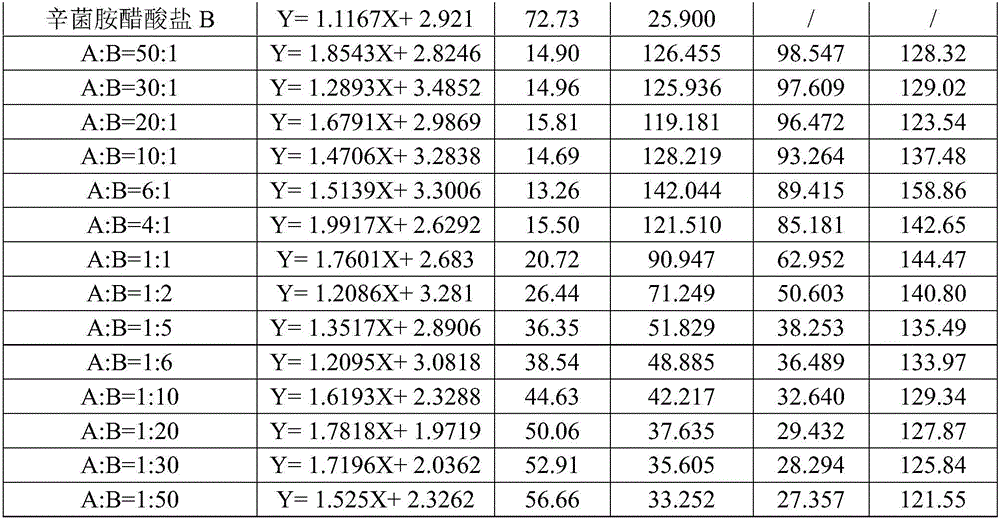
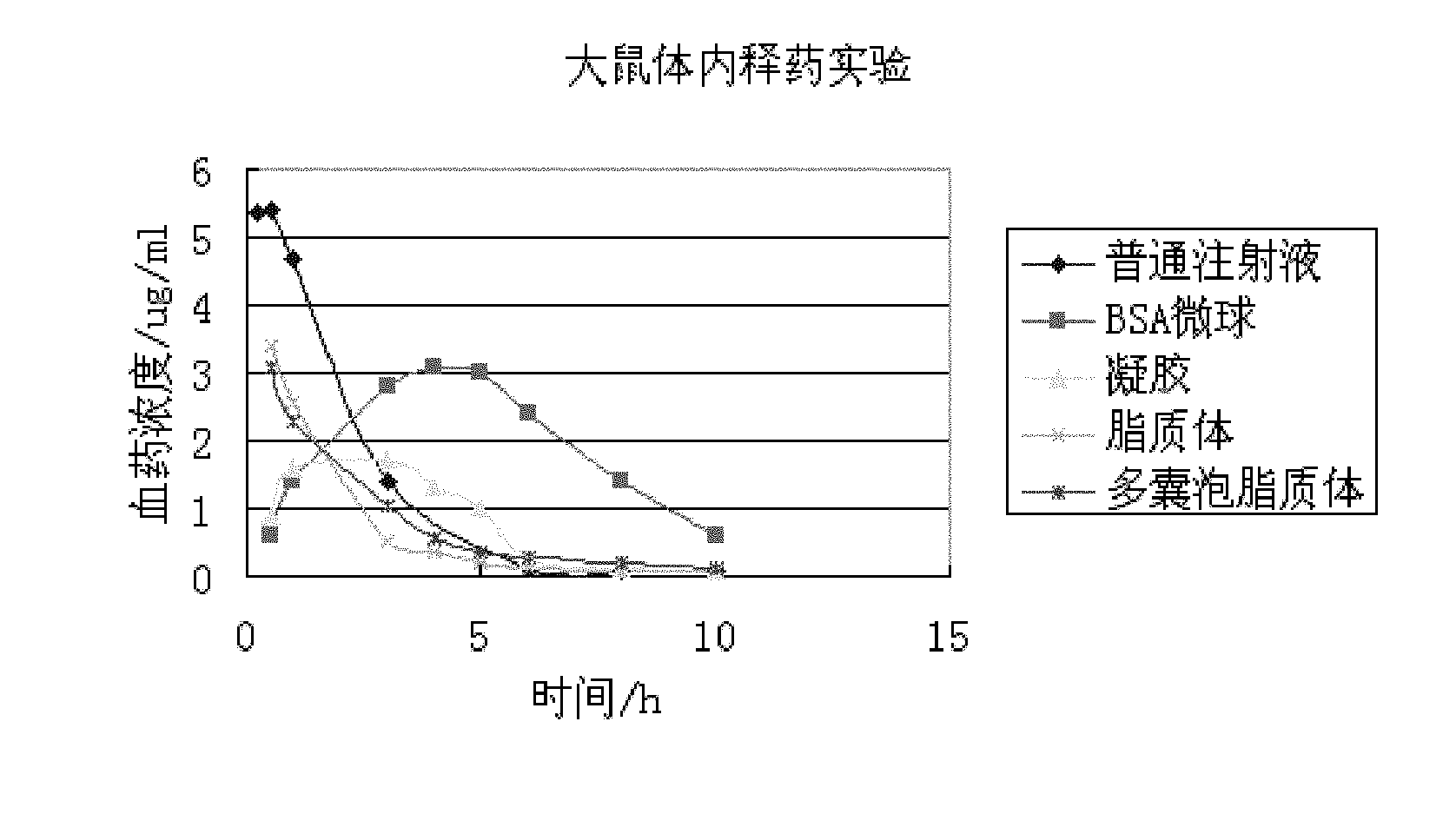
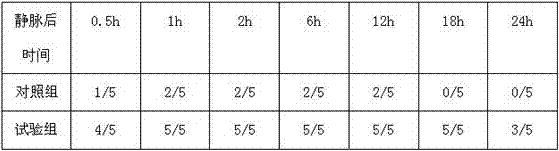
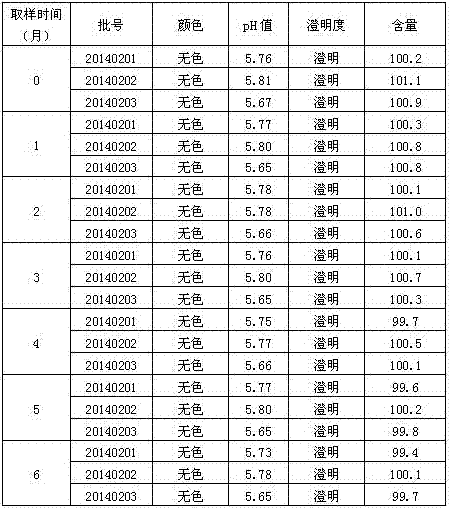
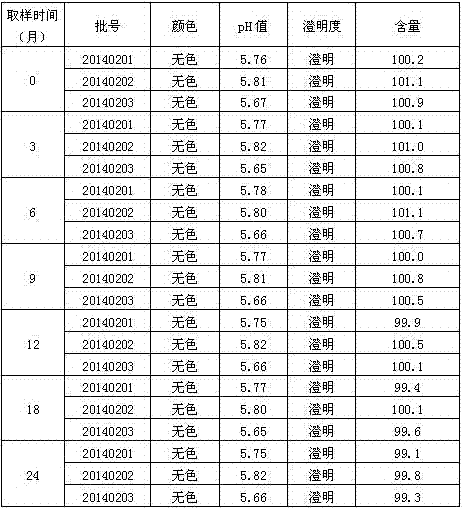
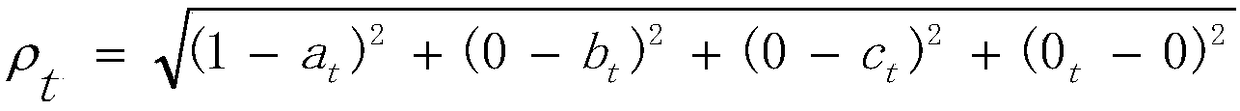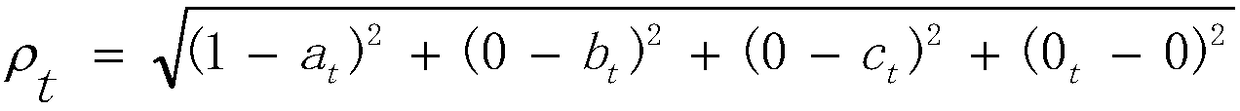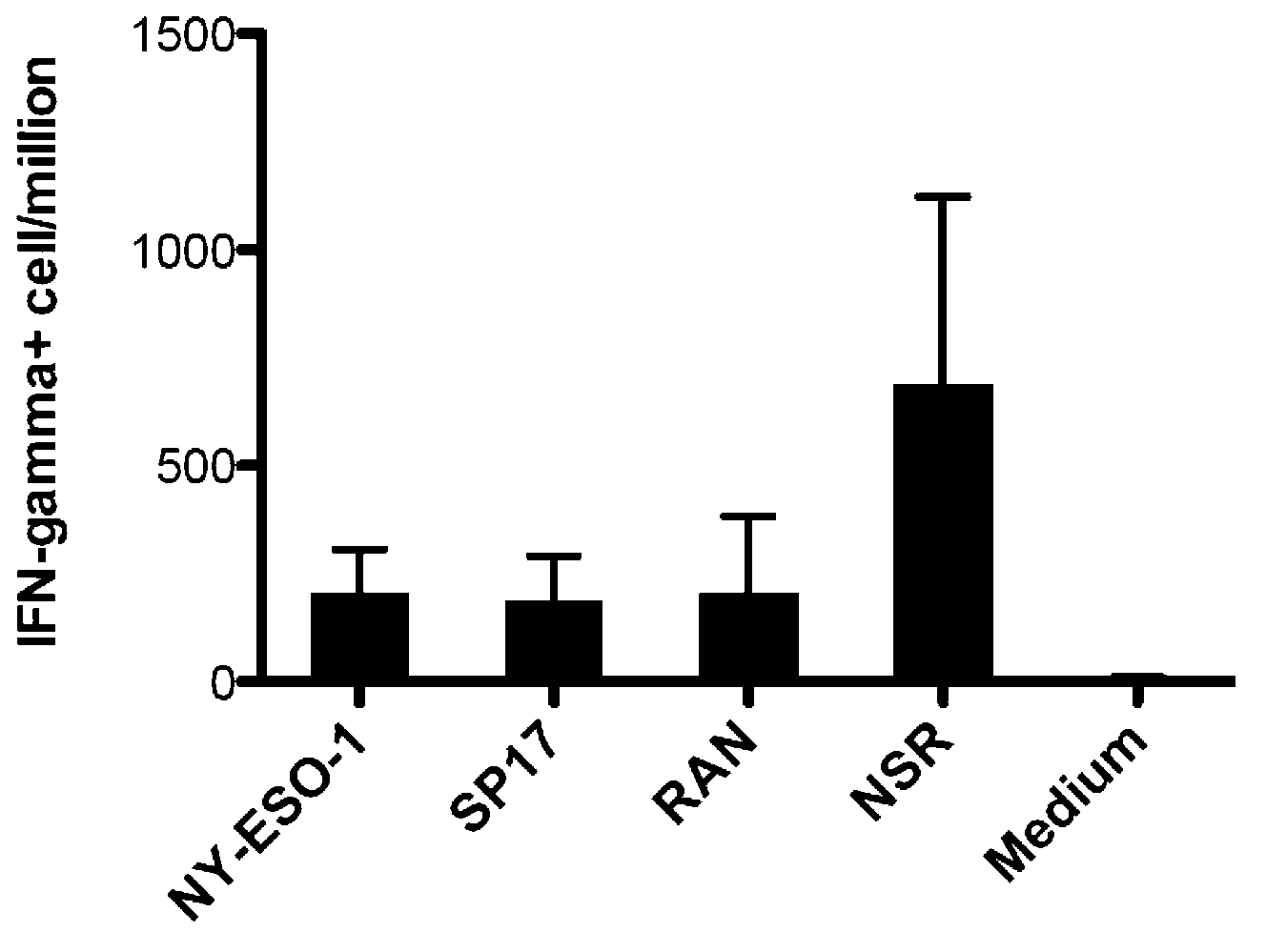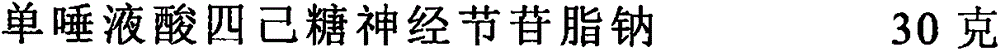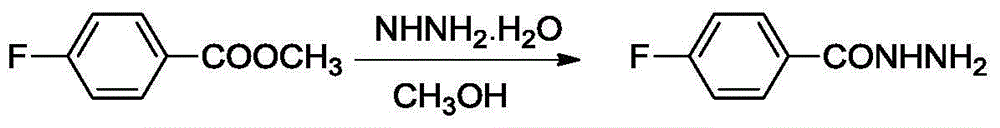Patents
Literature
56 results about "Medication frequency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
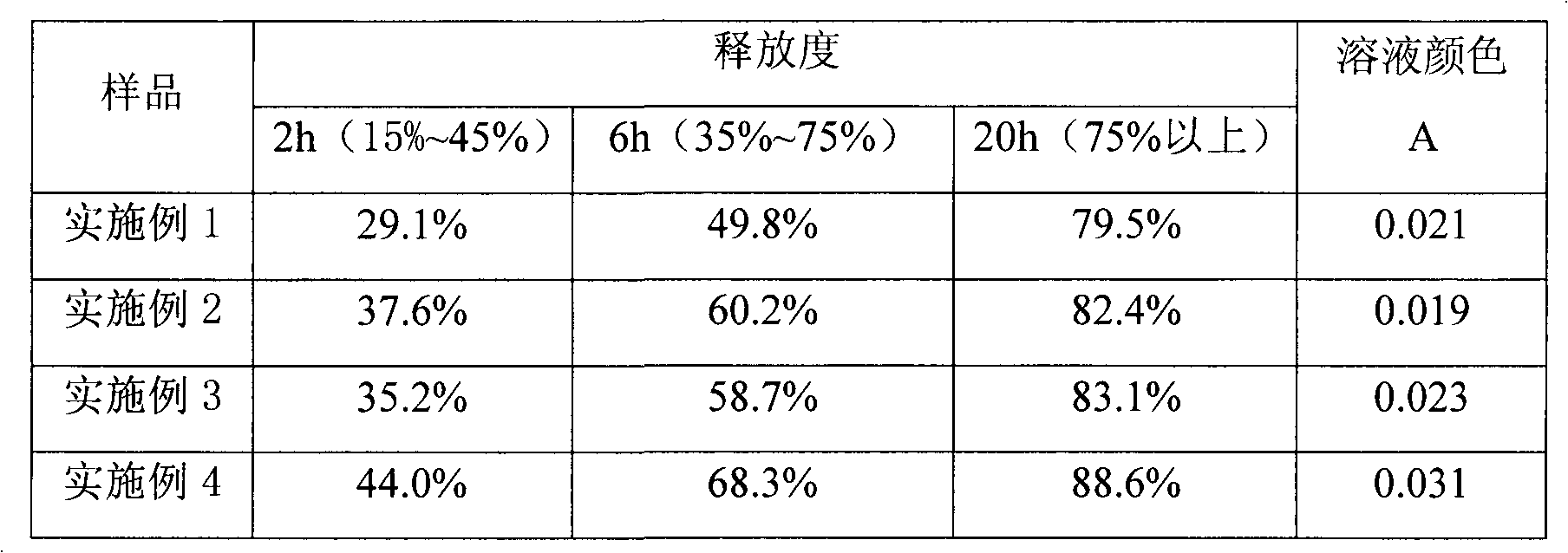
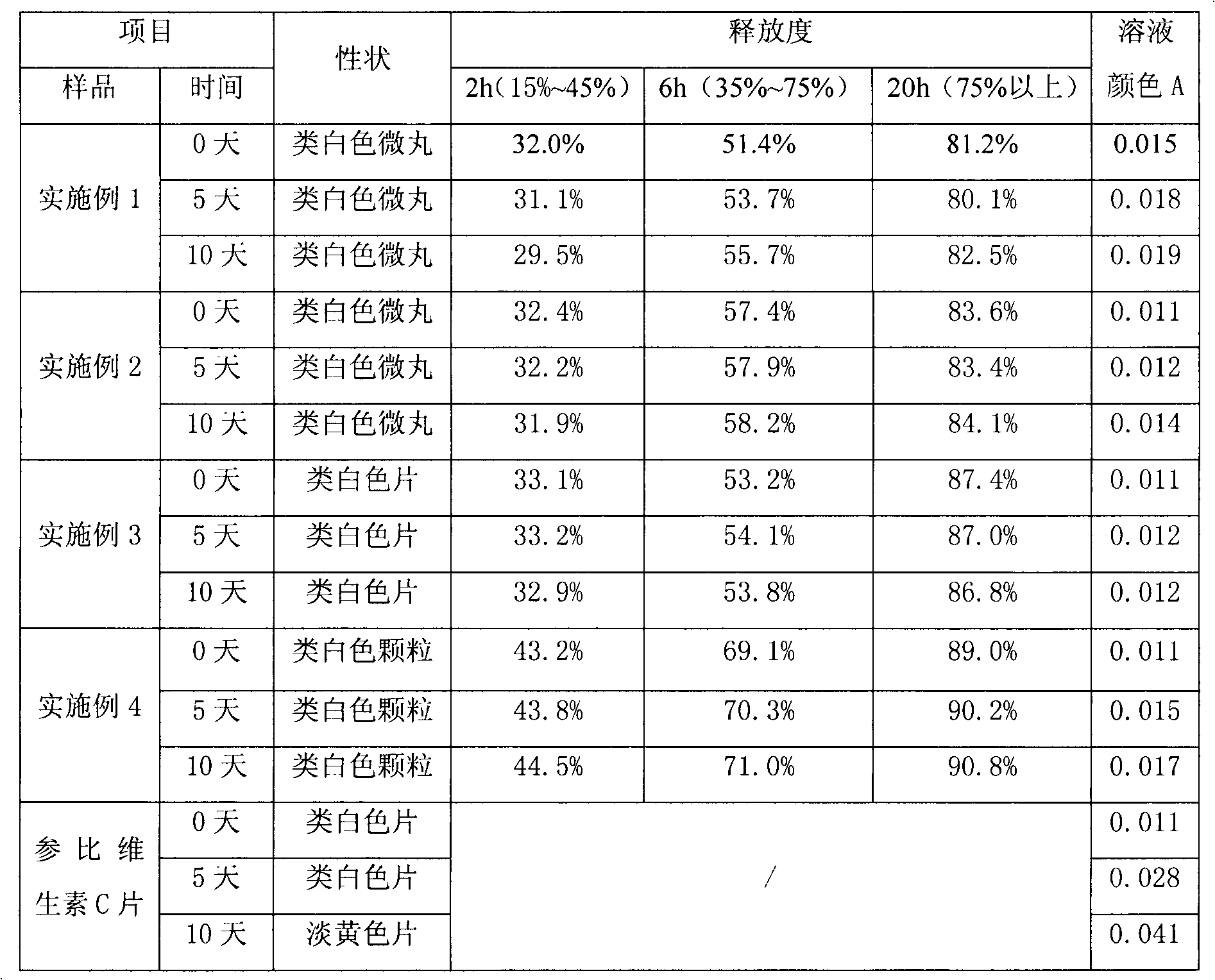
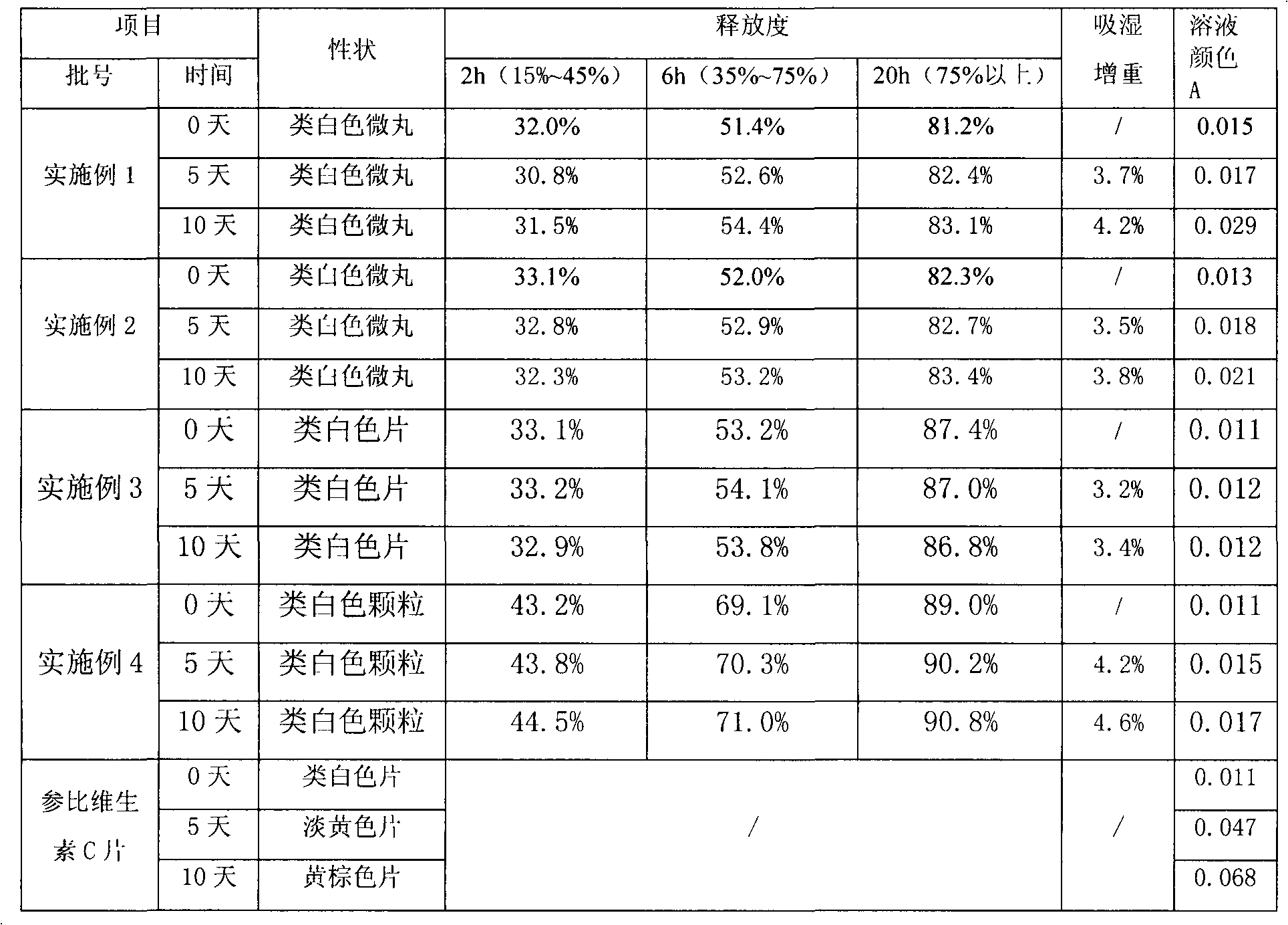
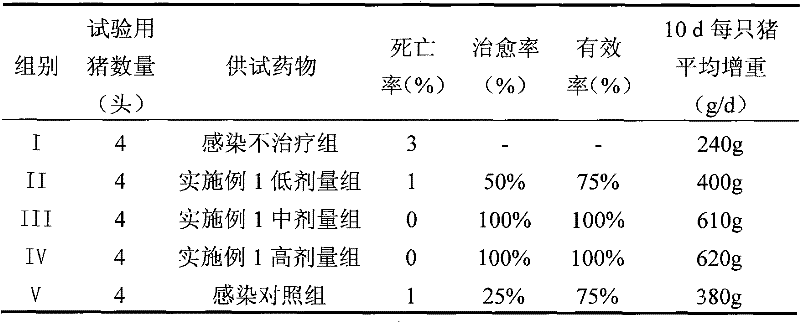
Stable vitamin C sustained release preparation and preparation method thereof
InactiveCN102579318AGuaranteed sustained release effectGuaranteed uniformityOrganic active ingredientsMetabolism disorderVitamin CPatient compliance
The invention relates to a stable vitamin C sustained release preparation and a preparation method thereof. The vitamin C sustained release preparation comprises vitamin C, an antioxidant synergist and a metal ion chelating agent. The stable vitamin C sustained release preparation has the advantages of high stability, insensitivity to change of external factors, long-acting sustained release and capabilities of uniformly, stably and gradually releasing vitamin C, the medication frequency can be reduced and bioavailability and patient compliance of the medicament can be improved. In addition, the preparation method disclosed by the invention has rational process design, is convenient to operate and is suitable for large-scale industrial production.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Cultivation method of double cropping rice
InactiveCN108012787APromote high and stable productionAvoid pollutionCereal cultivationHorticulture methodsNormal growthSoil quality
The invention discloses a cultivation method of double cropping rice. According to the cultivation method, the balanced fertilization is carried out according to a fertilization formula so as to meetthe fertility requirement of the double cropping rice, and the wasting of a fertilizer is avoided; a cultivation environment condition which is suitable for the normal growth and development of the double cropping rice and adverse to the occurrence and harm of diseases and pests is created, an insecticidal lamp is arranged for attracting and killing the pests, the selection