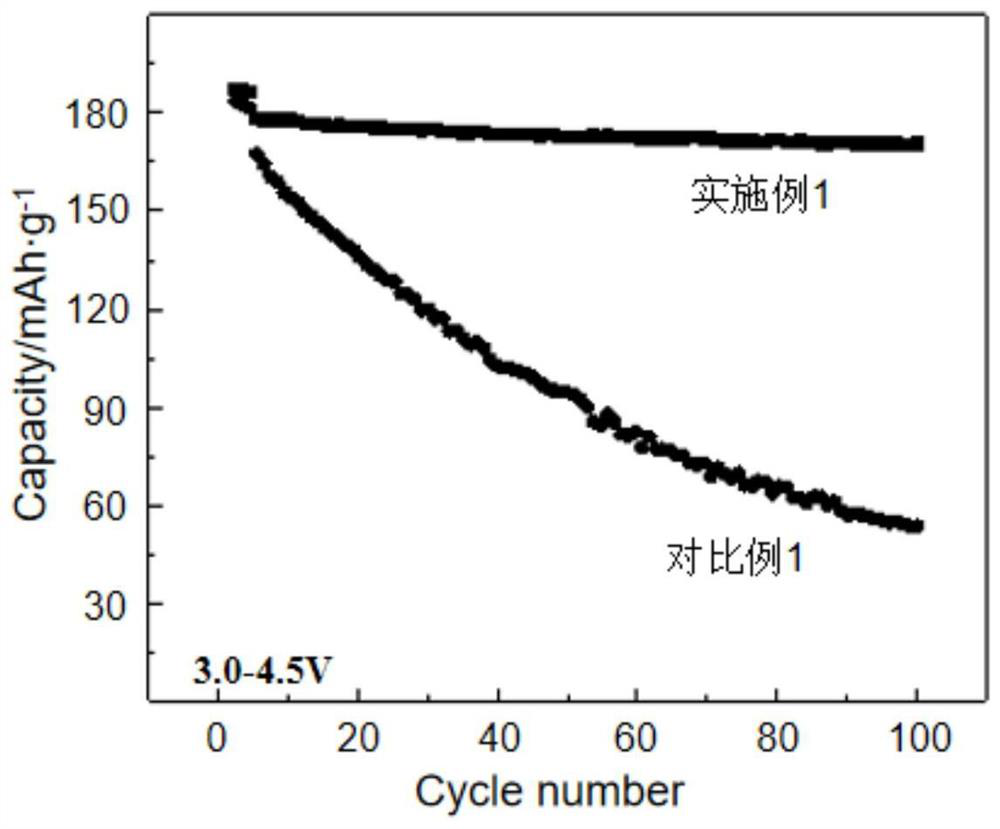
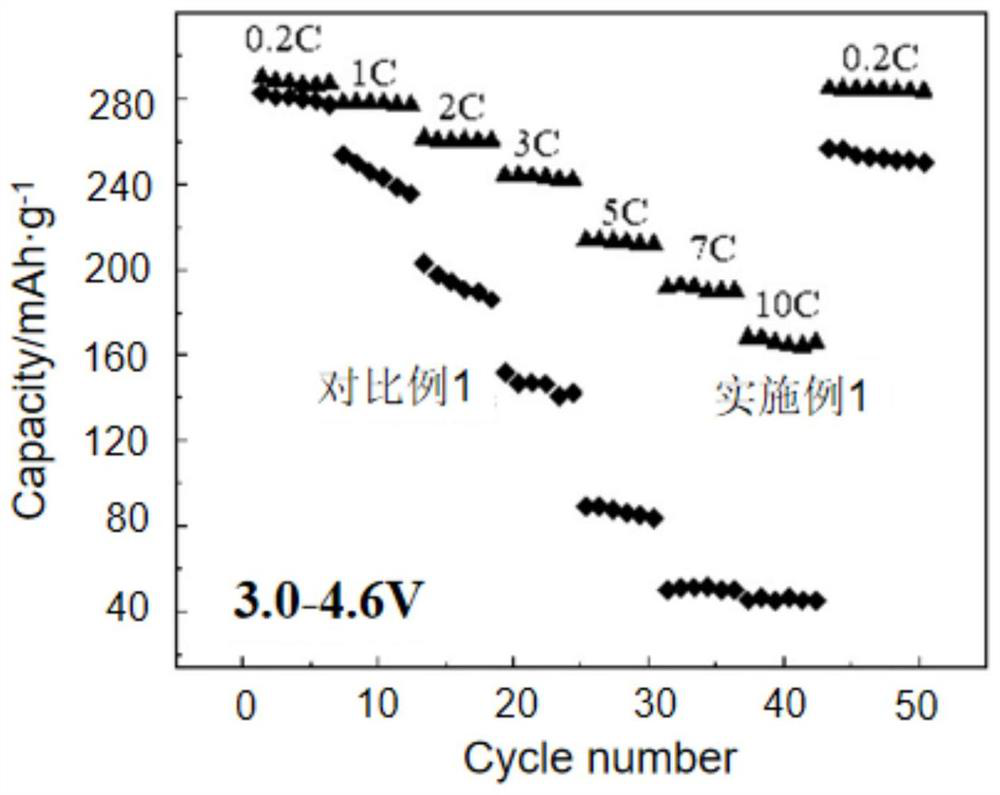
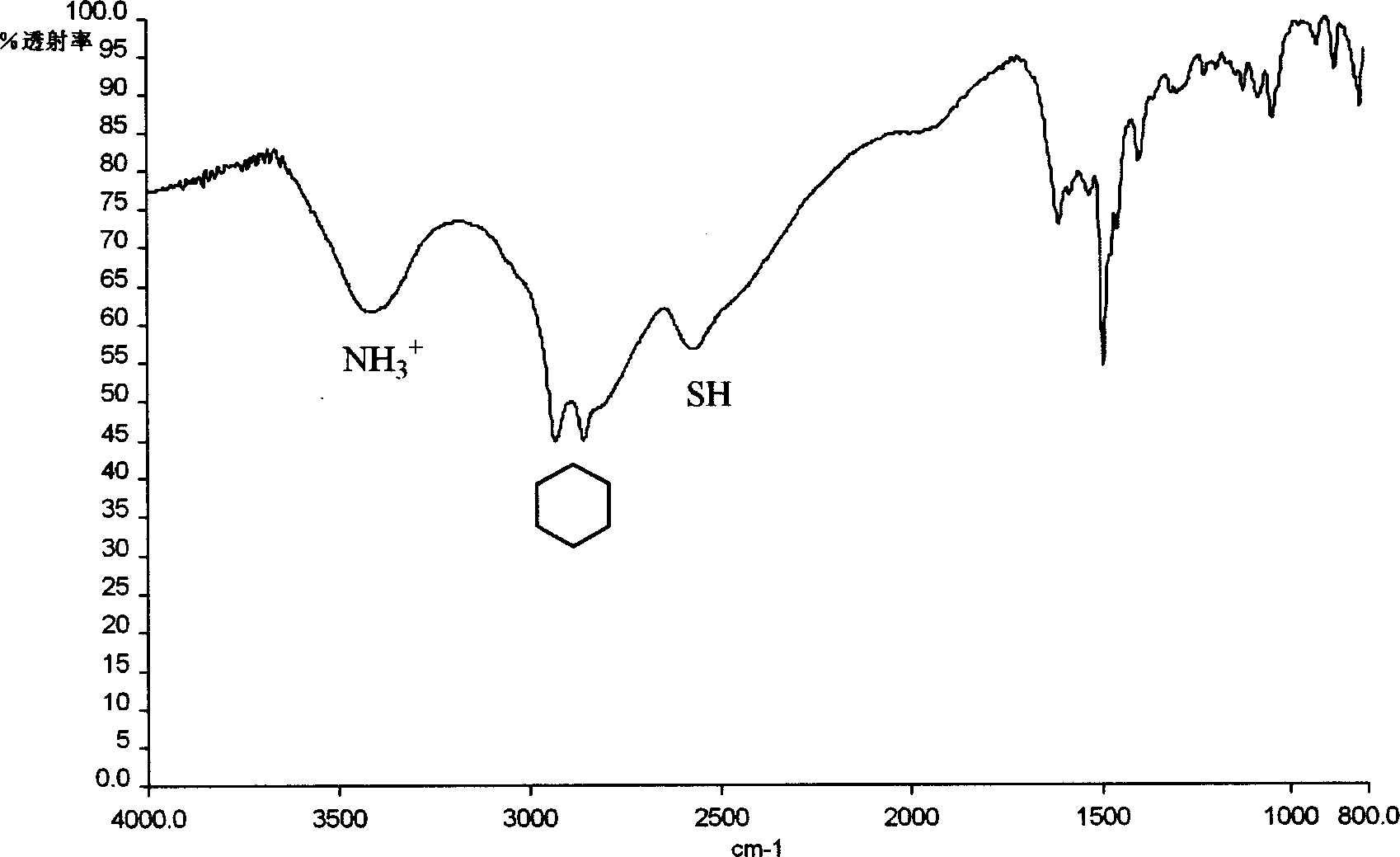
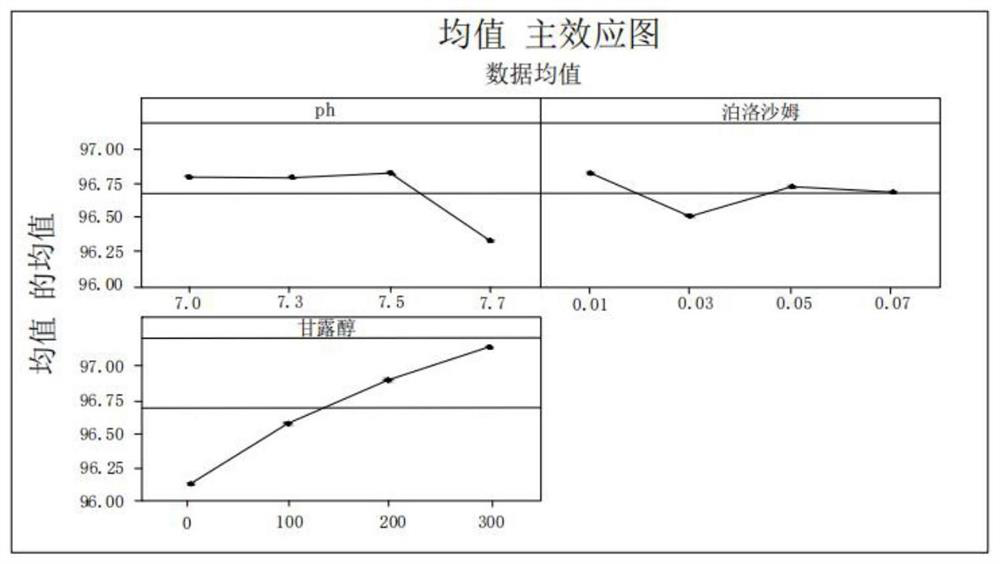
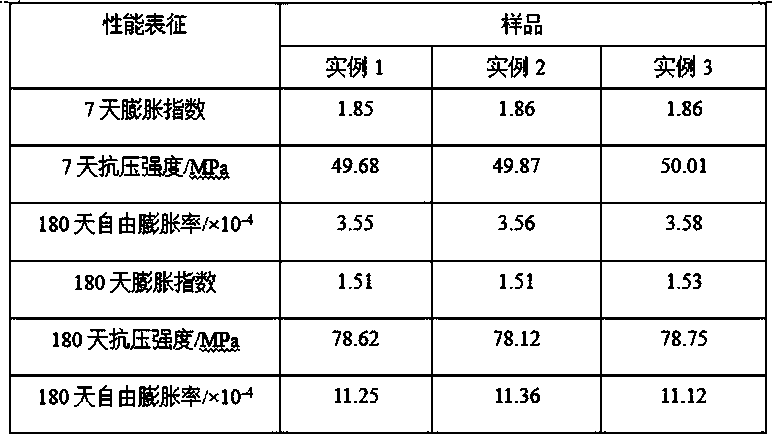
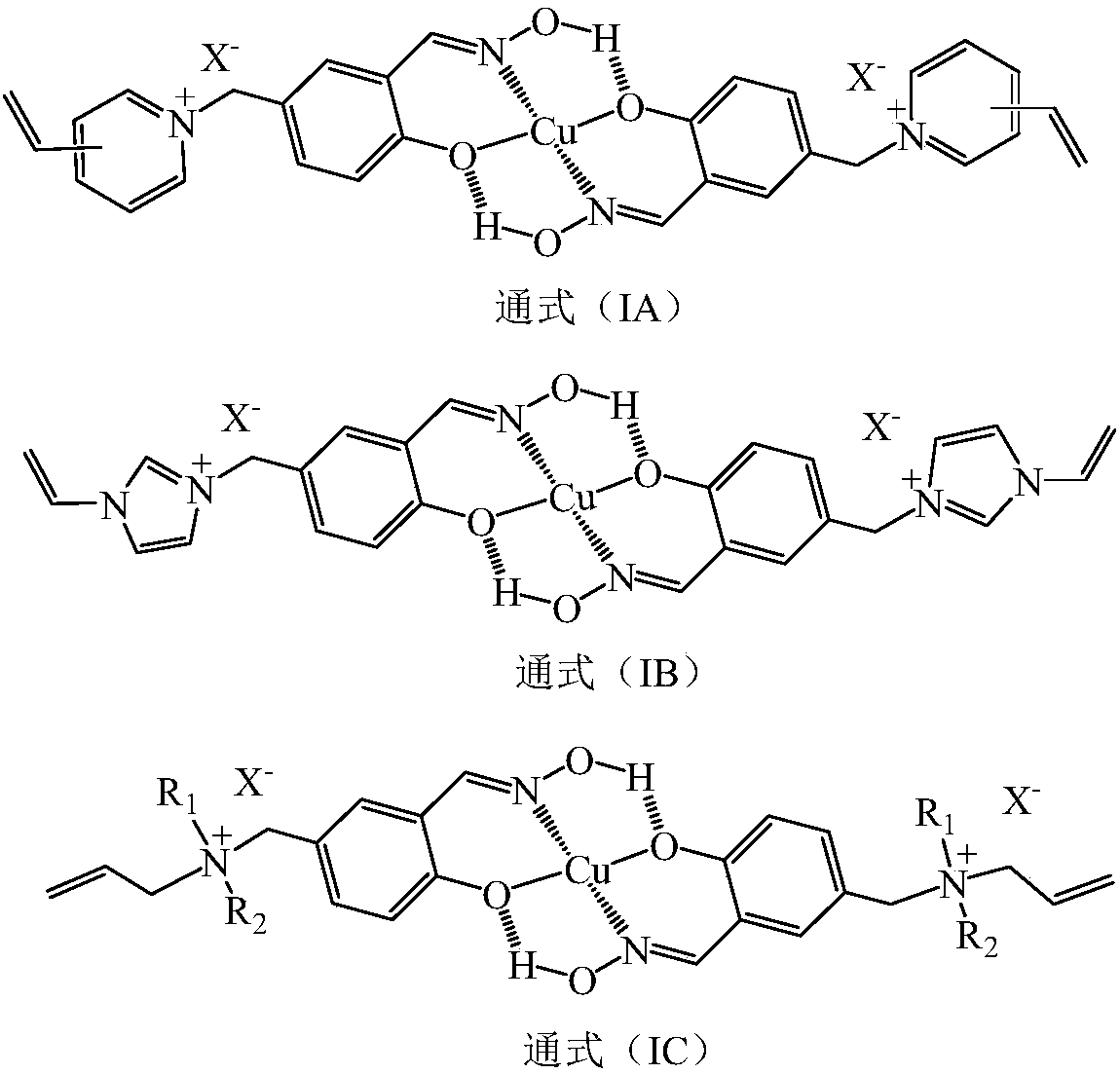
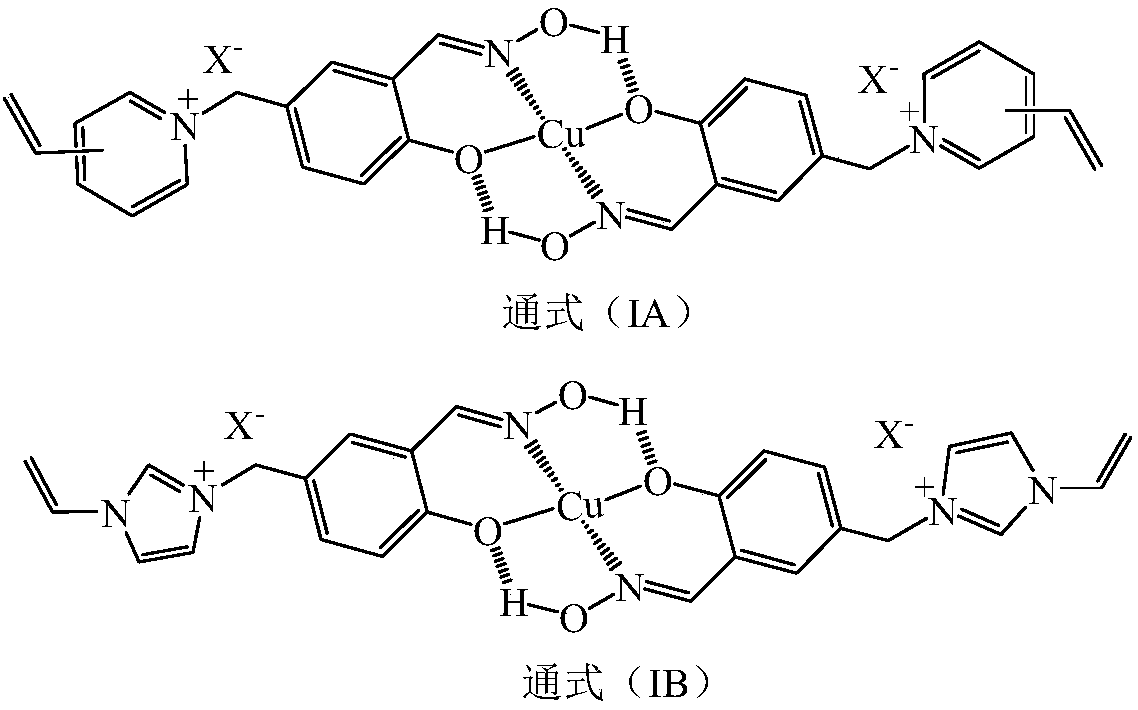
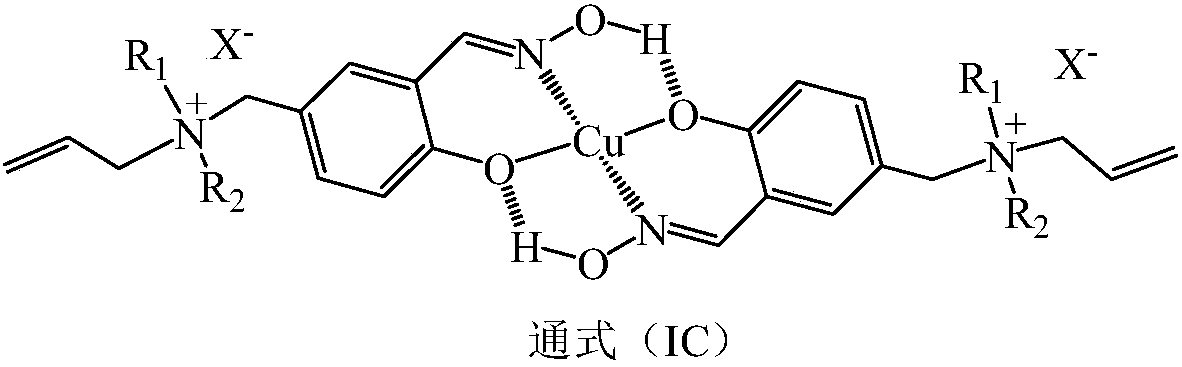
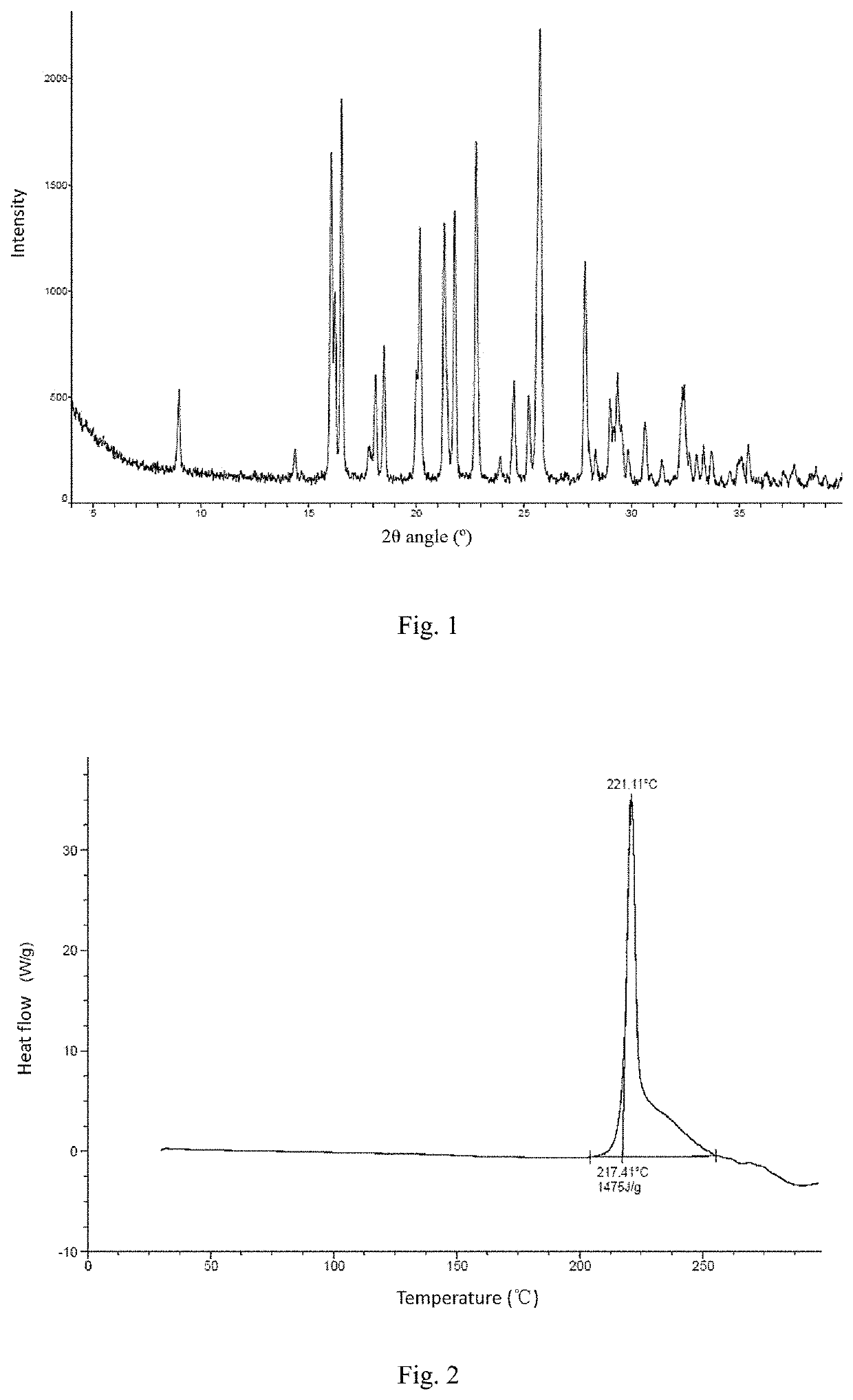
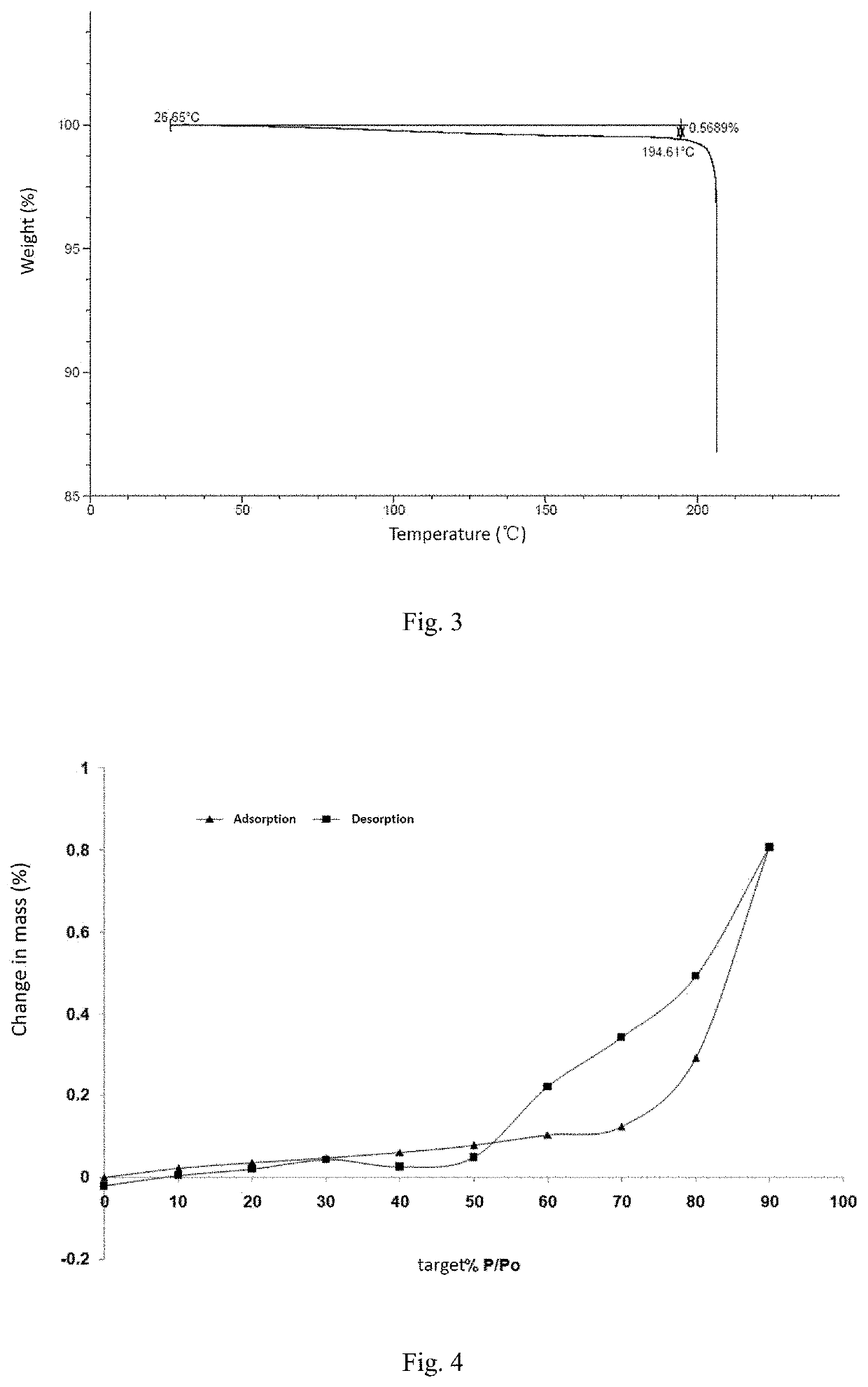
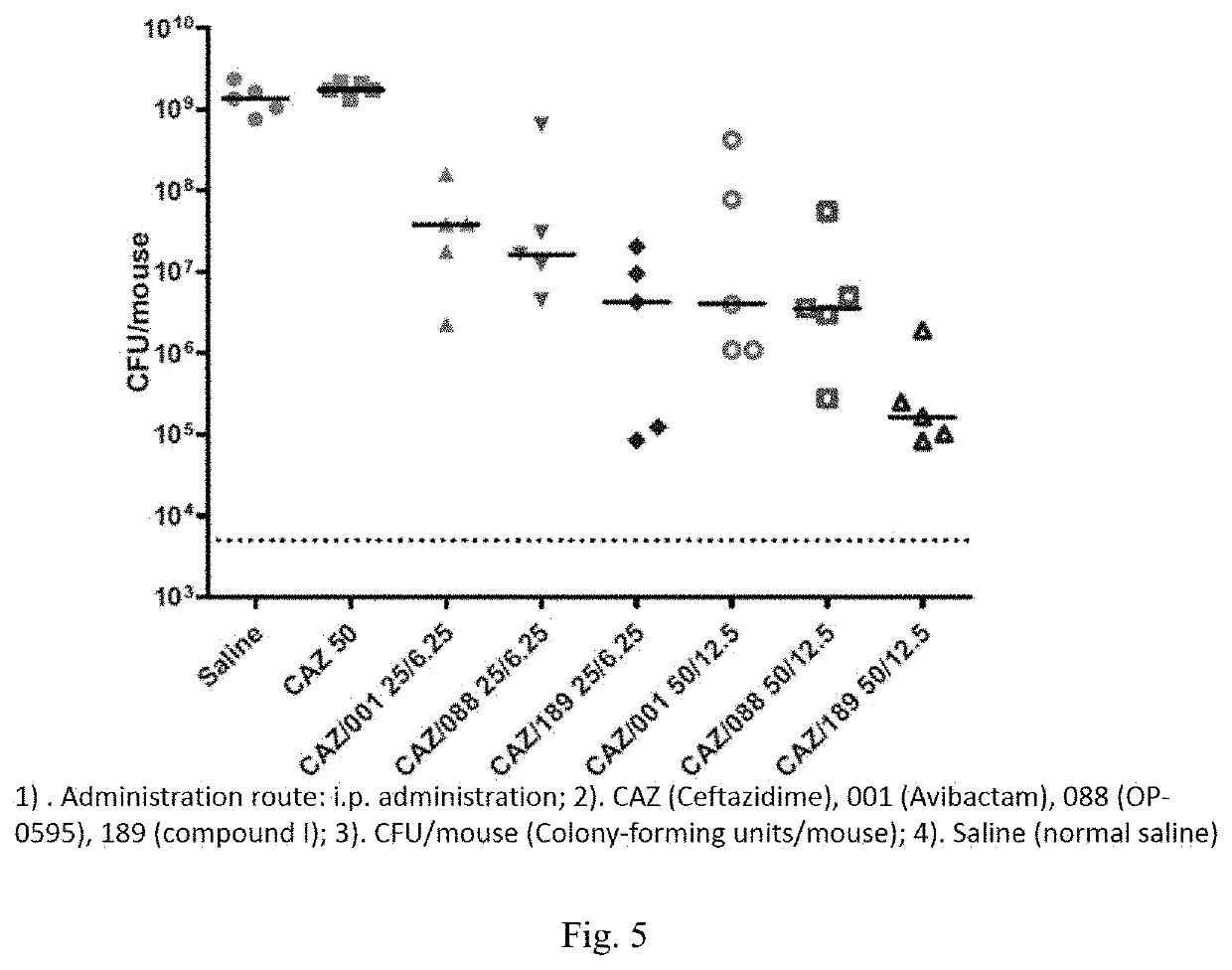
Patents
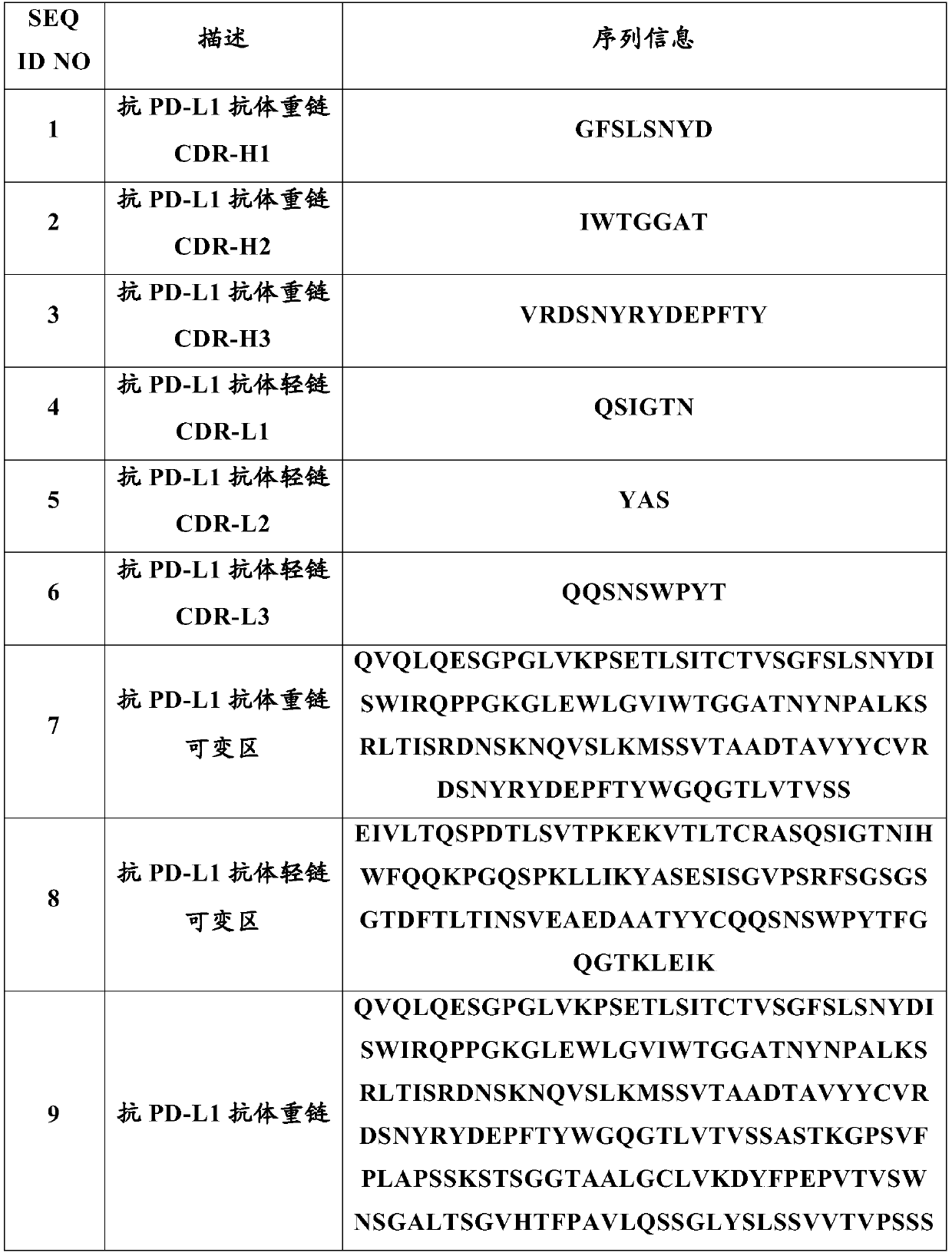
Literature
45results about How to "Maintain chemical stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
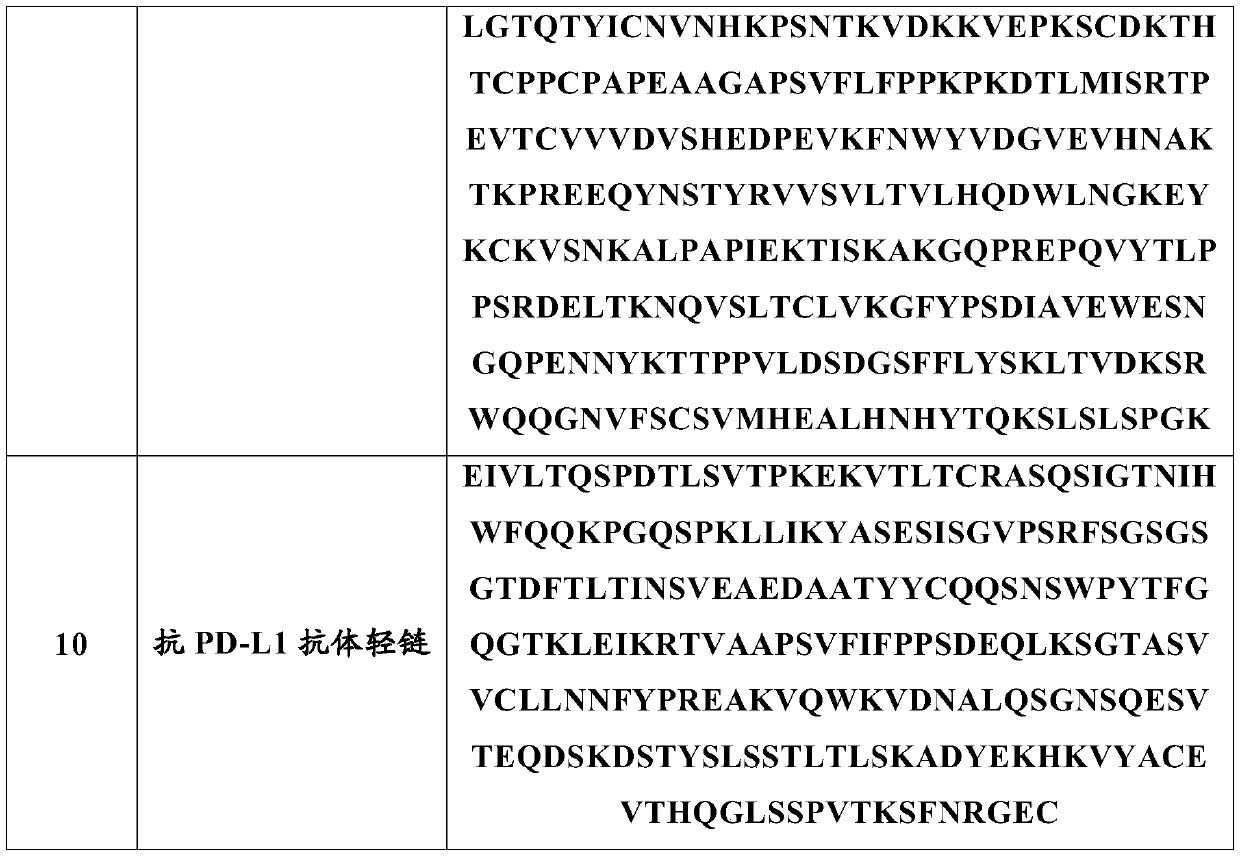
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
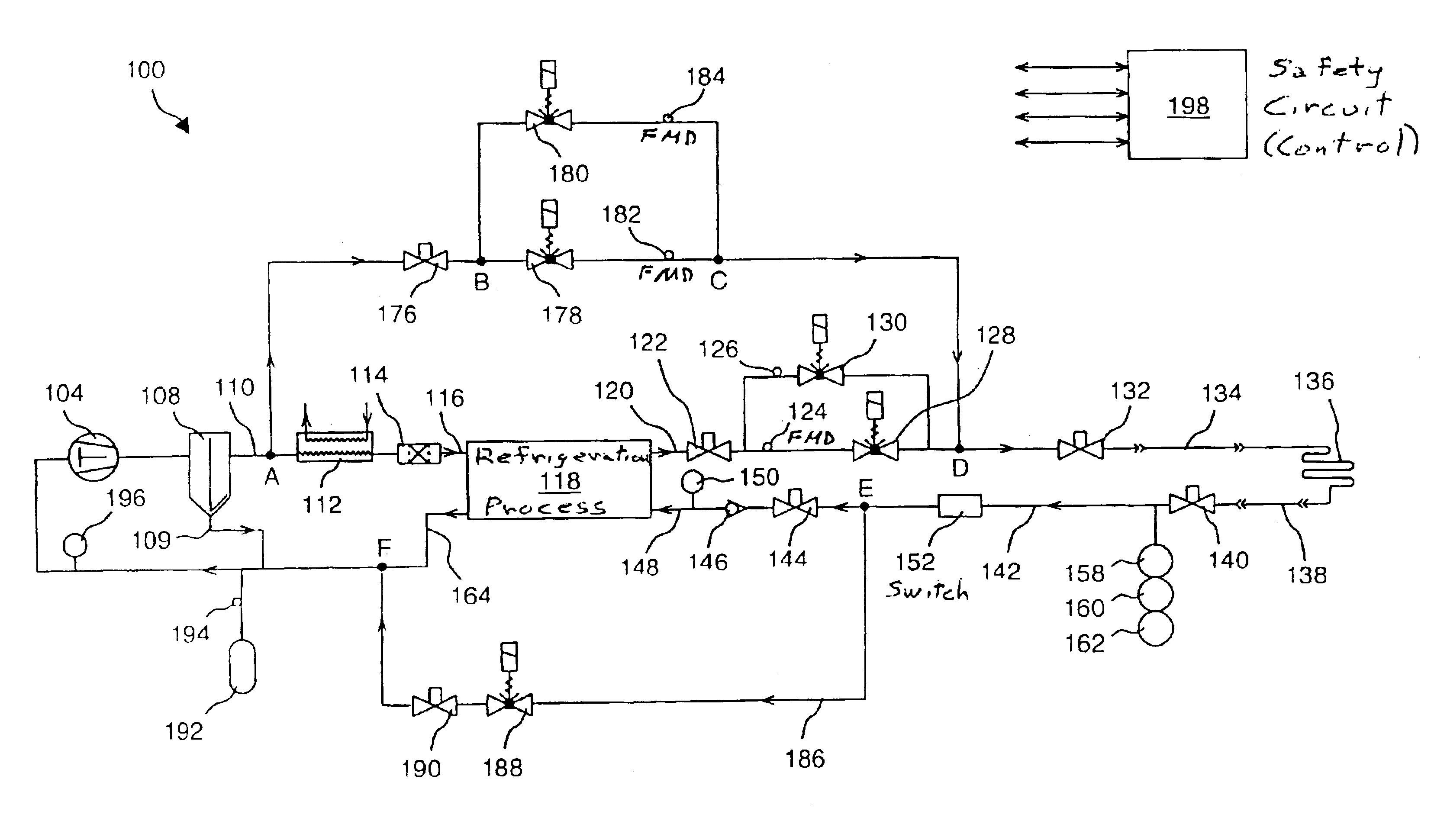
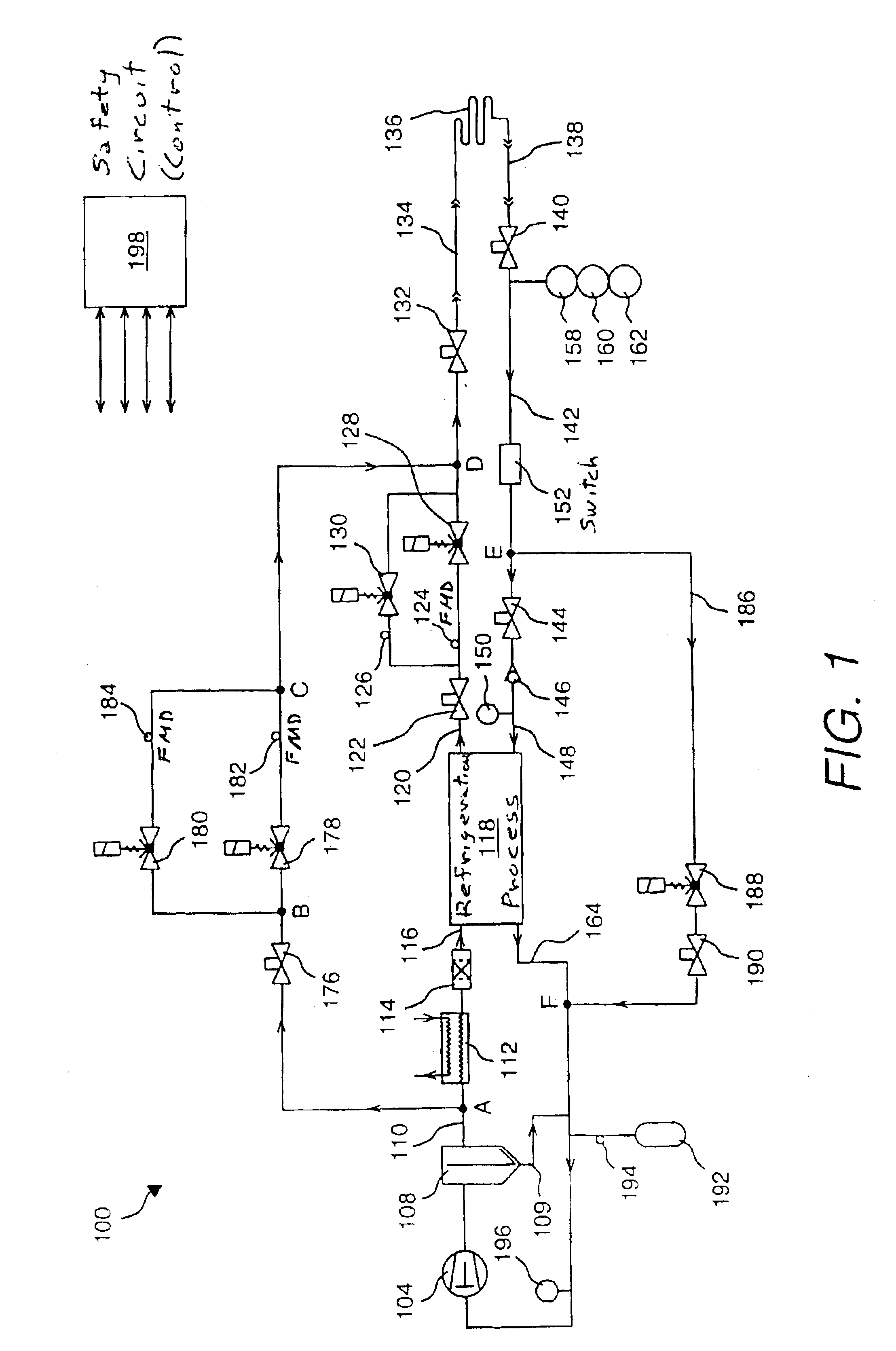
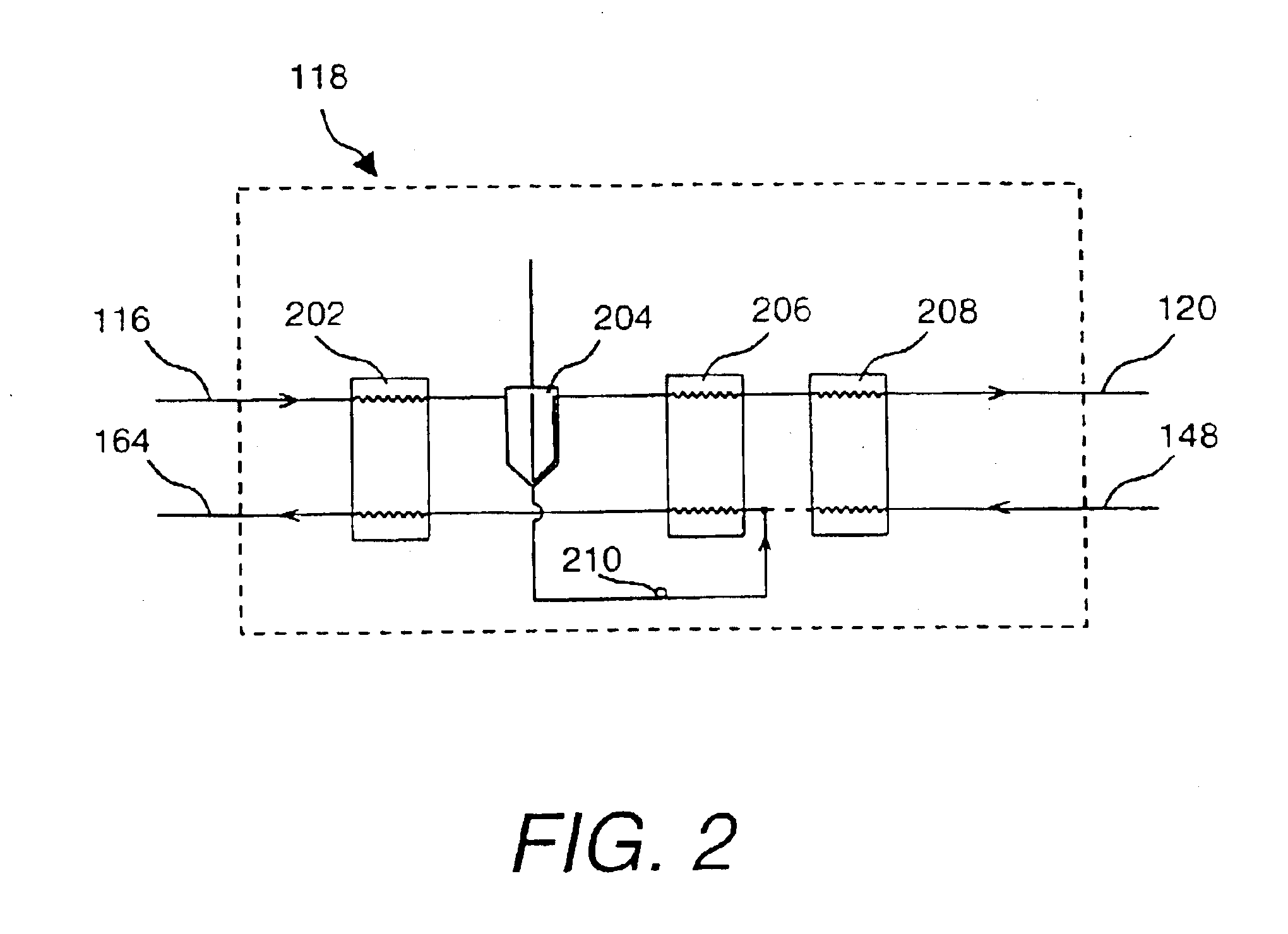
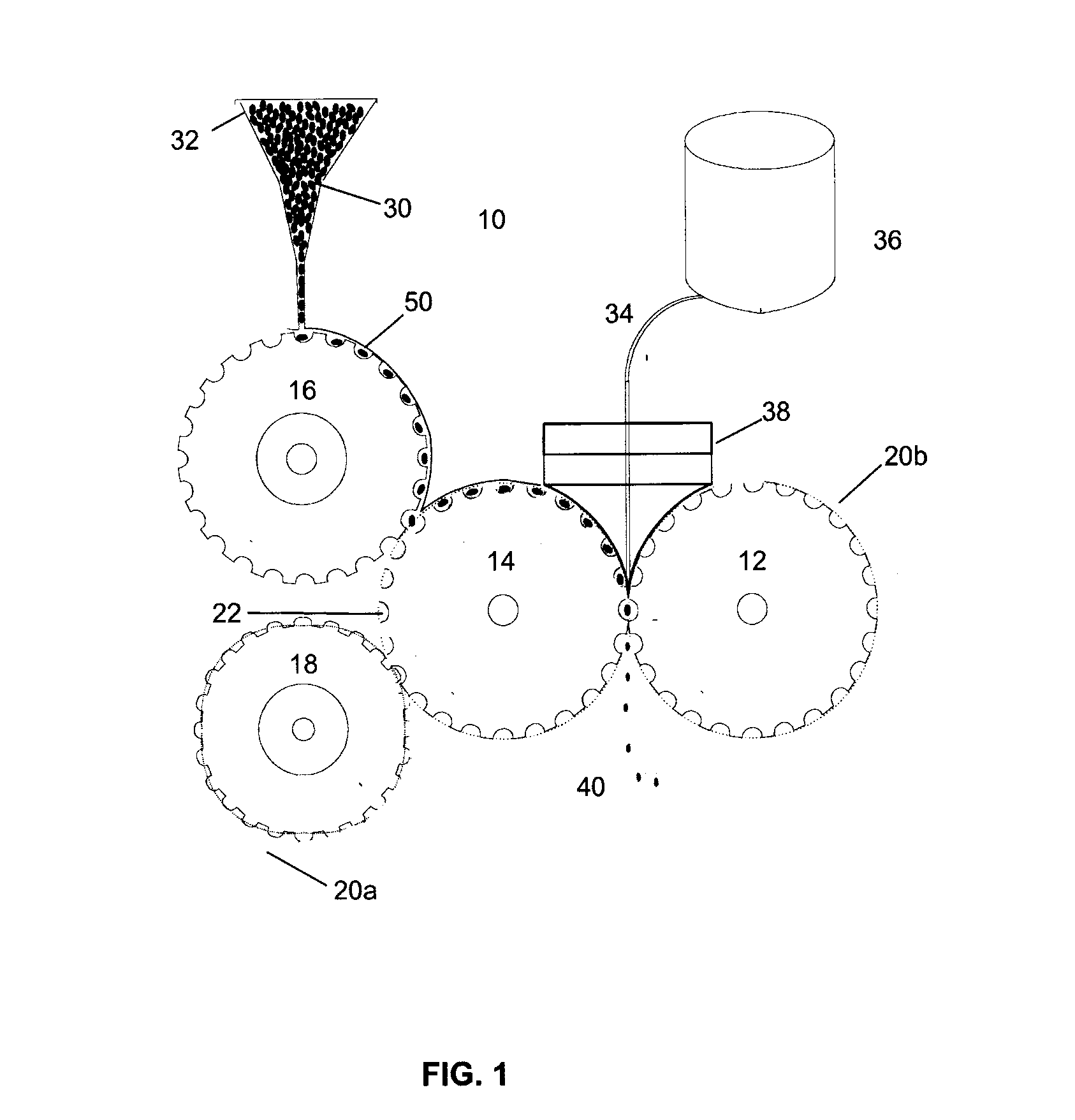
Very low temperature refrigeration system with controlled cool down and warm up rates and long term heating capabilities
