Exendin-4-Fc fusion protein injection preparation and preparation method thereof
An injection preparation and fusion protein technology, which is applied in the field of glucagon-like peptide analogs, can solve the problems of multiple components of excipients, poor stability of protein drugs, and discomfort of patients, and achieve simple components of excipients, stable and controllable quality, and reduced medication The effect of frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
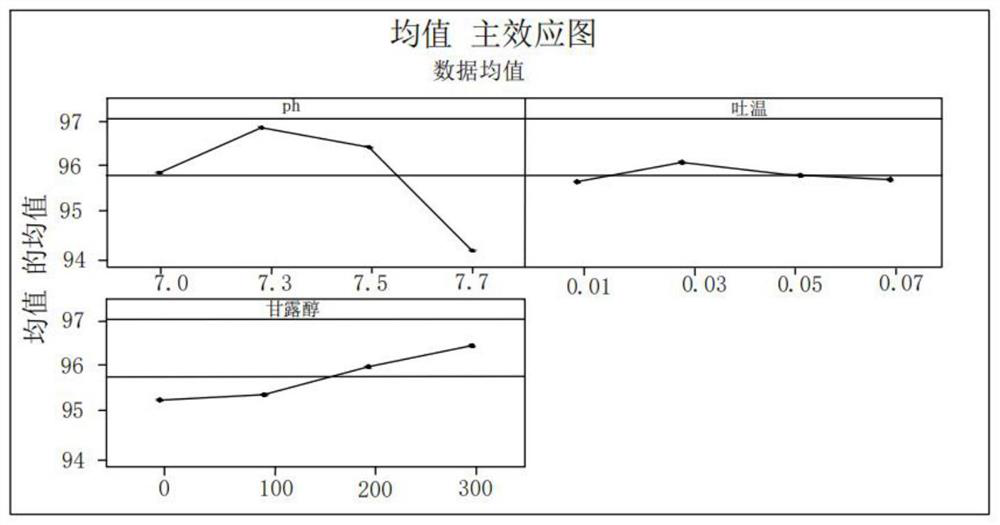
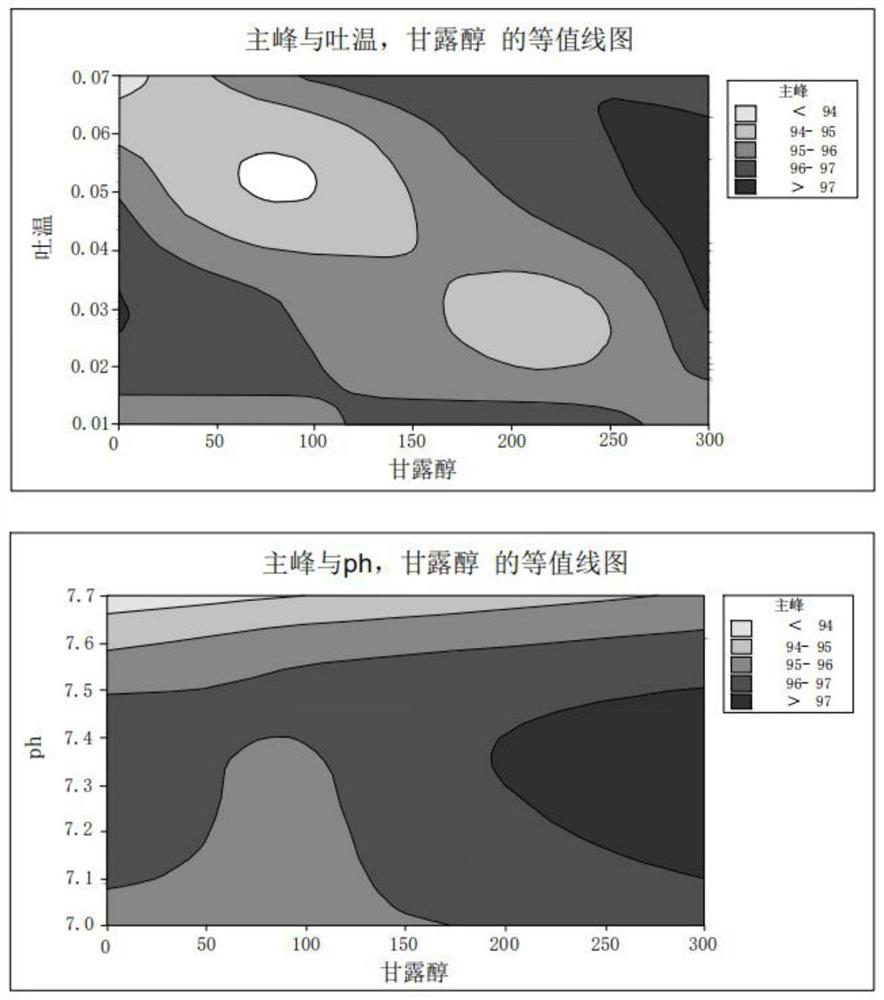
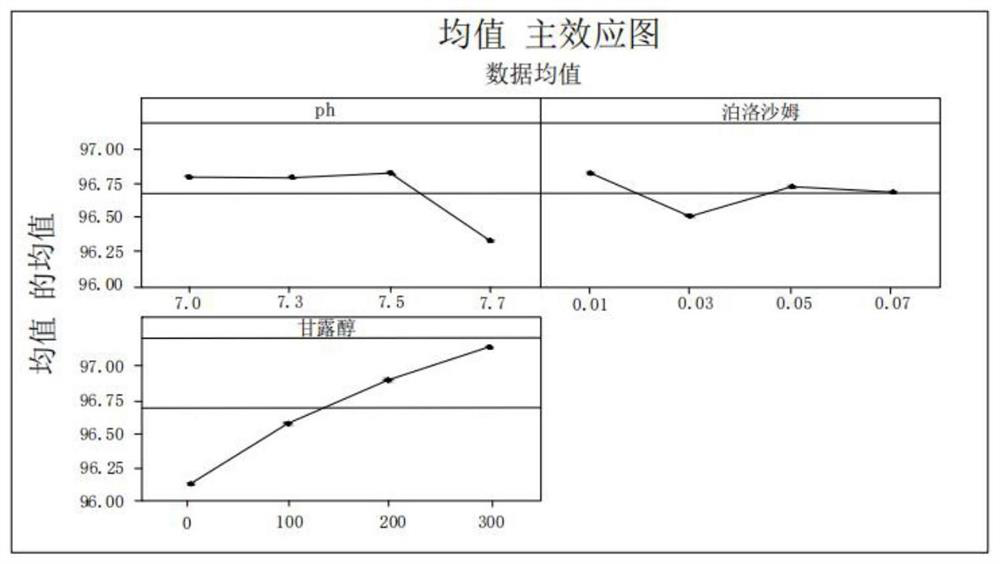
Image
Examples
Embodiment 1
[0031]
[0032] Preparation method: (1) Weigh the prescription amount of poloxamer-188, mannitol, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dodecahydrate and water for injection in proportion; (2) add injection Water, add pH adjuster - sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dodecahydrate and adjust the pH to 7.6, add stabilizer - mannitol, stir for 15 minutes until completely dissolved, as solution A; (3) take part Solution A, add poloxamer-188 to a concentration of 100g / L, stir for 10min, as solution B; (4) use ultrafiltration to replace the purified and handed-over sample with ultrafiltration to solution A, as solution C; ( 5) Mix solutions A, B, and C according to the amount, filter through a 0.22 μm filter membrane, and pass through N 2 filling;
Embodiment 2
[0034]
[0035]