treatment and the simultaneous treatment are grasped, and the medication area and the medication frequency are reduced. According to the technical scheme of the cultivation, a standard cultivation technique for cultivatingthe double cropping rice is established; and meanwhile, a total straw return manner is adopted during the harvesting of double cropping early-second rice, the double cropping early-second rice resources are reasonable utilized, and the straws are circularly utilized on site, so that the labor amount is reduced, the environmental pollution and soil hardening caused by the burning of the straws aresimultaneously avoided, the soil is improved, the soil fertility is reasonably cultured, the combined application amount of a chemical fertilizer is reduced, the soil quality is improved, and the highand stable yield of the double cropping early-second rice are promoted.
Owner:侯志平
Compound doxycycline hyclate suspension injection and preparation method thereof
InactiveCN102440998AFacilitated releaseHigh speedAntibacterial agentsTetracycline active ingredientsDoxycycline HyclateAntioxidant
The invention discloses a compound doxycycline hyclate suspension injection and a preparation process thereof. The suspension injection is prepared by taking doxycycline hyclate and tilmicosin phosphate as main medicines and adding appropriate medical accessories. The compound doxycycline hyclate suspension injection comprises the following components: 5-20 percent (W / V) of doxycycline hyclate, 5-20 percent (W / V) of tilmicosin phosphate, 0.5-2 percent (W / V) of a suspending aid, 0.01-0.2 percent (W / V) of an antioxidant, 0.1-2 percent (V / V) percent of a thickening agent and injection oil which supplements the balance to reach 100 percent. According to the invention, the doxycycline hyclate and the tilmicosin phosphate are evenly suspended into a solvent by a special process, and therefore the compound doxycycline hyclate suspension injection has good dispersibility and high bioavailability. Meanwhile, a feasible approach is provided for broadened production, and the advantages of increasing medical efficacy, reducing medication frequency and saving manpower and material resources are achieved.
Owner:TIANJIN RINGPU BIO TECH
Polyethylene glycol modified human serum albumin and preparation method thereof
ActiveCN101709085AExtended half-lifeAlleviate source tensionSerum albuminPeptide preparation methodsMonomethoxypolyethylene glycolHalf-life