InactiveUS6843065B2Continuous operationSmall temperature rangeCompression machinesValve members for absorbing fluid energyRecovery periodEngineering
Heating / defrost constructions of a very low temperature refrigeration system having a defrost supply circuit and a defrost return bypass circuit optimizing the heating / defrost cycle, preventing overload (excessive pressure) of its refrigeration process and protecting components from damaging temperatures. The defrost cycle operates continuously, when required, and provides a shorter recovery period between heating / defrost and cooling operating modes. The rate of the temperature change during cool down or warm up is controlled in an open loop fashion by controlled refrigerant flow in bypass circuits.
Owner:EDWARDS VACUUM LLC
Pharmaceutical formulations of statins and omega-3 fatty acids for encapsulation
PendingUS20130115281A1Avoid chemical reactionsMaintain chemical stabilityBiocideMetabolism disorderOmegaFatty acid
A multi phase soft gelatin dosage form comprising at least one preformed solid dosage form comprising a statin compound and at least one liquid fill phase comprising Omega-3 fatty acids. The multi phase soft gelatin dosage forms of the present invention are especially useful to combine at least one solid dosage form and at least one liquid phase for single ingestion. The solid phase, liquid phase or coatings may further comprise active pharmaceutical ingredients, nutraceuticals, nutritional supplements, or therapeutic substances, functional excipients or combinations thereof.
Owner:CATALENT ONTARIO LTD
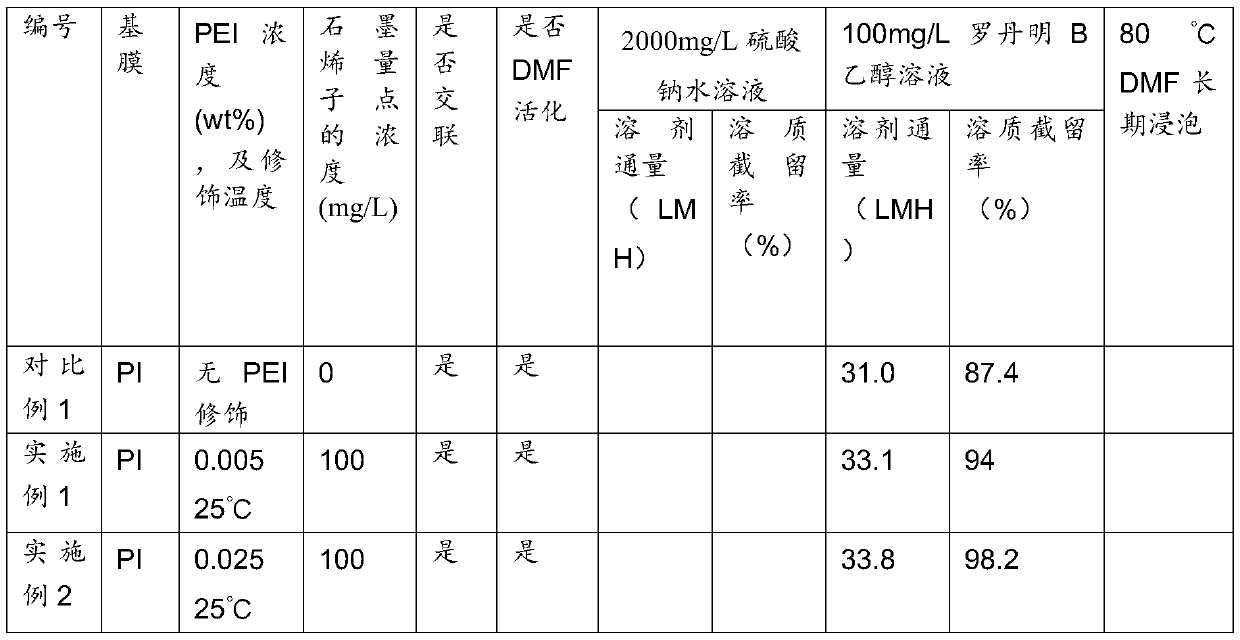
Preparation method for high flux solvent-resisting organic/inorganic hybridized compound film doped with aminated graphene quantum dot
ActiveCN107344074AEasy to separateGood solvent resistanceMaterial nanotechnologySemi-permeable membranesOrganic solventHigh flux
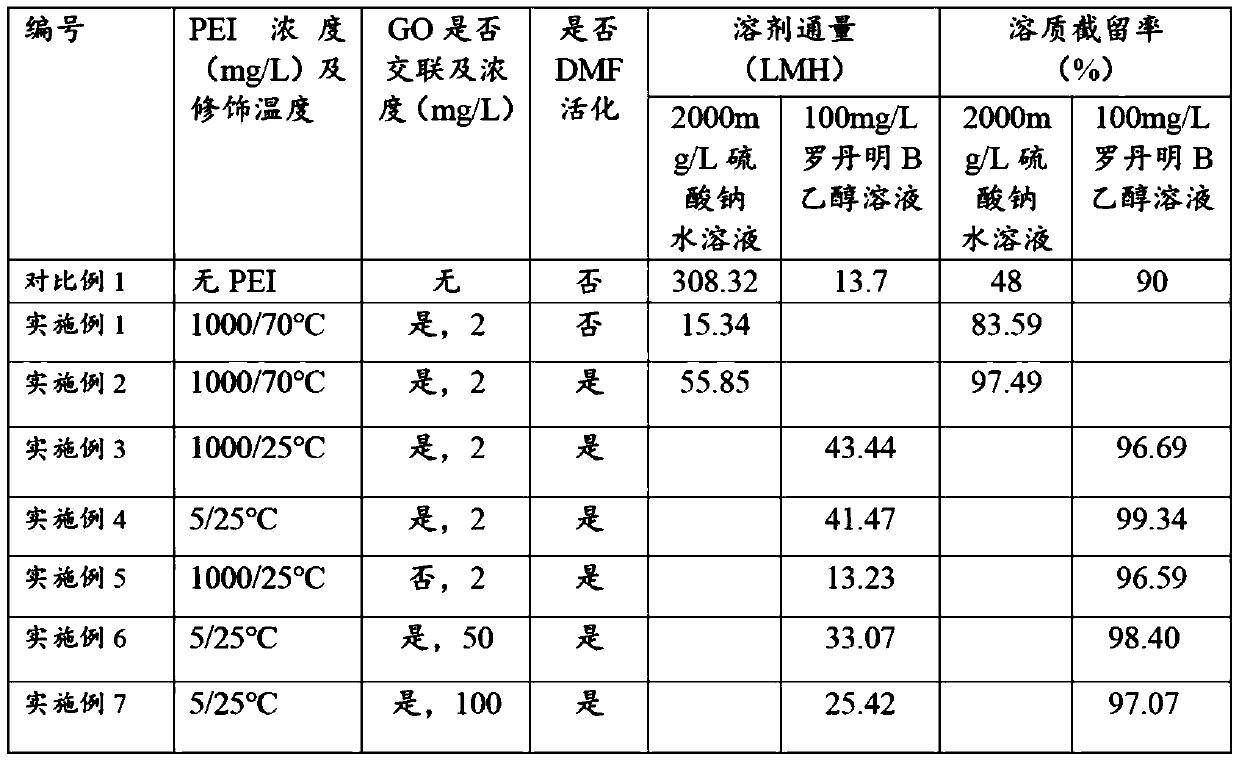
The invention discloses a preparation method for a high flux solvent-resisting organic / inorganic hybridized compound film doped with aminated graphene quantum dot, a prepared compound film and an application of the film. The preparation method for the compound film comprises the steps of interfacial polymerization reaction, chemical crosslinking and solvent activation. According to the invention, the aminated graphene quantum dot is added into an interfacial polymerization monomer solution, so that the separating property and solvent resistance of the film are obviously promoted; the film-making time is shortened in the manner of increasing the cross-linking and solvent activating time; a large quantity of amino groups are loaded on the surface of the aminated quantum dot and can react with an organic phase monomer so as to form a firm covalent bond, so that the performance of the film is promoted while the stability of the quantum dot in the film is obviously promoted. According to the invention, the preparation process is simple and the film has wide application prospect in the fields of organic solution system separation and organic solvent-containing water treatment.