[0036] Preparation method: (1) Weigh the prescription amount of poloxamer-188, mannitol, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dodecahydrate and water for injection in proportion; (2) add injection Water, add pH adjuster - sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dodecahydrate and adjust the pH to 7.7, add stabilizer - mannitol, stir for 10 minutes until completely dissolved, as solution A; (3) take part Solution A, add poloxamer-188 to a concentration of 100g / L, stir for 5min, as solution B; (4) use ultrafiltration to replace the purified and handed-over sample with ultrafiltration to solution A, as solution C; ( 5) Mix solutions A, B, and C according to the amount, filter through a 0.22 μm filter membrane, and pass through N 2 filling;
Embodiment 3
[0038]
[0039] Preparation method: (1) Weigh Tween-20, mannitol, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dodecahydrate and water for injection in proportion to the prescribed amount; (2) add water for injection into the preparation container, Add pH regulators - sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dodecahydrate and adjust the pH to 7.7, add stabilizer - mannitol, stir for 12 minutes until completely dissolved, as solution A; (3) take part of solution A , add Tween-20 to a concentration of 80g / L, stir for 5min, and use it as solution B; (4) use ultrafiltration to replace the purified sample with ultrafiltration to solution A, as solution C; (5) Mix solutions A, B, and C, filter through a 0.22 μm filter membrane, pass through N 2 filling.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com