The invention relates to the biomedical technical field, in particular to polyethylene glycol modified human serum albumin and a preparation method thereof. The polyethylene glycol modified human serum albumin comprises sulfydryl of 34-bit cysteine residues on albumin molecules and N-terminal amino or free amino group combined polyethylene glycol. Modifiers adopted by the fixed-point single modification are respectively polyethylene glycol-maleimide and polyethylene glycol-propionaldehyde; modifiers adopted by the free amino group random modification are respectively polyethylene glycol-succinimide active ester or methoxy-polyethylene glycol activated by cyanuric chloride; and the modification rate of the free amino group on the albumin molecules combined with the polyethylene glycol is 3%-7%. Compared with the natural albumin, the polyethylene glycol modified human serum albumin has the excellent characteristics of prolonged half-life period, slow plasma elimination, and the like and is beneficial to reducing the medication frequency and releasing the rare albumin source.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD +1
Cleaning and anti-scaling process without shut-down of gas-gas heater (GGH) and device thereof
The invention discloses a cleaning and anti-scaling process without shut-down of a gas-gas heater (GGH). A GGH system is provided with an on-line cleaning device and a high-pressure flusher, the on-line cleaning device comprises a medicating device and a medicine spraying device, the output end of the medicating device is connected with the medicine spraying device, and the high-pressure flusher comprises a high-pressure water outlet and a high-pressure water spraying device connected through a high-pressure water pipe. The process includes: the surface of a heating element is guaranteed to be moist, medication frequency is controlled, medicine pre-filling quantity and medicine spraying quantity are calculated, medicine enters to be sprayed for 5-30 minutes according to calculation quantity, an interval is 5-30 minutes after medicine spraying is finished, the medicine spraying device is used for supplementing water, water supplement is performed for 1-5 times continuously, the high-pressure washer is started, washing is performed for 30-60 minutes, the water supplement quantity is 0.01-0.5 time of the total quantity of medicine, and the medication frequency is controlled in 5-20 days.