Owner:OCEAN UNIV OF CHINA
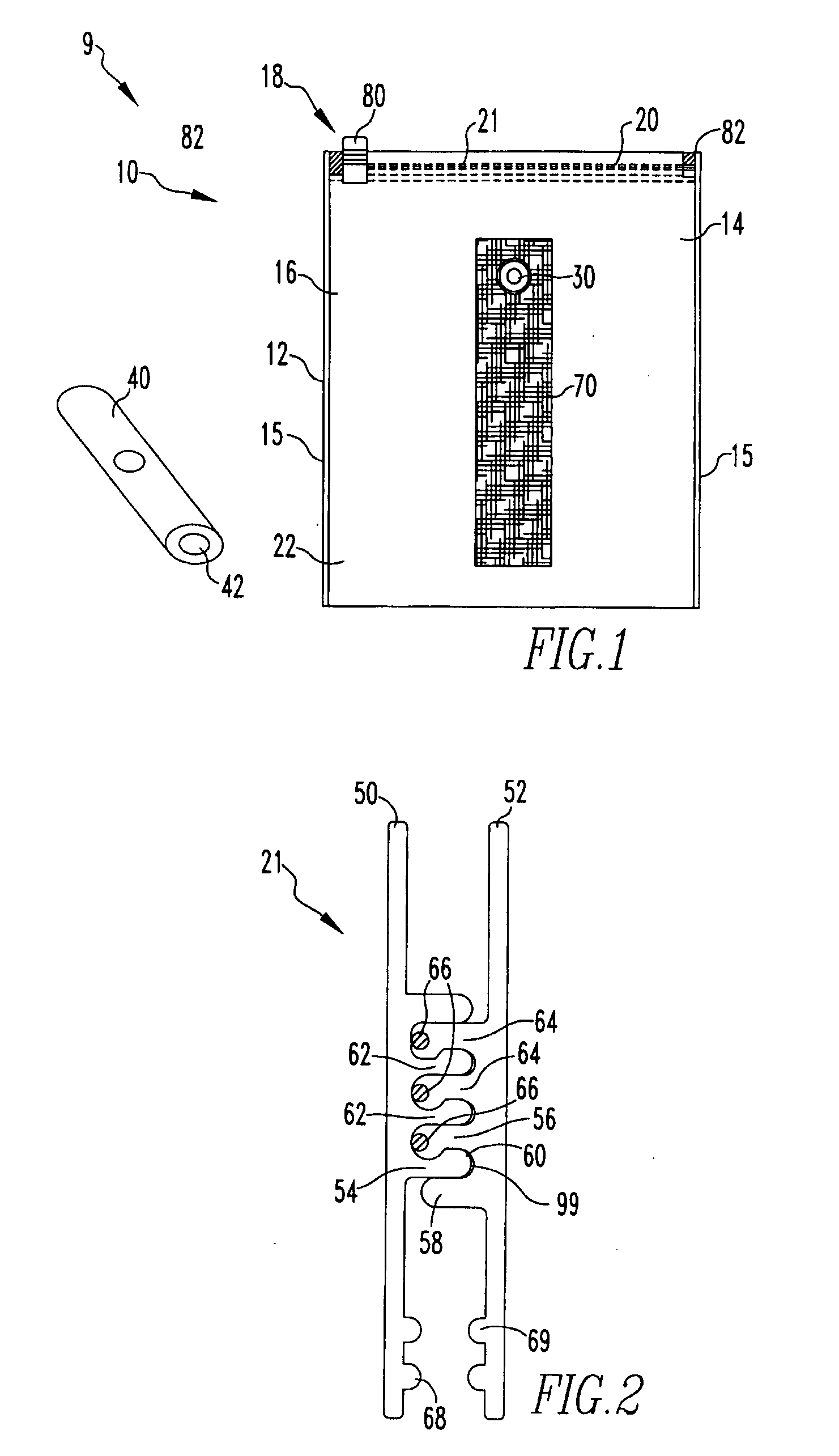
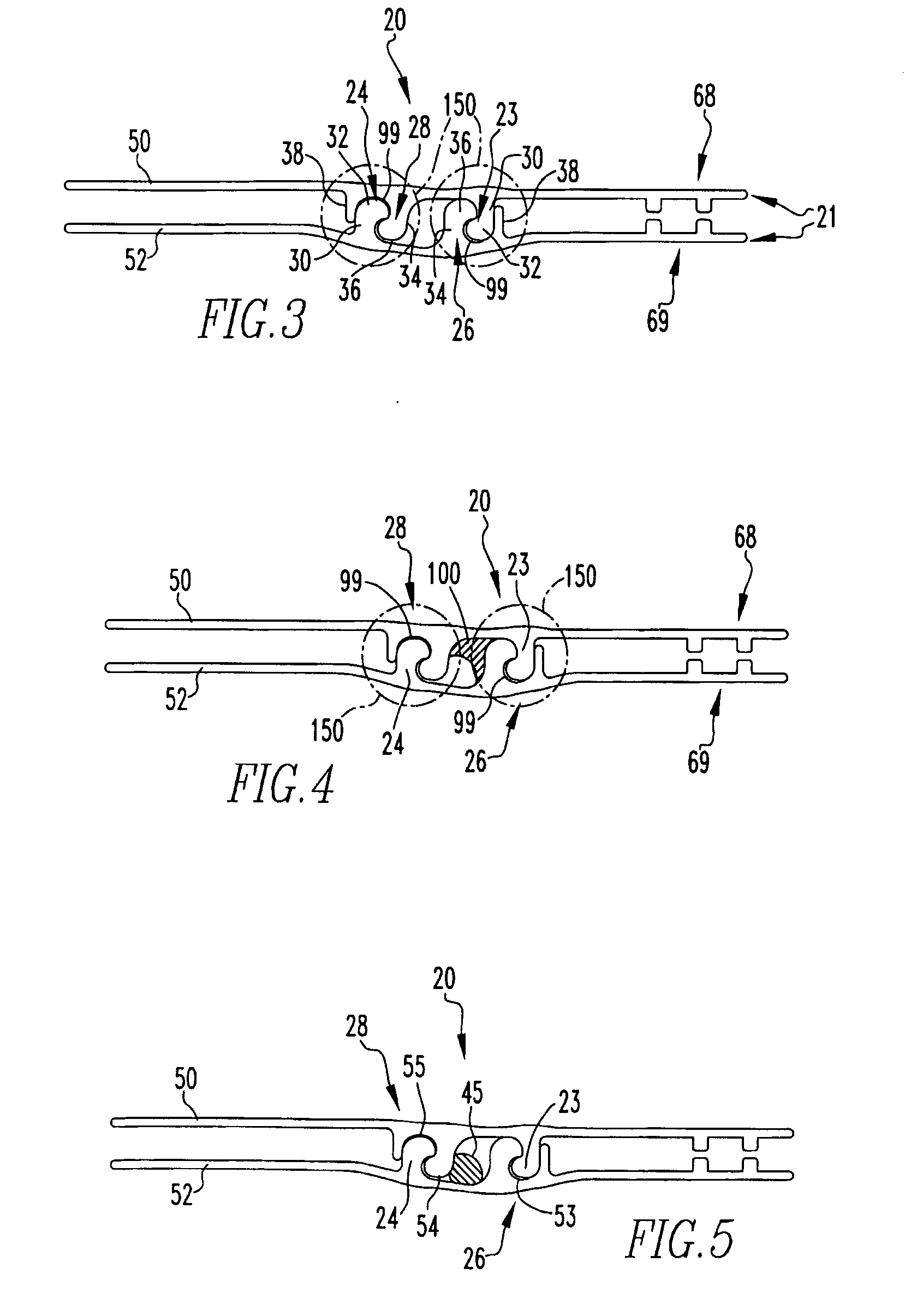
Storage system having a disposable vacuum bag
The present invention provides a storage system having a storage device having at least one polymeric sheet sealed along a portion of its' periphery to provide an opening to a storage space; a resealable closure structure adapted to seal the opening to the storage space, the resealable closure structure comprising selectively engaging male and female profiles and a sealing compound comprising liquid silicone and at least one filler in proportions suitable for at least incidental contact to food items contained within the storage space; a vacuum valve assembly disposed on the polymeric sheet; a stand-off structure disposed adjacent to the vacuum valve assembly, wherein the stand-off structure has a series of raised surfaces facing the vacuum valve assembly; a portable vacuum pump assembly structured to engage the vacuum valve assembly; and a liquid separator assembly coupled to the portable vacuum pump assembly.
Owner:REYNOLDS FOIL
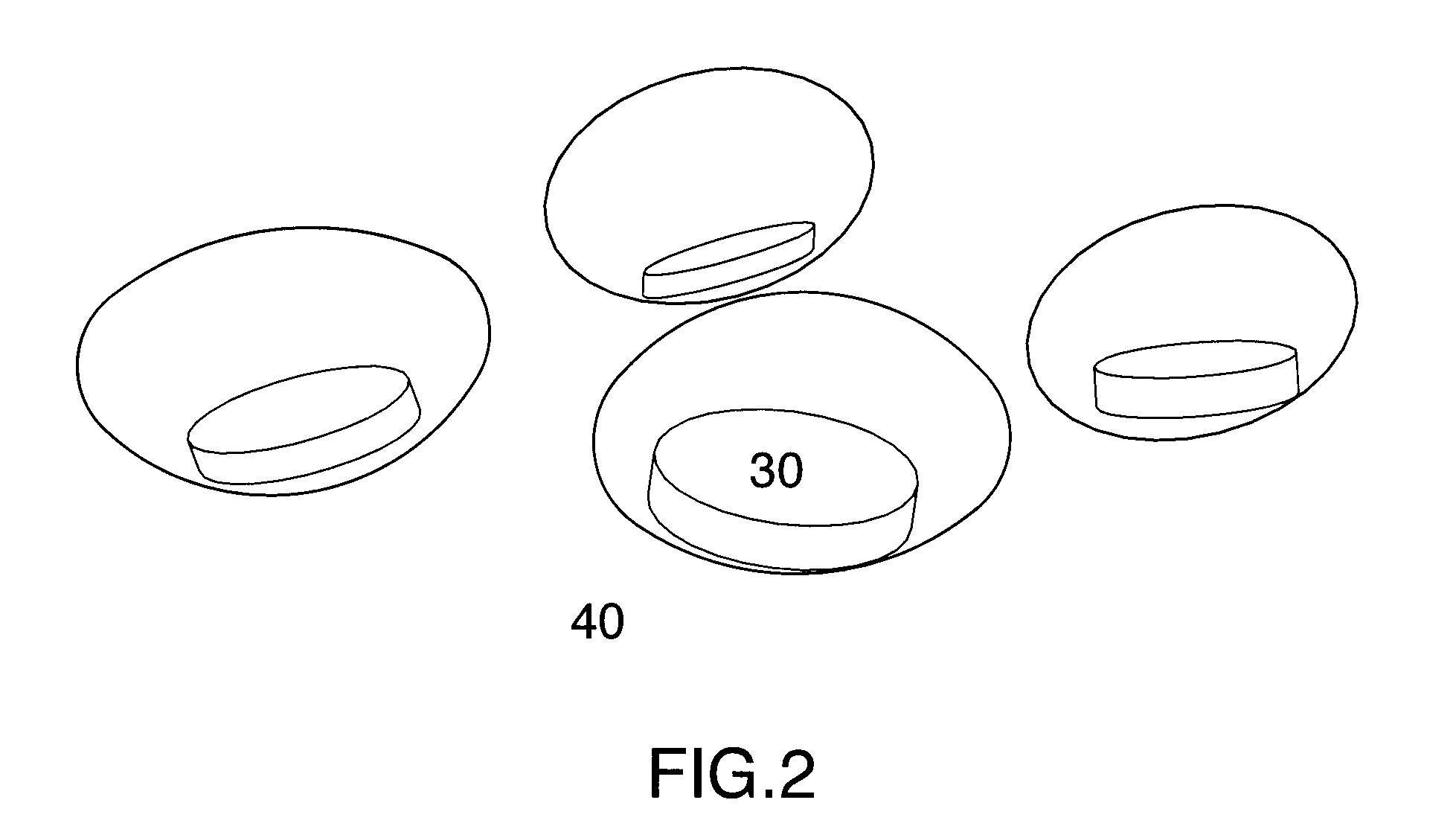
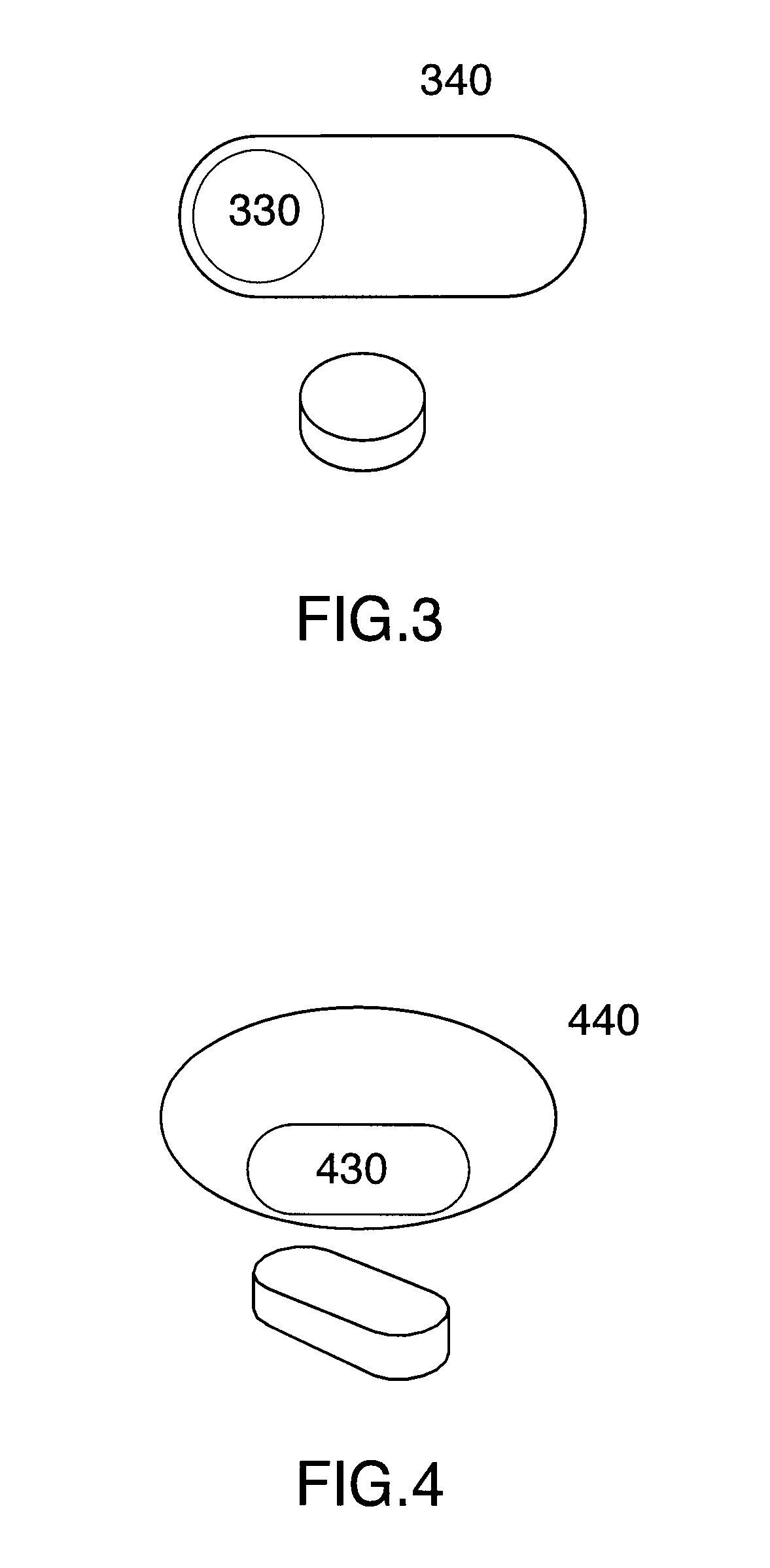
Multi phase soft gel capsules, apparatus and method thereof
ActiveUS20130136792A1Avoid chemical reactionsMaintain chemical stabilityBiocideAntipyreticExcipientMulti phase
A multi phase soft gelatin dosage form comprising at least one preformed solid dosage form and at least one liquid fill phase. The multi phase soft gelatin dosage forms of the present invention are especially useful to combine at least one solid dosage form and at least one liquid phase for single ingestion. Method and apparatus for manufacturing the multiphase soft gelatin dosage forms are also described. The solid phase, liquid phase or coatings may comprise active pharmaceutical ingredients, nutraceuticals, nutritional supplements, therapeutic substances, functional excipients or combinations thereof.