Owner:江苏肯创环境科技股份有限公司
Biological pesticide suitable for strawberry powdery mildew and preparation method of biological pesticide
InactiveCN104872200AGood function and effectEasy to prepareBiocideFungicidesTripterospermum taiwanenseMedication frequency
The invention discloses a biological pesticide suitable for strawberry powdery mildew and a preparation method of the biological pesticide. Aiming at the characteristics of the strawberry powdery mildew and the action mechanisms of various biological agents and herb extracts, the biological agents with different efficacies and characteristics, such as trichoderma, allicin, bacillus subtilis, low-poly-D glucosamine hydrochloride, a thunder god vine extract and a tomatidine extract, are reasonably screened and compounded. The invention further discloses an effective preparation method. The prepared biological pesticide is good in action effect on strawberry powdery mildew, nontoxic, harmless, ecological and environmentally friendly; the use amount, the medication frequency and the medication cost are significantly reduced; and the biological pesticide is convenient to prepare, long in storage time and very high in practical value.
Owner:苏州市新泾村农业基地专业合作社
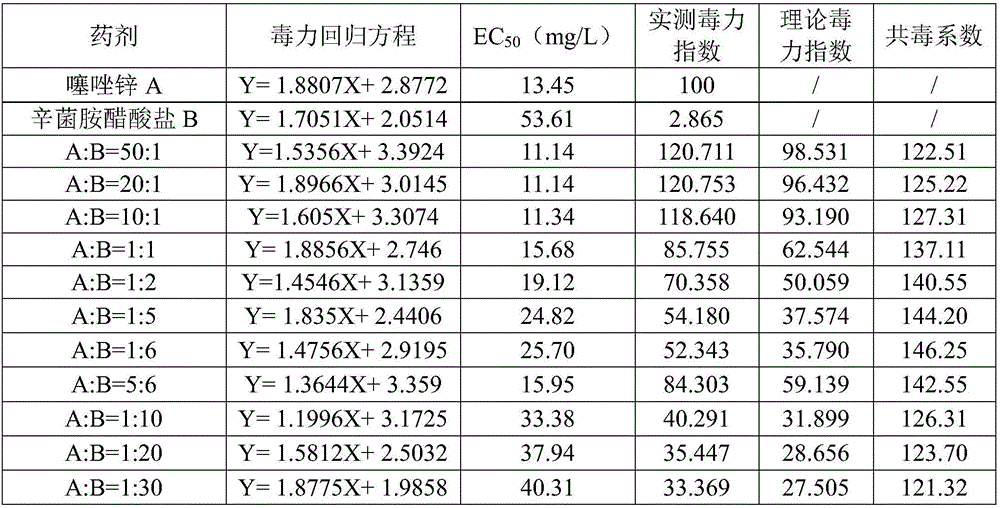
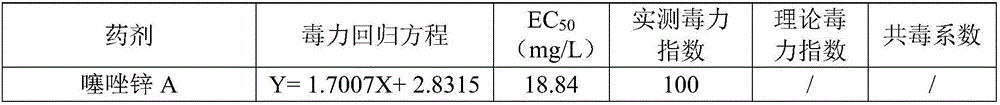
Composition containing zinc thiazole and xinjunan acetate, preparations and application thereof
InactiveCN106508919ASignificant synergyImprove the effect of prevention and controlBiocideDead animal preservationSuspending AgentsCanker
The invention discloses a composition containing zinc thiazole and xinjunan acetate, preparations and application thereof. The effective component of the bactericidal composition is compounded by zinc thiazole and xinjunan acetate, and the rest are auxiliary components, wherein the sum of the mass fraction of zinc thiazole and xinjunan acetate in the effective component of the bactericidal composition is 2%-90%. The dosage forms of the pesticide composition include suspending agents, wettable powder, water dispersible granules, and granules, etc., and the composition is mainly used for prevention and control of apple tree canker, pepper virus disease, citrus canker, tomato virus disease, bacterial canker of tomato, soft rot of Chinese cabbage, cotton blight and verticillium wilt, bacterial leaf streak, Erwinia chyrsanthemi, and rice bacterial leaf blight, etc. Mixing of zinc thiazole and xinjunan acetate can improve the control effect and expand the control spectrum, at the same time the medication frequency can be reduced, and the medication cost is lowered, thus reaching the effects of prevention first and comprehensive control.
Owner:JIANGSU XINNONG CHEM
Sinomenine hydrochloride slow-release injection and preparation method thereof
The invention discloses a sinomenine hydrochloride slow-release injection and a preparation method thereof. The slow-release injection comprises one of a synthetic high molecular polymer nanoparticle sinomenine hydrochloride slow-release injection, a natural high molecular material microsphere sinomenine hydrochloride slow-release injection, a liposome sinomenine hydrochloride slow-release injection, a multi-vesicle liposome sinomenine hydrochloride slow-release injection and a gel sinomenine hydrochloride slow-release injection. In the invention, various sinomenine hydrochloride slow-release injections prepared by different methods can prolong drug action time, reduce medication frequencies, maintain relatively stable in-vivo blood concentration and reduce toxic side effects of drugs.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
Fusion protein of human epidermal growth factor and metallothionein and preparation method and application thereof
The invention provides a fusion protein of a human epidermal growth factor and metallothionein, which comprises a first part and a second part, wherein the first part is the human epidermal growth factor, the second part is the human metallothionein, and the human metallothionein is positioned at the C terminal of the human epidermal growth factor. The invention further provides an application of the fusion protein in the preparation of drugs for treating burns or scalds or wounds or ulcers and the application of the fusion protein in the preparation of skin nursing drugs. Compared with the original human epidermal growth factor and the metallothionein, the fusion protein can increase the stability, reduce the clearance rate, reduce the degradation, bring benefits and convenience to the use of the EGF-MT fusion protein and further reduce the using dosage and the medication frequency. Simultaneously, the formed fusion protein is a bifunctional molecule which has double effects of EGF and MT.
Owner:SHANGHAI SIRUIBAO BIOTECH
Preparation method of puerarin sustained-release dropping pill
InactiveCN104622828AImprove Medication AdherenceReduce releaseOrganic active ingredientsSenses disorderWater bathsSide effect
The invention relates to a preparation method of a puerarin sustained-release dropping pill. The method comprises the following steps: (1) weighing hydrophobic matrix and hydrophilic matrix, fully melting and mixing evenly under a water bath heating condition, adding puerarin powder and mixing evenly; (2) starting the dropping pill, and preheating for 30 minutes; (3) starting a pill dropping machine stirring system and stirring for 10 minutes; (4) turning on a condensate liquid level adjusting knob and adjusting the dropping distance; (5) mounting a dripping head in a pill dropping machine; (6) dropping a liquid medicine into condensate; (7) collecting sustained-release dropping pills; and (8) sucking up condensate on the surfaces of the sustained-release dropping pills with filter paper and medical gauze, so as to obtain the puerarin sustained-release dropping pills. An optimal preparation technology of the novel puerarin sustained-release dropping pill is optimized; the targets of delaying drug release and reducing toxic and side effects can be reached; meanwhile, the medication frequency can be reduced; the medication compliance of patients is improved; the method has relatively large application value; and important reference is provided for pharmaceutical companies and clinical research and development.