Owner:CATALENT ONTARIO LTD
Storage system having a disposable vacuum bag
The present invention provides a storage system having a storage device having at least one polymeric sheet sealed along a portion of its' periphery to provide an opening to a storage space; a resealable closure structure adapted to seal the opening to the storage space, the resealable closure structure comprising selectively engaging male and female profiles and a sealing compound comprising liquid silicone and at least one filler in proportions suitable for at least incidental contact to food items contained within the storage space; a vacuum valve assembly disposed on the polymeric sheet; a stand-off structure disposed adjacent to the vacuum valve assembly, wherein the stand-off structure has a series of raised surfaces facing the vacuum valve assembly; a portable vacuum pump assembly structured to engage the vacuum valve assembly; and a liquid separator assembly coupled to the portable vacuum pump assembly.
Owner:REYNOLDS FOIL
Composite ultrafiltration membrane and preparation method thereof
InactiveCN101507904AEasy to useGuaranteed heat resistanceSemi-permeable membranesUltrafiltrationUltimate tensile strength
The invention discloses a composite ultrafiltration membrane and a preparation method thereof. The composite ultrafiltration membrane comprises the following raw materials in portion by weight: 6 to 9 portions of polyether sulfone and 1.4 to 2.2 portions of cellulose powder. The composite ultrafiltration membrane material has good hydrophilicity and overcomes the defects of poor hydrophilicity and weak pollution resistance of a polyether sulfone ultrafiltration membrane material, because cellulose molecular chains are provided with a plurality of hydroxyl groups. The composite membrane material has strong pollution resistance, high water flux, good retention characteristic and high strength, prolongs the service life of the ultrafiltration membrane, and saves the production cost.
Owner:INNER MONGOLIA MANZHOULI SENNUO BIOTECH CO LTD
Ultra-thin composite film and preparation method thereof
ActiveCN110141978AEasy to separateGood solvent resistanceSemi-permeable membranesWater contaminantsComposite filmSolvent
The invention discloses an ultra-thin composite film with graphene quantum dots as an intermediate layer and a preparation method of the composite film. The preparation steps of the ultra-thin composite film with the graphene quantum dots as the intermediate layer comprises a graphene quantum dot intermediate layer addition step, an interfacial polymerization reaction step, a chemical crosslinkingstep, and a solvent activating step. The separation performance of the film is significantly improved by adding the graphene quantum dot intermediate layer, the graphene quantum dots have a large amount of hydroxyl groups and carboxyl groups, the hydrophilicity of a base film can be increased, the pore size is reduced, the interfacial polymerization reaction is facilitated, and therefore the separation performance of the film is effectively improved. The preparation process is simple, and the composite film has a good application prospect in the field of organic solution system separation.
Owner:OCEAN UNIV OF CHINA
Injection preparation of anti-PD-L1 monoclonal antibody
ActiveCN110974958AHigh affinityHigh activityInorganic non-active ingredientsPharmaceutical delivery mechanismAntiendomysial antibodiesActive agent
The invention relates to the field of biological medicine, and particularly provides an injection preparation of an anti-PD-L1 monoclonal antibody, which comprises the anti-PD-L1 monoclonal antibody with the protein content of 10-60 mg / ml; a buffer agent having a concentration of 10-60 mM; a stabilizer with the concentration of 100 to 150 mM; an osmotic pressure regulator with the concentration of10-100 mM; and 0.005%-0.05% (w / v) of a surfactant; wherein a pH value of the injection preparation is 5.8 to 6.3. The anti-PD-L1 monoclonal antibody disclosed by the invention has relatively high affinity and good biological activity; meanwhile, different auxiliary materials are selected according to the characteristics of the anti-PD-L1 monoclonal antibody, so that the safety and effectiveness of the monoclonal antibody in the preservation process can be guaranteed, and the physical stability, chemical stability and biological stability of the anti-PD-L1 monoclonal antibody can be maintained.
Owner:BEIJING DONGFANG BIOTECH
Nanofiltration membrane for selectively separating trace organic substances and calcium-magnesium ions, and preparation method thereof
ActiveCN109046025AMaintain chemical stability and healthLow rejectionSemi-permeable membranesWater contaminantsIonChemistry
The invention provides a nanofiltration membrane for selectively separating trace organic substances and calcium-magnesium ions. The nanofiltration membrane comprises a porous support layer and a filter skin layer, a first solution and a second solution are subjected to interfacial polymerization reaction and heating treatment in sequence to form the filter skin layer, the first solution is a water solution containing polyamine monomers and acid organic matter monomers, and the second solution is formed by dissolving acyl chloride monomers in an organic solvent; in the first solution, the total mass concentration of the polyamine monomers and the acid organic matter monomers is 0.4-1%, and the acid organic matter monomers account for 35-65%; in the second solution, the mass concentration of the acyl chloride monomers is 0.1-0.2%. The invention further provides a preparation method of the nanofiltration membrane for selectively separating the trace organic substances and the calcium-magnesium ions. The nanofiltration membrane has high rejection rate for the trace organic substances but low rejection rate for the calcium-magnesium ions, and chemical stability and health of nanofiltration water are maintained while the trace organic substances are effectively removed.
Owner:TSINGHUA UNIV
Hybrid composite membrane, preparation method and application thereof
ActiveCN109925896ASignificant progressSubstantialSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisOrganosolvEngineering
The invention discloses a hybrid composite membrane, a preparation method and application of the prepared hybrid composite membrane. The preparation method of the hybrid composite membrane comprises an interface polymerization step, a polyamine modification step, a chemical crosslinking step and a solvent activation step. According to the invention, low-concentration interfacial polymerization iscarried out on a graphene oxide intermediate layer, so that the flux and solvent resistance of the membrane are obviously improved. Because the graphene oxide intermediate layer has a large number ofoxygen-containing functional groups, the hydrophilicity of a base membrane is greatly increased, the interfacial polymerization process of low-concentration monomers is regulated and controlled, the crosslinking degree of an interfacial separation layer is enhanced, and the solvent resistance of the membrane is improved. By adopting the low concentration monomer interfacial polymerization, the surface roughness of the membrane can be effectively improved. The hybrid composite membrane has simple preparation process and good application prospect in the field of organic solvent system separationand water treatment containing organic solvents.
Owner:OCEAN UNIV OF CHINA
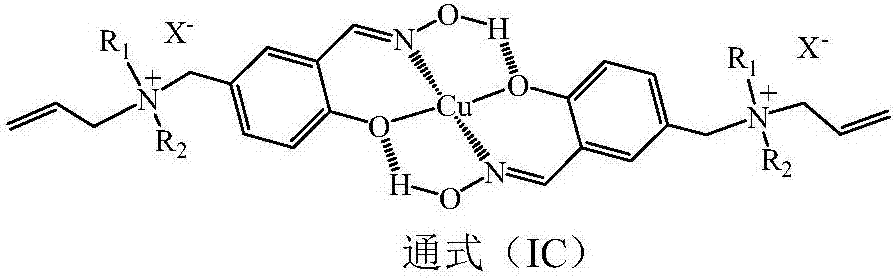
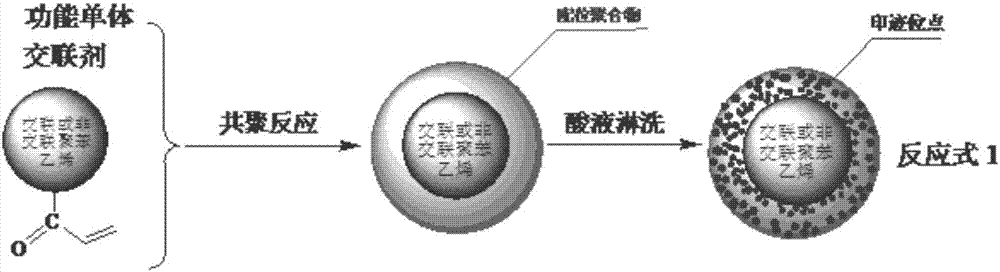
Copper (II) ion surface imprinted polymer and preparation method thereof
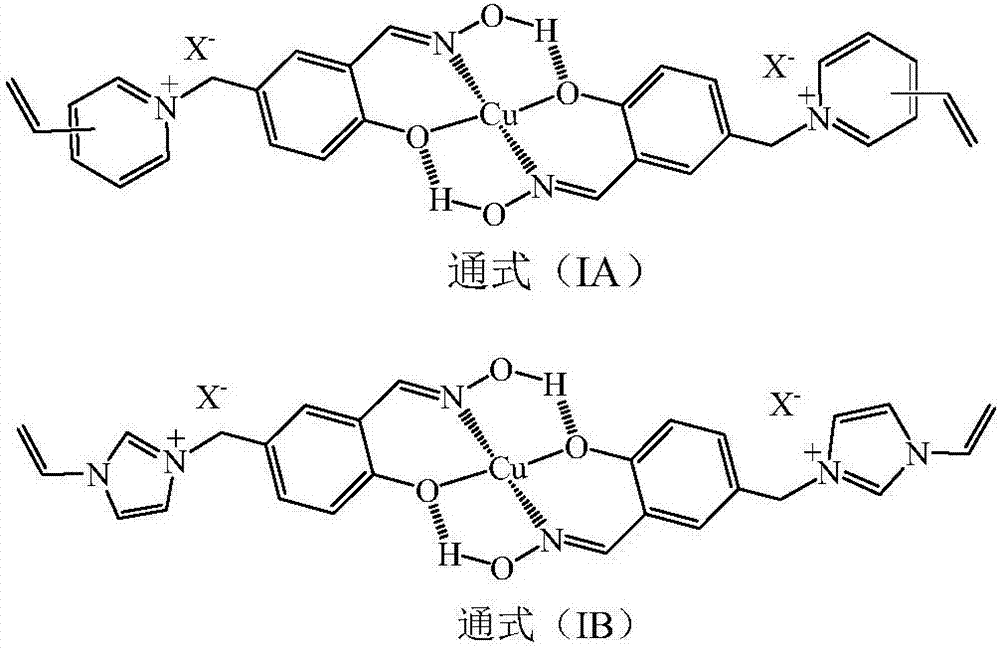
ActiveCN107573462AImprove stabilityMaintain chemical stabilityWater contaminantsWater/sewage treatment by sorptionSolid phasesFunctional monomer
The invention provides a copper (II) ion surface imprinted polymer supported by polystyrene. The copper (II) ion surface imprinted polymer is prepared by using an unsaturated quaternary ammonium cation modified salicylaldoxime copper (II) complex as a functional monomer and polyene propyl ammonium salt as a cross-linking agent through copolymerization of framework monomers on the acryloyl polystyrene surface; the imprinted polymer is used as a solid phase imprinted two-position extracting agent of copper (II) and is used for selective enrichment and separation of copper (II) ions in each watersystem; the characteristic and the function of simultaneously extracting counterbalancing anions are realized. An effective method is provided for removing copper (II) ions in various water systems and realizing the auxiliary detection, separation and enrichment of copper (II) ions in various water systems; the copper (II) ion surface imprinted polymer can show the foam, diaphragm plate, filamentor particle forms.
Owner:HUAIHAI INST OF TECH
Anti-programmed cell death-ligand 1 (PD-L1) antibody preparation
ActiveCN111228479AGuaranteed physical stabilityMaintain chemical stabilityInorganic non-active ingredientsPharmaceutical delivery mechanismTherapeutic antibodyAntiendomysial antibodies
The invention relates to the field of therapeutic antibodies, in particular relates to an anti-programmed cell death-ligand 1 (PD-L1) antibody preparation. The preparation comprises an anti-PD-L1 antibody, a buffer solution and sodium chloride, and added sugar and / or sugar alcohols are absent. The anti-PD-L1 antibody preparation of the invention can maintain antibody stability after long-term storage. In addition, the invention further relates to a use of the anti-PD-L1 antibody preparation in preparing drugs used for the prevention and / or treatment and / or adjuvant treatment and / or diagnosis of tumors or anemia
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Protein a mutants having high alkali resistance and methods of use thereof
ActiveUS20190048046A1Maintain chemical stabilityAntibody mimetics/scaffoldsPeptide preparation methodsHigh resistanceComplementarity determining region
A series of protein A mutants having high alkali resistance, and methods of using the protein A mutants are provided. The protein A mutants have a high binding affinity for regions of immunoglobulin proteins other than the complementarity determining regions. The protein A mutants can be coupled to a solid support for immunoglobulin isolation, or conjugated to a label for immunoglobulin detection. This series of protein A mutants have high chemical stability under alkaline conditions of pH 13-14, and can also be used as chromatography ligands for purification procedures that use alkaline solutions under harsh conditions, such as Clean-In-Place (CIP). Also provided are methods of immunoglobublin separation and purification, and alkali regeneration of affinity chromatography medium that uses protein A as a ligand.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
Preparation method of hydrophilic poly(vinylidene fluoride) microfiltration membrane
ActiveCN108905647AWay of increaseImprove hydrophilicityMembranesUltrafiltrationHydrophilic coatingChemical reaction
The invention discloses a preparation method of a hydrophilic poly(vinylidene fluoride) microfiltration membrane. A heterogeneous modification process of conventional polydopamine-modified PVDF is promoted to a homogeneous process, and in the process of homogeneous polymerization of dopamine, polydopamine molecules combine with PVDF molecular chains through strong non-covalent bonds; and then water-soluble polymer is introduced to chemically react with polydopamine so as to obtain a raw material of the hydrophilic PVDF, finally the raw material of the hydrophilic PVDF is redissolved, and a pore-forming agent and additive are added so as to obtain the microporous membrane. Through the method, the efficiency of hydrophilic modification is improved, the obtained hydrophilic PVDF microfiltration membrane has excellent hydrophilicity, the long-term stability and chemical stability of a hydrophilic coating on the PVDF microfiltration membrane are improved remarkably, the problems of a long operation period of the polydopamine-modified PVDF microfiltration membrane, desorption of the polydopamine coating after long-term use is conducted and the like are solved, and the preparation efficiency of the hydrophilized PVDF microfiltration membrane is improved greatly.
Owner:杭州安诺过滤器材有限公司
Macrocyclic amides, pharmaceutical compositions, preparation methods and uses thereof
ActiveUS8680087B2Easy to prepareGood handling characteristicsAntibacterial agentsBiocidePhotorhabdus speciesTransgenic technology
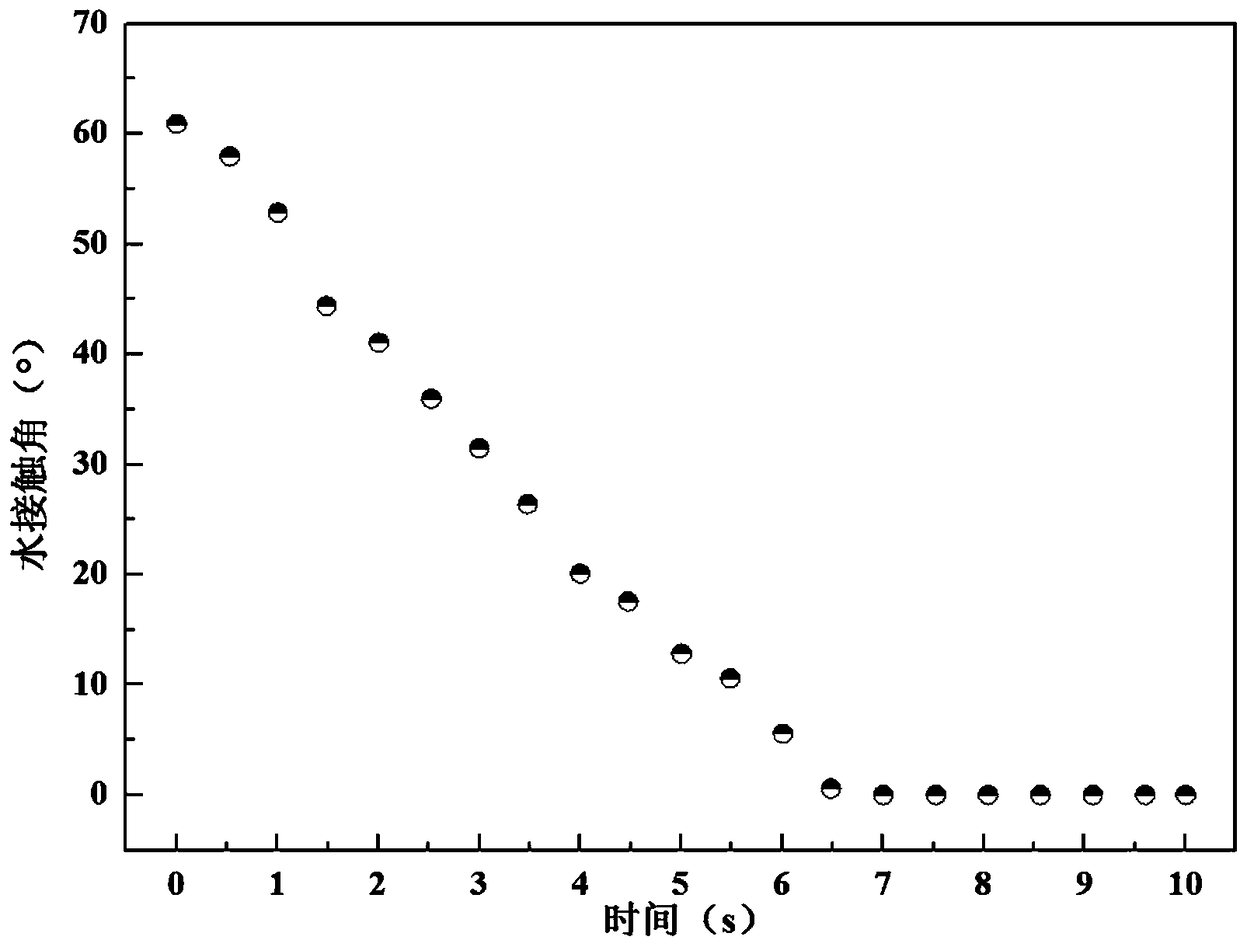
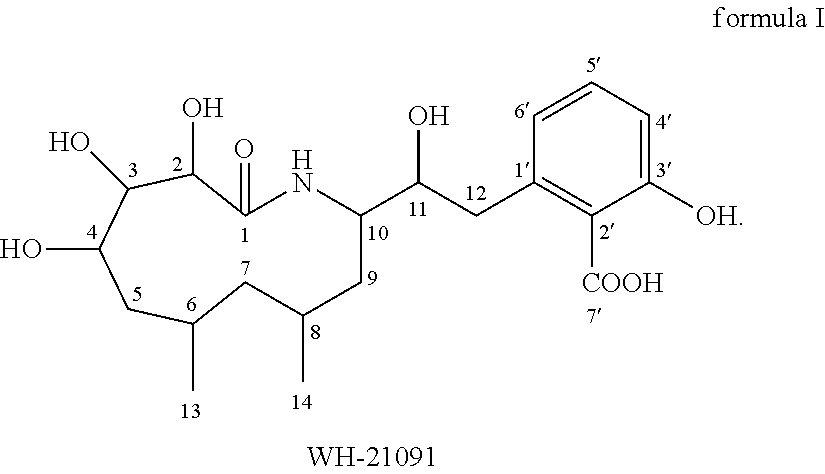
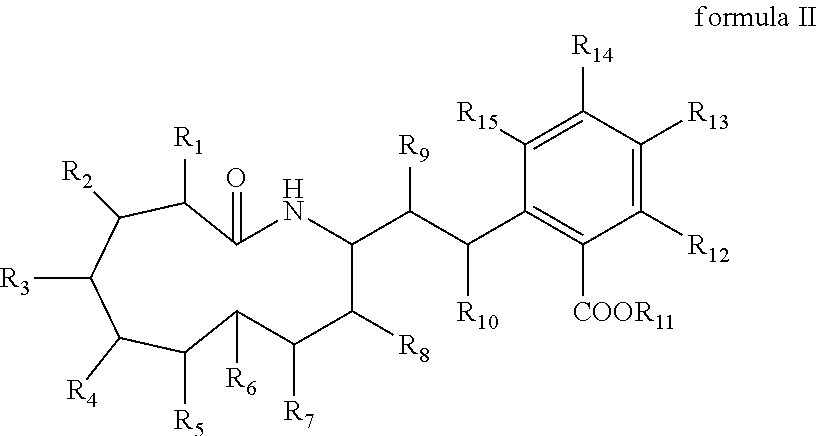
Macrocyclic amide WH-21091 with antibacterial and antitumor activities, its analogs, preparation methods and uses thereof. The said macrocyclic amides are prepared by microbes of Xenorhabdus and Photorhabdus, or they can be prepared by other living beings through transgenic techniques. The compositions of the said macrocyclic amide and its analogs can be used as drugs and / or agricultural chemicals for treatment of microbial infections, especially for treatment of infectious diseases of Staphylococcus aureus with drug resistance. The said compositions can also be used as drugs for treatment of cancers of human beings or animals.
Owner:YUXI WINHEY BIO TECH
Lithium ion battery electrode binder and application thereof
InactiveCN111710864AGuaranteed specific capacityGuaranteed cycle performanceNon-aqueous electrolyte accumulator electrodesLi-accumulatorsLithium-ion batteryButadiene-styrene rubber
The invention discloses a lithium ion battery electrode binder and application thereof. The lithium ion battery electrode binder is obtained by performing hydrogenation treatment on a binder containing unsaturated bonds, and the hydrogenation treatment is used for partially or completely changing the unsaturated bonds in the binder containing the unsaturated bonds into saturated bonds so as to form the lithium ion battery electrode binder; the binder containing the unsaturated bond comprises one or a combination of several of butadiene styrene rubber, carboxymethyl cellulose sodium, lithium polyacrylate, polyacrylonitrile and sodium alginate.
Owner:TIANMU LAKE INST OF ADVANCED ENERGY STORAGE TECH CO LTD
Reinforcing sheet for solid electrolyte membrane
InactiveUS20130040225A1Prevent increase in strength and dimensional stabilityInfluence chemicalElectrolytic capacitorsCell component detailsGlass fiberPolymer science
The reinforcing sheet according to the present invention includes glass fibers and an organic binder that coats the glass fibers, and has voids to be filled with a solid electrolyte. The organic binder is (i) an organic polymer containing no element other than carbon, hydrogen and fluorine; or (ii) an organic polymer having a main chain and side chains, the main chain being perfluoroalkylene, and at least one of the side chains being terminated with a sulfonic acid group or a carboxylic acid group. The reinforcing sheet according to the present invention is suitable for improving the durability of a solid electrolyte membrane and maintaining the chemical stability thereof.
Owner:NIPPON SHEET GLASS CO LTD
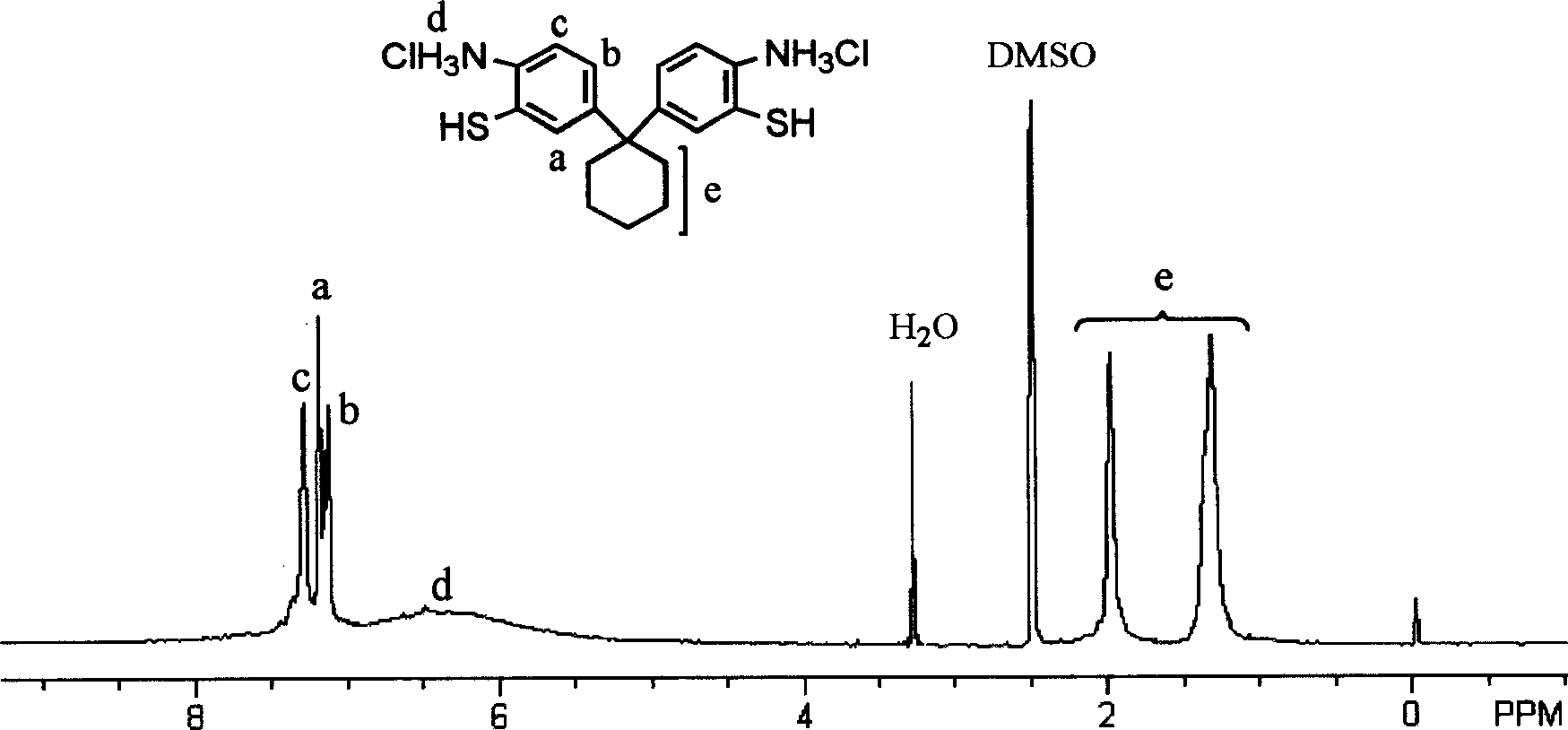
Substituted di(amino mercapto) benzene hydrochloride and process for preparing same
InactiveCN1699336AImprove solubilityMaintain thermal stabilityThiol preparationCarboxylic acidAniline
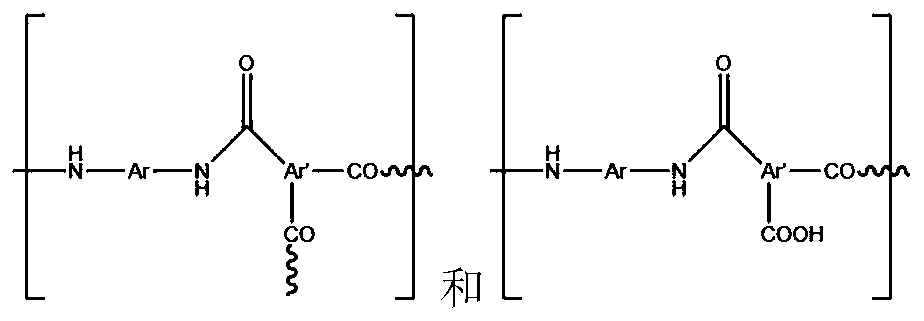
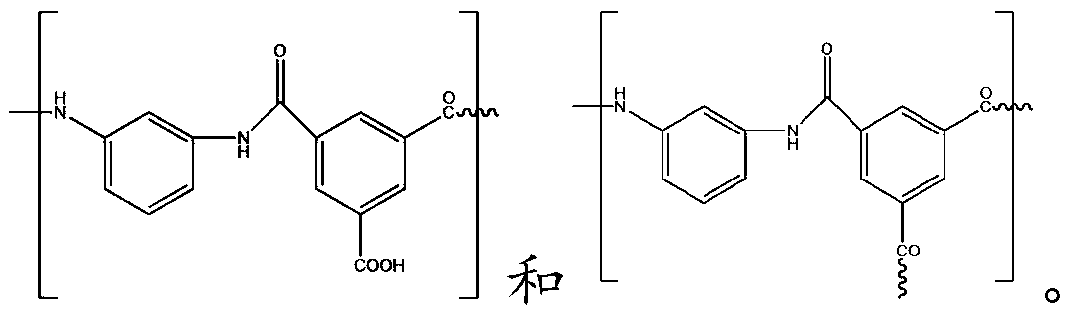
The invention discloses a substituted di(amino mercapto) benzene hydrochloride and process for preparation, which is prepared by using compounds containing benzeneamine and carbonyl as starting raw material through three-step reactions. It can be used for preparing novel benzopyrene polymers together with dicarboxylic acids through polycondensation, the dissolving property of these polymers can be increased, and fine properties of high thermal stability and chemical stability can be achieved.
Owner:SHANGHAI JIAO TONG UNIV
A kind of injection preparation of anti-PD-L1 monoclonal antibody
ActiveCN110974958BGuaranteed stabilityGuaranteed efficacyInorganic non-active ingredientsPharmaceutical delivery mechanismAntiendomysial antibodiesActive agent
The invention relates to the field of biological medicine, and particularly provides an injection preparation of an anti-PD-L1 monoclonal antibody, which comprises the anti-PD-L1 monoclonal antibody with the protein content of 10-60 mg / ml; a buffer agent having a concentration of 10-60 mM; a stabilizer with the concentration of 100 to 150 mM; an osmotic pressure regulator with the concentration of10-100 mM; and 0.005%-0.05% (w / v) of a surfactant; wherein a pH value of the injection preparation is 5.8 to 6.3. The anti-PD-L1 monoclonal antibody disclosed by the invention has relatively high affinity and good biological activity; meanwhile, different auxiliary materials are selected according to the characteristics of the anti-PD-L1 monoclonal antibody, so that the safety and effectiveness of the monoclonal antibody in the preservation process can be guaranteed, and the physical stability, chemical stability and biological stability of the anti-PD-L1 monoclonal antibody can be maintained.