Owner:TAIYUAN INST OF TECH
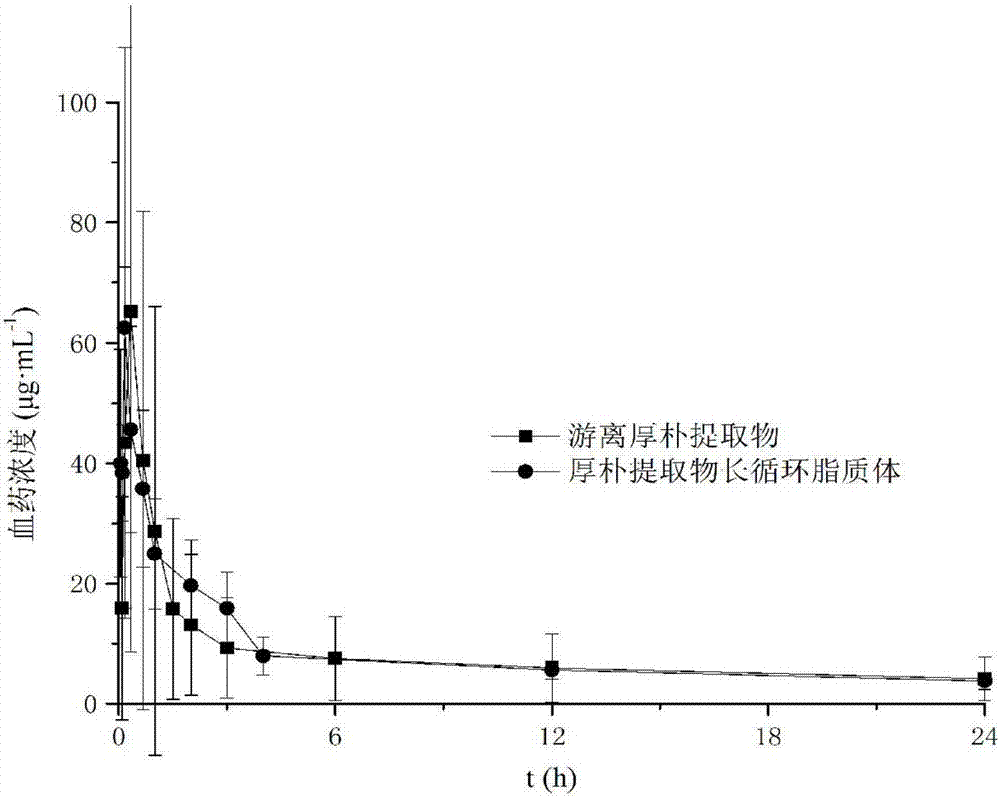
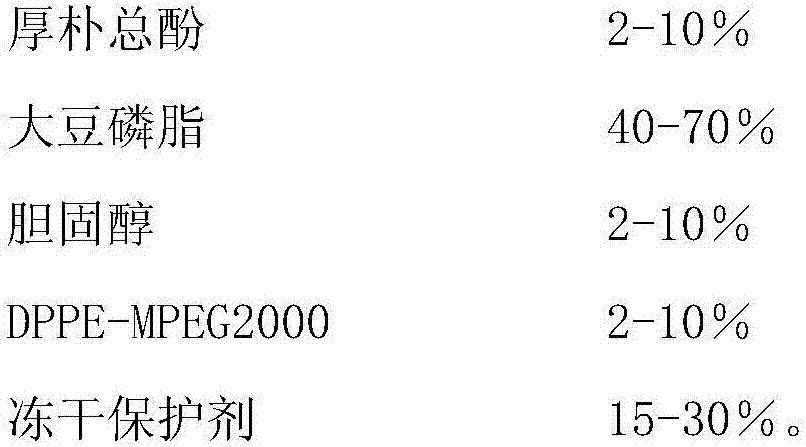
Long-circulating liposome freeze-drying oral preparation of magnolia cortex total phenol and preparation method of freeze-drying oral preparation
InactiveCN107998079AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPowder deliveryCholesterolRetention time
The invention discloses a long-circulating liposome freeze-drying oral preparation of magnolia cortex total phenol, which comprises the following ingredients by weight percentage: 2-10% of magnolia cortex total phenol, 40-70% of soybean lecithin, 2-10% of cholesterol, 2-10% of DPPE-MPEG (dipalmitoylphosphatidylethanolamine-methoxy polyethylene glycol) 2000 and 15-30% of freeze-drying protective agent. The liposome freeze-drying preparation of the magnolia cortex total phenol has a slow release effect in vitro; a medicine can be slowly released within 24h, so that the in-vivo retention time ofthe medicine is prolonged; and the medication frequency is reduced. A pharmacokinetic experiment result shows that the liposome freeze-drying preparation of the magnolia cortex total phenol has a higher blood concentration and a longer half life after intragastric administration; the medicine is absorbed quickly and eliminated slowly, is high in bioavailability and can serve as a long circulatingoral preparation; a new magnolia cortex total phenol dosage form that is safer and more effective and has a lasting effect is provided clinically.
Owner:HUBEI UNIV
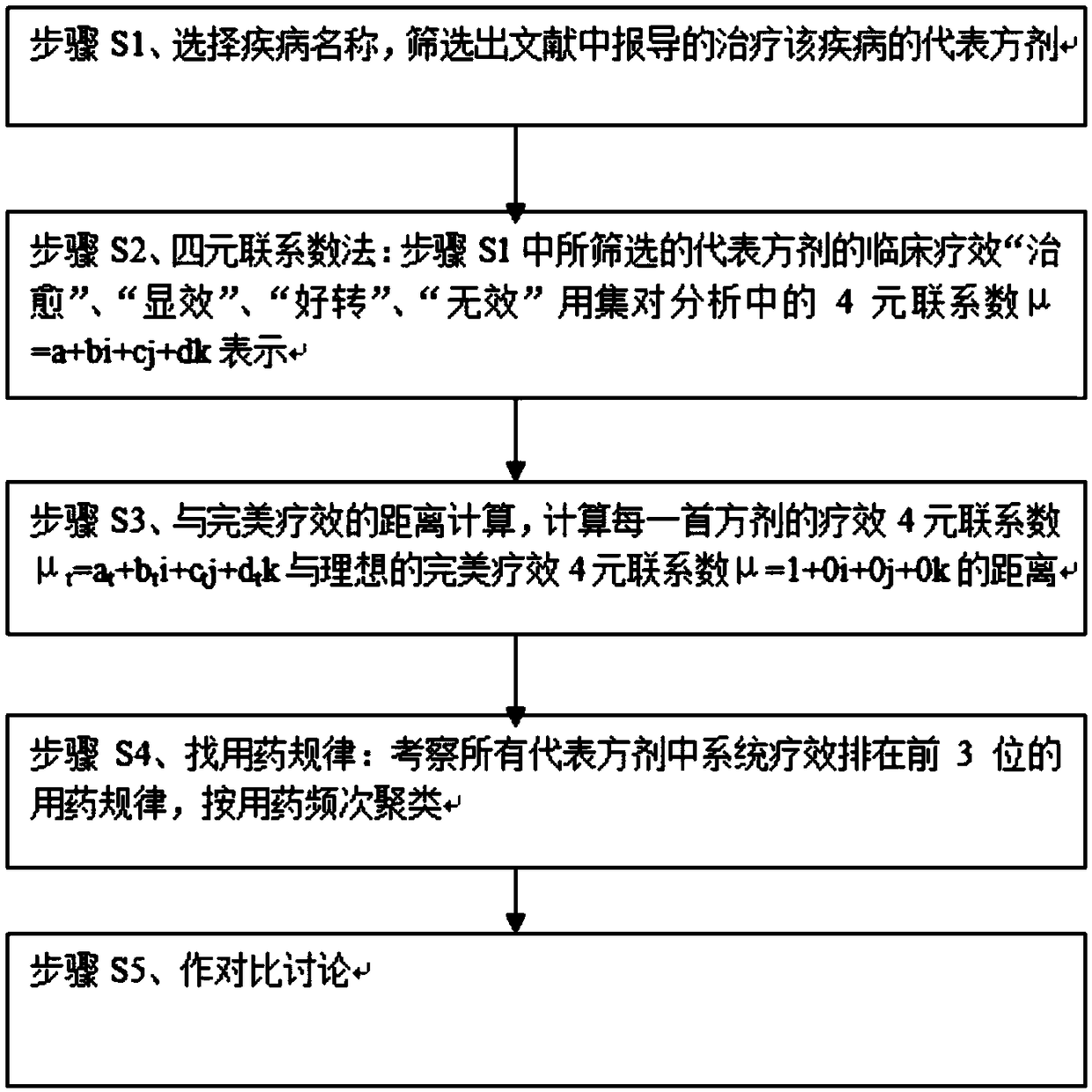
Application of set pair analysis in representative prescription use law research
InactiveCN109192320AGood curative effectImproving the Clinical Curative Effect of TCM Dialectical TherapyDrug referencesDiseaseClinical efficacy
The invention relates to application of set pair analysis in representative prescription use law research. The application includes the following steps that: step S1, the name of a disease is selected, representative prescriptions which are reported in literature and are used for treating the disease are screened out; step S2, a four-element connection number method is adopted: the clinical efficacies of the representative prescriptions screened out in the step S1, namely "cured", "significant effect", "improved" and " inefficient " are a four-element connection number which is expressed as anequation that Mu=a+bi+cj+dk in set pair analysis; step S3, a distance to a perfect efficacy is calculated: a distance between the four-element connection number of each prescription which is expressed as an equation that Mut=at+bti+ctj+dtk and the four-element connection number of the ideal perfect efficacy which is expressed as an equation that Mu=1+0i+0j+0k; and step 4, medication laws are searched: the medication laws of some prescriptions in all the representative prescriptions are examined, wherein the system efficacies of the certain prescriptions rank first three places, and the prescriptions are clustered according to medication frequencies; and step 5, comparative discussion is carried out. With the application of the invention adopted, general medication laws in different syndrome differentiation and treatment prescriptions are explored; the efficacies of all the representative prescriptions are sequenced according to the merits and demerits of the representative prescriptions on the basis of total efficacy rates; and therefore, clinical efficacies can be improved.
Owner:YUEYANG INTEGRATED TRADITIONAL CHINESE & WESTERN MEDICINE HOSPITAL SHANGHAI UNIV OF CHINESE TRADITIONAL MEDICINE
Intelligent medicine box
ActiveCN109077929AAchieve recordRealize managementPharmaceutical containersMedical packagingMedicineComputer module
The invention discloses an intelligent medicine box. A bottom plate with a medicine outlet base is adopted, the medicine outlet base is provided with a photoelectric sensor for monitoring the medicinedischarge frequency, medicine discharge information data is processed by a microprocessor and then transmitted by a wireless communication module, and then recording, management and early warning forthe medication frequency are realized; a communicating component with a first limit position and a second limit position is adopted, the communicating component is communicated with an inner medicinebox and a medicine outlet at different limit positions respectively, then medicine can be accurately, quantitatively and manually discharged, the power consumption is low, and recording is accurate.The intelligent medicine box is used for intelligent management of chronic disease medication and is easy to operate, convenient to use and low in cost.
Owner:深圳市全逸鑫医药研究有限公司
Application of macrocyclic host molecule as medicinal solubilizing agent
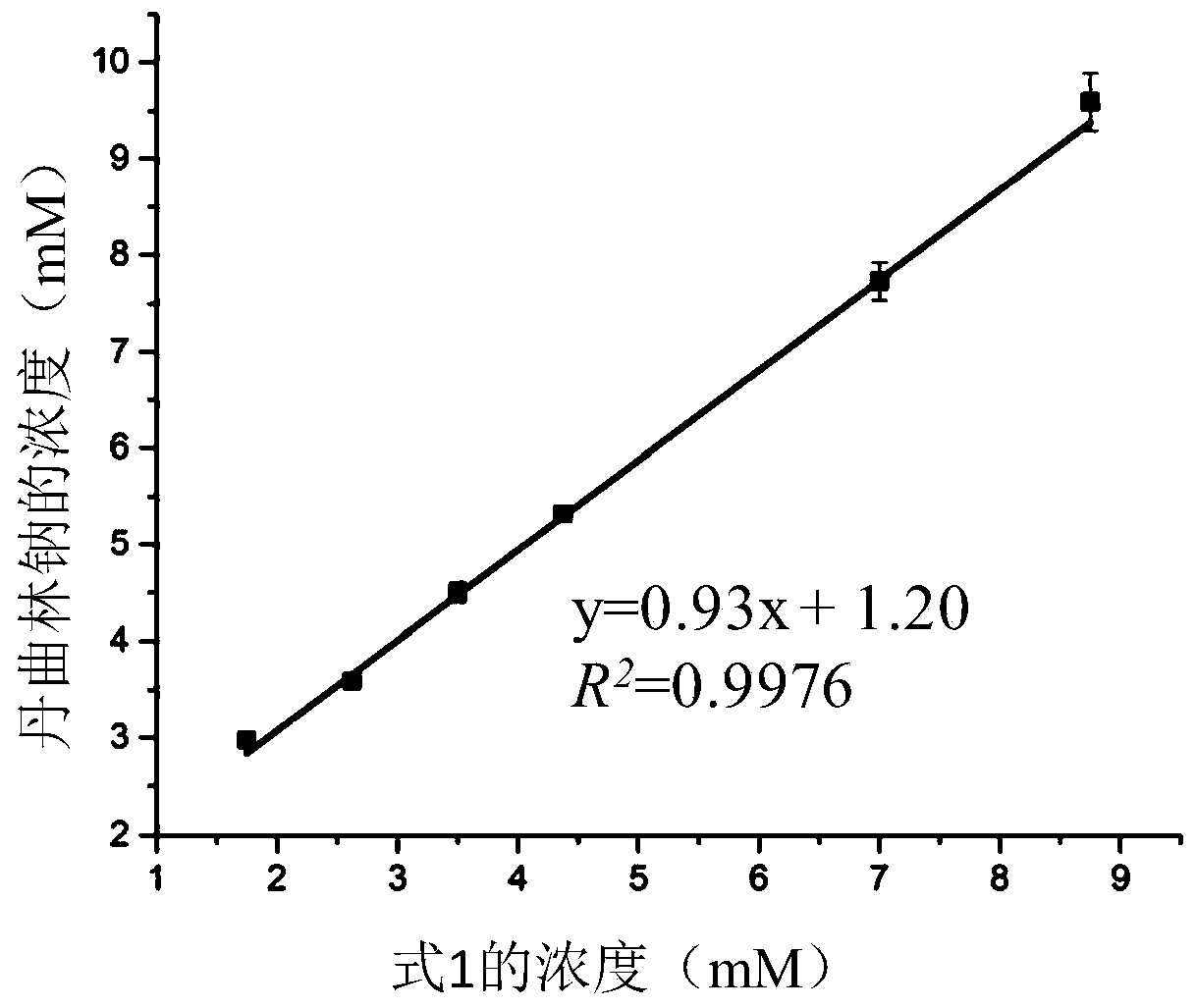
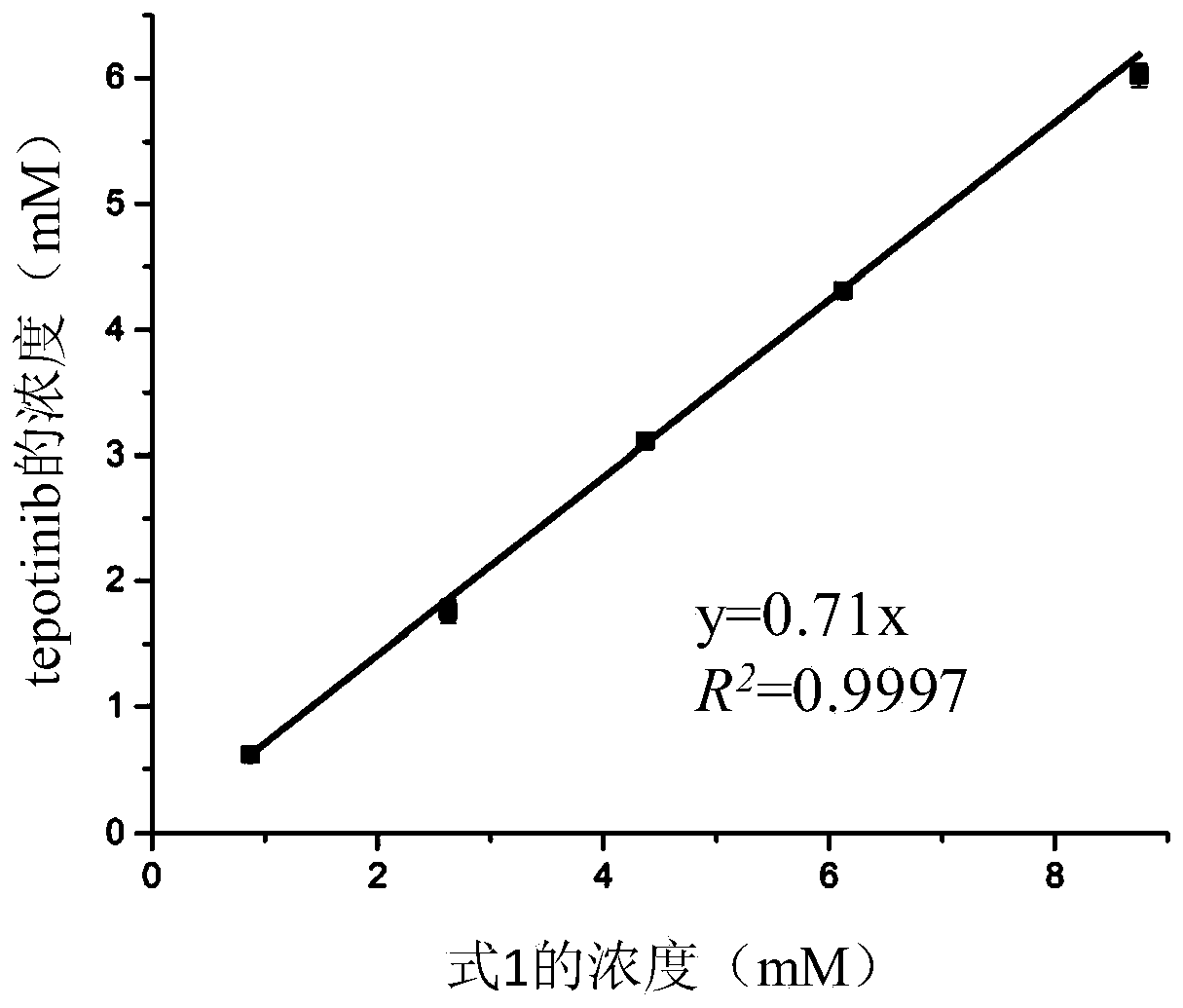
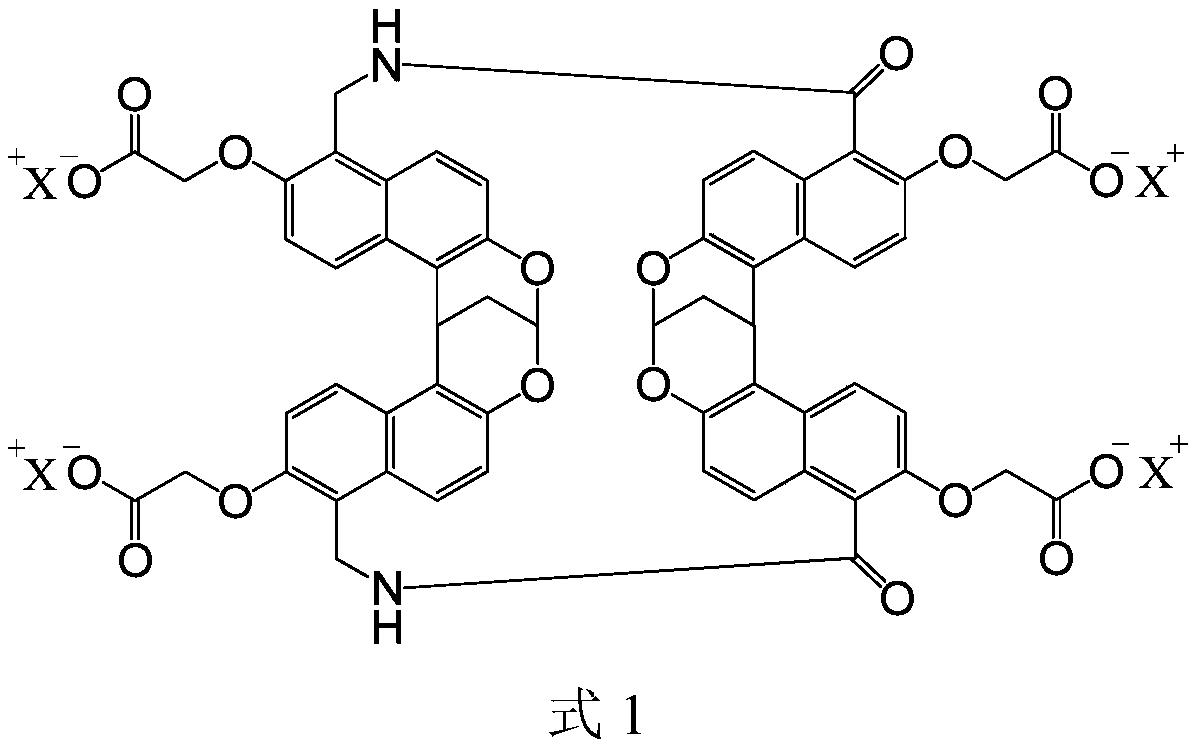
ActiveCN110384803AImprove solubilityIncrease blood concentrationPowder deliveryOrganic active ingredientsSolubilityBlood concentration
The invention relates to application of a macrocyclic host molecule as a medicinal solubilizing agent. The macrocyclic host molecule is provided with the structure shown in the formula 1 (please see the specification for the formula 1). The invention specifically further relates to a medicine solution. The medicine solution includes an active component and the solubilizing solvent, the active component includes dantrolene sodium or tepotinib, and the solubilizing solvent has the structure shown in the formula 1 (please see the specification for the formula 1). According to the application andthe medicine solution, the macrocyclic host molecule shown in the formula 1 with high solubility in water and low toxicity to cells is used, solubility of a medicine in a water phase can be significantly improved, thus blood concentration of the medicine is increased to reduce medication frequency, and the side effect does not exist.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Hydroxychloroquine sulfate and polyglutamic acid polymer, as well as preparation method and application thereof
InactiveCN105924641AHigh activityGood sustained release effectOrganic active ingredientsSkeletal disorderCross-linkSide effect
The invention discloses a hydroxychloroquine sulfate and polyglutamic acid polymer, as well as a preparation method and application thereof. The hydroxychloroquine sulfate and polyglutamic acid polymer is a polymer formed by carrying out esterification reaction between hydroxychloroquine sulfate and polyglutamic acid according to a weight ratio of 1:(1-6). The hydroxychloroquine sulfate and polyglutamic acid polymer is applied to treatment of discoid lupus erythematosus and systemic lupus erythematosus. The hydroxychloroquine sulfate and the polyglutamic acid are cross-linked to obtain the hydroxychloroquine sulfate and polyglutamic acid polymer, and hydroxyls of the hydroxychloroquine sulfate are modified to change certain original physiochemical properties thereof, improve pharmaceutical activity, enhance curative effects, reduce side effects, improve the sustained release effects of the drug and reduce the medication frequency of a patient; when the hydroxychloroquine sulfate and polyglutamic acid polymer is used for treating discoid lupus erythematosus and systemic lupus erythematosus, the drug can reach a destination lesion location under protection of the polyglutamic acid in a slow degradation process, so that the aim of accurate treatment is fulfilled, and the hydroxychloroquine sulfate and polyglutamic acid polymer has a certain clinical application value.