Owner:BEIJING DONGFANG BIOTECH
Preparation method for forming magnetite nanoparticles in cellulose acetate membrane
InactiveCN110833773AImprove hydrophilicityMaintain physical and chemical stabilitySemi-permeable membranesOther chemical processesQuinolinePolyvinyl alcohol
The invention relates to a preparation method for forming magnetite nanoparticles in a cellulose acetate membrane. The preparation method comprises: preparing a cellulose acetate membrane; adding 8-hydroxyquinoline, and casting the solution on a glass plate base material through a casting knife; washing the prepared membrane, and soaking in water; drying the film; violently mixing a polyvinyl alcohol polymer, and dissolving in water to obtain a uniform solution; adding ferric chloride, and dropwisely adding sodium hydroxide into the mixed solution; carrying out ultrasonic treatment on the solution through an ultrasonic instrument oscillator, and breaking the material aggregate; and immersing the cellulose acetate membrane into the nanometer composite material solution, discharging the excessive solution, and drying to obtain the cellulose acetate membrane. According to the invention, the membrane prepared by adopting the preparation method is improved in ion removal performance, improved in membrane surface hydrophilicity, good in adsorption performance and mechanical integrity, and capable of being repeatedly used.
Owner:广州市思创信息技术有限公司
Exendin-4-Fc fusion protein injection preparation and preparation method thereof
ActiveCN114053217AMaintain physical stabilityMaintain chemical stabilityPeptide/protein ingredientsMetabolism disorderIrritationActive agent
The invention provides an Exendin-4-Fc fusion protein injection preparation and a preparation method, the injection preparation comprises 0.28%-0.35% (w / v) of Exendin-4-Fc fusion protein, 0.01%-0.05% (w / v) of a surfactant, 1.8%-5.5% (w / v) of a stabilizer, a pH regulator and a solvent, and the pH of the injection preparation is 7.0-7.7. The Exendin-4-Fc fusion protein injection preparation is free of preservative, colorless to faint yellow, and is a clear to slightly opalescent liquid, the physical and chemical stability of the fusion protein can still be maintained within a long time, and the storage period is effectively prolonged; the Exendin-4-Fc fusion protein injection preparation provided by the invention is relatively high in fusion protein content and pH, can reduce the medication frequency and reduce the irritation of low pH to blood vessels, is suitable for blood vessel injection, relieves the pain of patients caused by high-frequency and dependent injection of hypoglycemic injections such as insulin and the like, and is good in patient medication compliance.
Owner:HUALAN BIOLOGICAL ENG INC
Preparation method of durable high-strength composite concrete expansive material
The invention relates to a preparation method of a durable high-strength composite concrete expansive material and belongs to the technical field of expansive agents. The preparation method includes:using raw powder of sepiolite as a raw material, modifying the raw material by acidolysis so that sepiolite fiber tows are stripped from particles of the powder, combining the sepiolite fiber tows with blast furnace slag to form high-specific-surface-area fiber tow aggregate by melt drawing, making a network framework with the aggregate while chemical stability and mechanical strength of the blastfurnace dry slag fibers are maintained. Pores among the composite fibers support water-absorbing gel to fill the material herein; the material can effectively absorb water and be expansively modifiedduring use. Composite magnesia is used herein, early expansion of the composite magnesia provides sufficient expansion, the composite magnesia can react continuously later to expand, concrete structure damage due to stress relaxation caused by excessive early expansion is avoided, contraction of concrete can be compensated, and durability of the expansive material herein can be effectively improved.
Owner:FOSHAN CHAOHONG NEW MATERIAL TECH CO LTD
Anion-cation double exchange type copper (II) ion surface imprinted polymer and preparation method thereof
ActiveCN107641174AImprove stabilityMaintain chemical stabilityOther chemical processesWater contaminantsCross-linkQuaternary ammonium cation
The invention provides polystyrene-supported anion-cation double exchange type copper (II) ion surface imprinted polymer which is prepared by performing copolymerization with skeleton monomers on polyenoic allyl ammoniated polystyrene surfaces by taking an unsaturated quaternary ammonium cation-modified sulfated aldoxime copper (II) complex as a functional monomer and polyenoic allyl ammonium saltas a cross-linking agent; the polymer can be used as solid-phase blotting bi-site extractant for copper (II) to perform selective enrichment and separation of copper (II) ions in various aqueous systems; the polymer has the function and characteristic of simultaneously extracting counter-anions and can be used in methods for removing the copper (II) ions in the various aqueous systems and methodsfor detecting, and separating and enriching the copper (II) ions in the various aqueous systems in an auxiliary manner; and the anion-cation double exchange type copper (II) ion imprinted polymer canbe in forms of foam, diaphragm plates, fibrils or particles.
Owner:HUAIHAI INST OF TECH
Preparation method of resin beads
The invention relates to a preparation method of resin beads, which mainly solves the problems that the existing catalytic hydrogenation technology is relatively complicated in process, high in cost,high in solvent extraction cost and difficult in effectively utilizing and treating acid residues obtained by a sulfonation method. The preparation method comprises the following steps: adding styrenemonomers into a solvent in the presence of nitrogen, adding a catalyst and triethylamine, dropwise adding a mixed solution of a sulfonating agent and solvent, separating and purifying a product by utilizing a column chromatography, obtaining sulfuryl-containing styrene monomers, adding the styrene monomers and the sulfuryl-containing styrene monomers into a reaction apparatus, adopting a polyvinyl alcohol aqueous solution as the solvent, introducing nitrogen, adding a cross-linking agent, reacting to obtain solid beads, and filtering to obtain the resin beads. The preparation method of the resin beads has the advantages that the heat stability and chemical stability of the resin beads can be kept, and the adsorption efficiency can be increased; and the reusability of the resin beads can be ensured.
Owner:任丽丽
Crystal form of β-lactamase inhibitor and preparation method therefor
ActiveUS11180501B2Easy to prepareImprove stabilityAntibacterial agentsOrganic active ingredientsPharmaceutical drugMedicinal chemistry
Disclosed in the present disclosure are a crystal form of a β-lactamase inhibitor and a preparation method therefor, as well as an application of the crystal form in preparing a β-lactamase inhibitor drug. (I)
Owner:QILU PHARMA CO LTD
Composite ultrafiltration membrane and preparation method thereof
InactiveCN101507904BEasy to useGuaranteed heat resistanceSemi-permeable membranesUltrafiltrationUltimate tensile strength
Owner:INNER MONGOLIA MANZHOULI SENNUO BIOTECH CO LTD
High-voltage-resistant insulation material and preparation method thereof
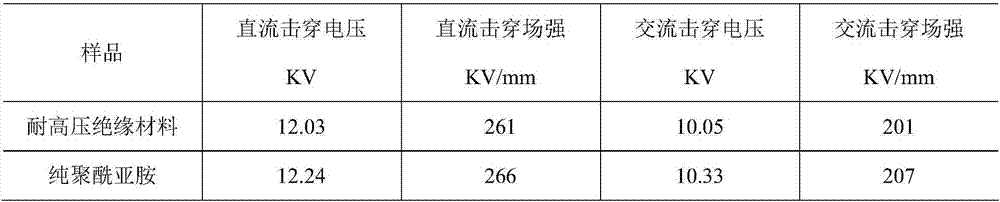
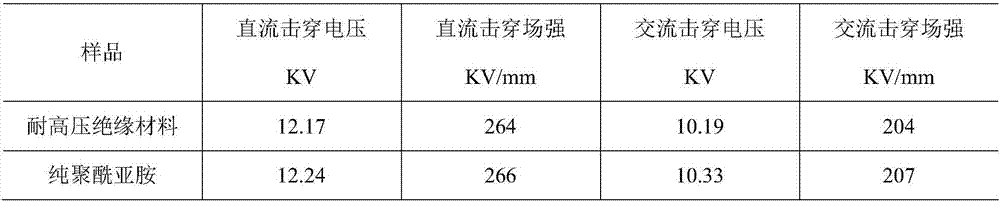
The invention relates to a high-voltage-resistant insulation material and a preparation method thereof, and belongs to the field of insulation material preparation. The high-voltage-resistant insulation material is mainly prepared from the following raw materials in parts by weight: 15 to 30 parts of trimellitic anhydride, 20 to 30 parts of 5-norbornene-2,3-dicarboxylic monomethylester, 30 to 70 parts of N-methyl pyrrolidone, 10 to 20 parts of xylene, 15 to 30 parts of N,N-dimethylacetamide, 5 to 10 parts of phenol, 20 to 100 parts of 4,4'-diphenylmethane diisocyanate, 0.5 to 10 parts of modified nanometer particles and 0.5 to 10 parts of modified fiber. The high-voltage-resistant insulation material has the advantages that the high-voltage-resistant performance is unchanged; the corona-resistant capability and the ablation-resistant capability are improved.
Owner:HARBIN UNIV OF SCI & TECH
Solid dispersoid of amorphous-form Simeprevir or Simeprevir salt acceptable to pharmacy and pharmaceutical auxiliary materials and preparation method of solid dispersoid
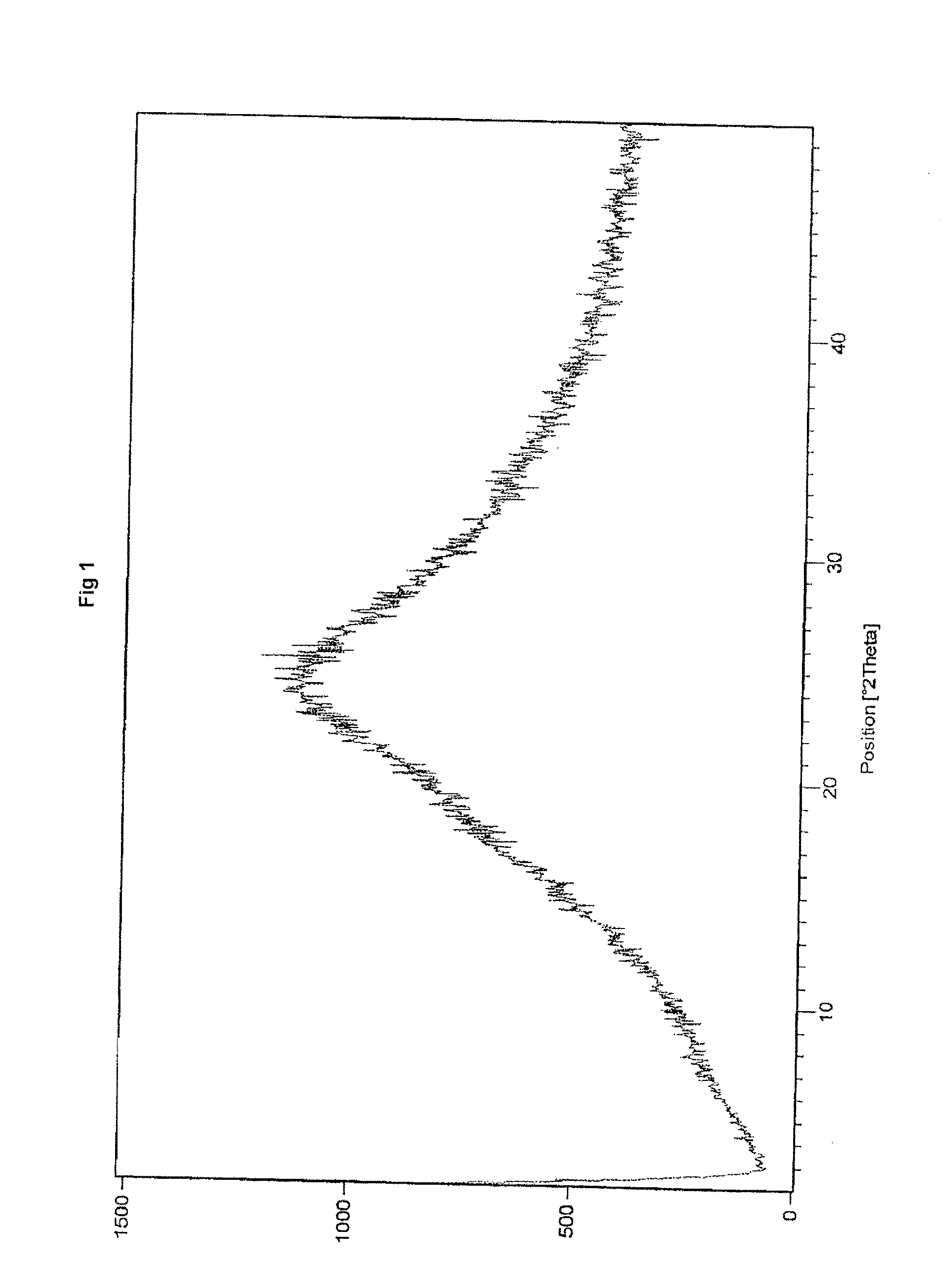
InactiveCN106539760AGuaranteed physical stabilityMaintain physical stabilityPowder deliveryOrganic active ingredientsMedicineX-ray
The invention discloses solid dispersoid of amorphous-form Simeprevir or Simeprevir salt acceptable to pharmacy and pharmaceutical auxiliary materials and a preparation method of the solid dispersoid. The solid dispersoid comprises the Simeprevir or Simeprevir salt acceptable to pharmacy and the pharmaceutical auxiliary materials, wherein the weight ratio of the Simeprevir or Simeprevir salt acceptable to pharmacy to the pharmaceutical auxiliary materials is 1: (0.1-100). The Simeprevir or Simeprevir salt acceptable to pharmacy is in the amorphous form. After a background peak of the pharmaceutical auxiliary materials is deducted from an X-ray powder diffraction spectrum of the solid dispersoid, no characteristic peak of crystals of the Simeprevir or Simeprevir salt is generated. The solid dispersoid of the amorphous-form Simeprevir or Simeprevir salt acceptable to pharmacy and the pharmaceutical auxiliary materials is good in stability and dispersibility; the dissolution rate of the Simeprevir or Simeprevir salt is increased; bioavailability of pharmaceutical preparations and pharmaceutical absorption of organisms can be improved more conveniently; and under the accelerated test conditions, good physical stability and chemical stability can be maintained. The preparation method of the amorphous-form solid dispersoid is simple, low in cost, good in reproducibility, easy to implement and suitable for industrial production.
Owner:CHANGZHOU FANGNAN MEDICINE TECH CO LTD
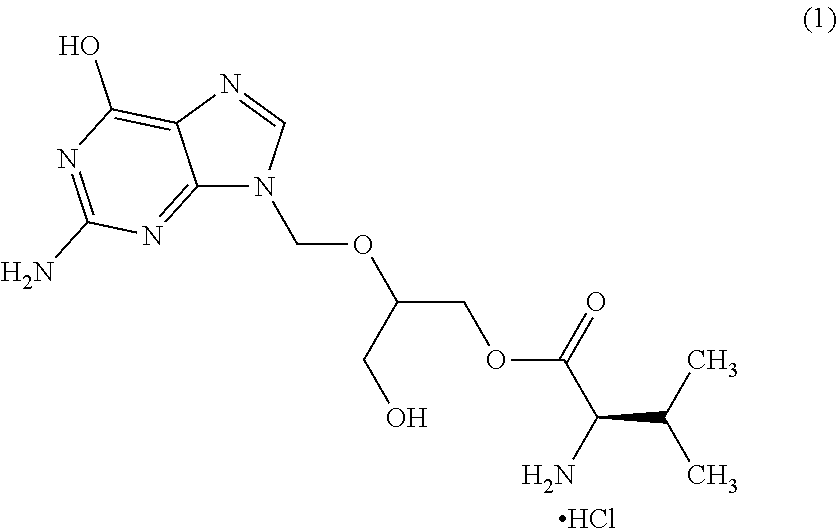
Process for the preparation of amorphus valgancyclovir hydrochloride
InactiveUS20130133289A1Maintain polymorphicMaintain chemical stabilityOrganic chemistrySynthetic resin layered productsValganciclovir HydrochlorideSolvent
Amorphous valgancyclovir hydrochloride has a median particle size of below 100 μm. A process for the preparation of the compound includes dissolving valgancyclovir hydrochloride in at least one solvent, removing the solvents under moisture controlled conditions, and drying the wet mass.
Owner:MYLAN LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com