Owner:陕西省生物农业研究所
Imidacloprid and buprofezin compound pesticide
InactiveCN103222473AEffective expansionResidue reductionBiocideAnimal repellantsPesticide residueBuprofezin
The invention discloses an imidacloprid and buprofezin compound pesticide which is characterized in that effective components of the pesticide are imidacloprid and buprofezin. The imidacloprid and buprofezin compound pesticide provided by the invention has advantages as follows: insecticidal spectrum can be effectively expanded, the control range is increased, a plurality of insects can be controlled by one-time medication, and generation of resistance can also be decreased. In addition, persistent period can be prolonged, medication frequency can be minimized, agricultural cost is reduced, and pesticide residue of crops is lowered.
Owner:NANTONG JINLING AGROCHEM
Pharmaceutical composition containing colistin sulfate and preparation method of pharmaceutical composition
InactiveCN104721809AProlong the action timeStable blood concentrationAntibacterial agentsDigestive systemBlood concentrationColistin Sulfate
The invention relates to a pharmaceutical composition containing colistin sulfate and a preparation method of the pharmaceutical composition. The pharmaceutical composition containing colistin sulfate comprises the following components in parts by weight: 10-50 parts of colistin sulfate and 50-90 parts of an embedding medium. According to a colistin sulfate micro-capsule, the problems that the traditional colistin sulfate powder is poor in palatability, is easy to block, and not easily and evenly mixed with a feed are solved; the colistin sulfate provided by the invention is combined with the embedding medium through a molecular state; the action time of the colistin sulfate is prolonged by the sustained-release framework property of the embedding medium; the blood concentration is stable; the medication frequency is reduced; the medication cost of a farmer is reduced; and the economic benefits are improved.
Owner:JINHE ANIMAL PHARMA
Veterinary long-acting analgin injection and preparation method thereof
ActiveCN107028877AReduce human stressPromote recoveryOrganic active ingredientsAntipyreticRetention timeVeterinary Drugs
The invention relates to a veterinary long-acting analgin injection and a preparation method thereof and belongs to the technical field of veterinary drug preparations. Each 1000L of the injection contains the following components by weight: nearly 200kg-400kg of injection level analgin, 50kg-150kg of PVP-K30, 1kg-5kg of sodium hydrogen sulfite, 50kg-150kg of propylene glycol and the balance of fresh prepared injection water. Compared with a national standard analgin injection, the veterinary long-acting analgin injection has the advantages that the effectively drug peak concentration retention time is doubled, the fever-abatement pesticide effect is prolonged and durable, the medication frequency is reduced, the human stress caused to diseased pigs is reduced, the recovery of the diseased pigs is promoted, and the analgin injection is stable in quality and unlikely to oxidize. Meanwhile, specific auxiliary materials, controlled release agents and production processes are selected according to requirements of a vein injection, and the prepared analgin injection can be used for carrying out intravenous injection and has a good effect on treating hyperpyrexia of sows when being used in cooperation with cephalosporin antibiotics.
Owner:浙江大飞龙动物保健品股份有限公司
A kind of monosialotetrahexosyl ganglioside sodium sustained-release capsule and preparation method thereof
ActiveCN106309410BImprove stabilitySimple methodOrganic active ingredientsNervous disorderSustained Release CapsuleMedication frequency
The invention provides a monosialotetrahexosylganglioside sodium slow-release capsule and a preparation method thereof. The slow-release capsule is composed of a capsule filler and a capsule shell. The capsule filler is mainly composed of the following components in parts by weight: 10-30 parts of monosialotetrahexosylganglioside sodium, 10-20 parts of transesterified polyglycerol ricinoleate, 10-15 parts of methyl poly-L-hydroxyethylacrylate, 10-15 parts of hydroxypropyl methylcellulose, 5-8 parts of guar gum and 5-10 parts of chitosan. The capsule provided by the invention is convenient for oral administration, can effectively enhance the stability of monosialotetrahexosylganglioside sodium in the gastrointestinal tract, can ensure the regular slow constant-speed release of monosialotetrahexosylganglioside sodium, effectively prevents drugs from being quickly decomposed in the gastrointestinal tract, and keeps the drugs in the organism at a relatively constant concentration. The slow-release capsule can effectively reduce the medication frequency, reduces the pains of the patient, and enhances the drug effects.
Owner:赛隆药业集团股份有限公司
Combined CTL antigenic epitope and its application
ActiveCN103254313AImprove complianceExtended half-lifeAntibody medical ingredientsHybrid peptidesSolubilityPatient compliance
The invention relates to the field of immunology, in particular to a combined antigenic epitope of tumor antigen, and also relates to application of the combined antigenic epitope. The combined CTL (cytotoxic T lymphocyte) antigenic epitope contains at least one polypeptide selected from SEQ ID NOS:1-3. The derivative is formed through modification of the epitope peptide by polyethylene glycol (PEG). After modification by PEG, the polypeptide has a longer half-life period, a low maximum plasma concentration, small plasma concentration fluctuation, less enzymolysis, less immunogenicity and antigenicity, less toxicity, better solubility, reduced medication frequency, and can improve patient compliance, improve the quality of life, and reduce the cost of treatment. A multi-epitope vaccine can stimulate human peripheral blood mononuclear T cells, can strongly induce the generation of antigen specific CTL, detects the ability of secreting functional cytokine IFN-gamma, and can cause specific dissolution breaking of a target cell in a target cell co-culture process. Thus, the multi-epitope vaccine is expected to become a vaccine for treatment of tumors.
Owner:ARMY MEDICAL UNIV
Burst release-free irinotecan microsphere and preparation method thereof
InactiveCN104352449AUniform appearanceRelieve painOrganic active ingredientsPharmaceutical non-active ingredientsAcetic acidSide effect
The invention discloses a burst release-free irinotecan microsphere and a preparation method thereof. The irinotecan microsphere comprises irinotecan and a polylactic acid-glycolic acid copolymer (poly(lactic-co-glycolicacid), PLGA), wherein the weight ratio of the irinotecan to the PLGA is 1 to (10-40), and the grain size of the irinotecan microsphere is 1-5 micrometers. A long-acting burst release-free irinotecan microsphere can be prepared from the irinotecan and the PLGA at a specific ratio, and the slow release period of the irinotecan microsphere is longer than 40 days, so the medication frequency is obviously reduced, a peak and valley phenomenon of the medicine is reduced, the bioavailability of the irinotecan is improved and toxic and side effects of the medicine are reduced.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Traditional Chinese medicine formula for lactational acute mastitis
InactiveCN108743850AEnhance antipyretic effectImprove the effect of treating diseasesUnknown materialsSexual disorderDiseaseHerb medicine
The invention discloses a traditional Chinese medicine formula for lactational acute mastitis. The formula comprises 9 to 12g of trichosanthes hisrilowii, 9 to 12g of fructus arctii, 6 to 12g of radixbupleuri, 6 to 10g of pericarpium citri reticulatae viride, 9 to 12g of nepeta cataria, 15 to 30g of taraxacum mongolicum, 15 to 30g of lonicera japonica, 9 to 12g of vaccariae semen, fructus liquidambaris, 9 to 12g of radix rhapontici, 9 to 12g of cornu cervi, 12 to 15g of roasted malt, 12 to 15g of roasted rice sprout, and 3 to 6g of radix glycyrrhizae. The formula has the advantages that application of drugs for relieving exterior syndrome is enhanced, meanwhile herbal medicine is decocted through a short-time decoction method, medication frequency and dose are adjusted according to the variation of the body temperature of a patient on the premise of closely observing the body temperature, the efficacy of allaying fever is enhanced during treatment, the effect of treating diseases is improved, and Western medicine antibiotics and antifebric are replaced by the formula to treat lactational acute mastitis.
Owner:蔡国英
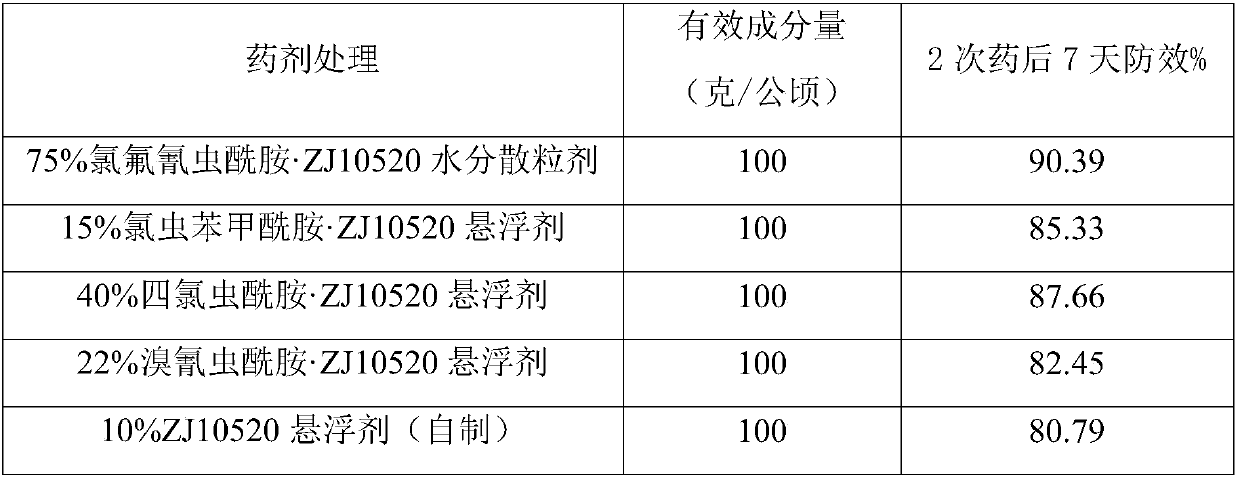
Composition with ZJ10520 and ryanodine receptor insecticide
ActiveCN107616176AMeet security requirementsAchieve bactericidalBiocideFungicidesSKELETAL MUSCLE RYANODINE RECEPTORMedication cost
The invention discloses a bactericidal and insecticidal composition with ZJ10520 and ryanodine receptor insecticide. The bactericidal and insecticidal composition has the advantages that good bactericidal and insecticidal effects can be realized by the bactericidal and insecticidal composition, the bactericidal and insecticidal composition is low in medication frequency and medication cost, and the safety requirements on pesticide preparations can be met.
Owner:ZHEJIANG RES INST OF CHEM IND CO LTD +2
Monosialotetrahexosylganglioside sodium slow-release capsule and preparation method thereof
ActiveCN106309410AImprove stabilitySimple methodOrganic active ingredientsNervous disorderOral medicationSustained Release Capsule
The invention provides a monosialotetrahexosylganglioside sodium slow-release capsule and a preparation method thereof. The slow-release capsule is composed of a capsule filler and a capsule shell. The capsule filler is mainly composed of the following components in parts by weight: 10-30 parts of monosialotetrahexosylganglioside sodium, 10-20 parts of transesterified polyglycerol ricinoleate, 10-15 parts of methyl poly-L-hydroxyethylacrylate, 10-15 parts of hydroxypropyl methylcellulose, 5-8 parts of guar gum and 5-10 parts of chitosan. The capsule provided by the invention is convenient for oral administration, can effectively enhance the stability of monosialotetrahexosylganglioside sodium in the gastrointestinal tract, can ensure the regular slow constant-speed release of monosialotetrahexosylganglioside sodium, effectively prevents drugs from being quickly decomposed in the gastrointestinal tract, and keeps the drugs in the organism at a relatively constant concentration. The slow-release capsule can effectively reduce the medication frequency, reduces the pains of the patient, and enhances the drug effects.
Owner:赛隆药业集团股份有限公司
Pharmaceutical composition for treating pigeon body psoroptic mange and preparation method of pharmaceutical composition
InactiveCN104815217AImprove immunityImprove disease resistanceAntiparasitic agentsPlant ingredientsDiseasePolygonum aviculare
The invention discloses a pharmaceutical composition for treating pigeon body psoroptic mange and a preparation method of the pharmaceutical composition. Raw materials of the pharmaceutical composition for treating the pigeon body psoroptic mange comprise cortex meliae, garlic, ox's thyroid gland, aloe, lilac daphne flower bud, sweet vernal grass, bambusa glaucophylla, polygonum aviculare, fructus evodiae, thelephora vialis, scirpus subcapitatus, lythrum, ailanthus altissima, diuranthera major, lycopodium phlegmaria, red-knees herb, radish leaves, lysimachia foenum-graecum, costustoot, turbinate aster, rhizoma anemarrhenae, rhizoma phragmitis, draba nemorosa, root of Chinese trichosanthes, glabrous greenbrier rhizome, dayflower, medicago lupulina, dandelion, sesbania, japanese violet herb or root, scandent schefflera stem and leaf and edible tulip. The pharmaceutical composition for treating the pigeon body psoroptic mange has the beneficial effects that the pharmaceutical composition has the effects of clearing away heat and toxic materials and expelling parasites and is mainly used for treating pigeon body psoroptic mange of pigeons 2-3 years in age, so that immunity of the pigeons is improved, and disease resistance of the pigeons can alsobe improved; the advantages of strong targeted property, obvious curative effect, difficulty in occurrence, short medication time interval, low medication frequency and no toxic or side effect are realized, and hatchability and laying rate of female pigeons can be well guaranteed.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Profenofos microcapsule suspension concentrate and preparation method thereof
InactiveCN102210323AReduce pollutionImprove efficiencyBiocideAnimal repellantsAnti freezingOrganic solvent
The invention relates to a profenofos microcapsule suspension concentrate which comprises the following components by weight percent: 5-15% of profenofos, 1-2% of solvent, 1.5-2.5% of capsule wall material, 3-5% of wetting dispersant, 2-3% of anti-freezing agent, 0.3-1% of thickening agent, 0.1-0.5% of organic silicon defoaming agent, 1-3% of curing agent and the balance of water, wherein the total weight percent is 100%. The water is used for replacing a large quantity of organic solvents, the aromatic organic solvent is not used or the usage amount of the aromatic organic solvent can be reduced as far as possible, on the one hand, the environmental pollution is reduced, and the profenofos microcapsule suspension concentrate is safe to operation staff, on the other hand, the valid period is prolonged, the medication frequency and the medication quantity can be reduced, and the using efficiency of agricultural chemicals can be improved.
Owner:SOUTHWEST UNIV
Herbicidal composition, preparation and application thereof
InactiveCN106538576AImprove closureStrengthen the killing effect of stems and leavesBiocideAnimal repellantsChemical compositionPhytotoxicity
The invention discloses a herbicidal composition. The herbicidal composition contains active ingredients isoproturon, tralkoxydim and florasulam in a mass ratio of 0.1-90:0.1-80:0.1-50, preferably 5-80:1-60:0.2-20, most preferably 5-60:5-50:0.2-10. A herbicidal composition preparation includes the herbicidal composition and a preparation accessory, by mass percentage, the herbicidal composition accounts for 1-90% of the total amount, preferably 5-75%. The herbicidal composition provided by the invention has the advantages of: 1. high safety to wheat; 2. weeding spectrum, comprehensive killing of various weeds including Gramineae and broadleaf weeds; 3. good weeding effect, and high control effect on wheat and barley field weed; 4. simple and convenient use, soil sealing treatment after wheat sowing or postemergence stem and leaf treatment of weeds; 5. environmental friendliness, difficult drifting, and environmental safety; and 6. quick effect and durable effect, reduction of medication frequency, lowering of work amount and production cost, decrease of phytotoxicity risk, and delaying of weed resistance to herbicides.
Owner:杜金河
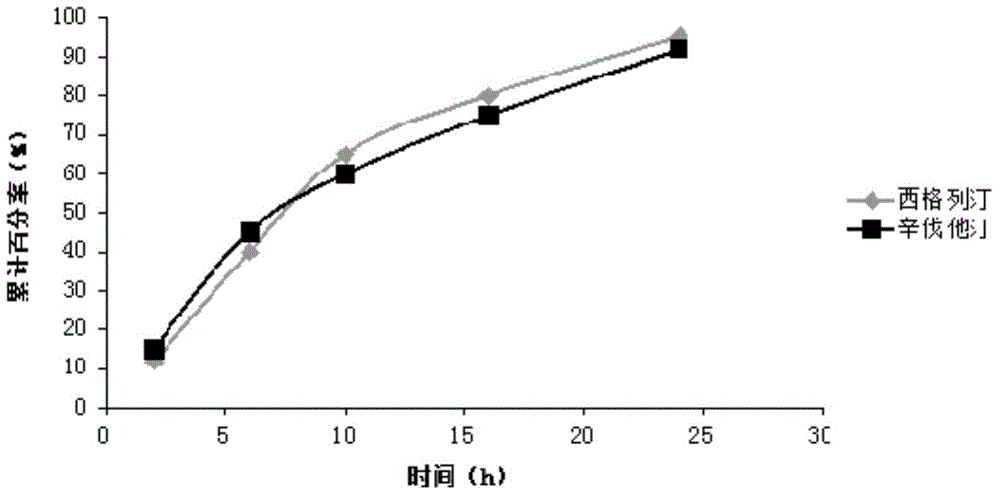
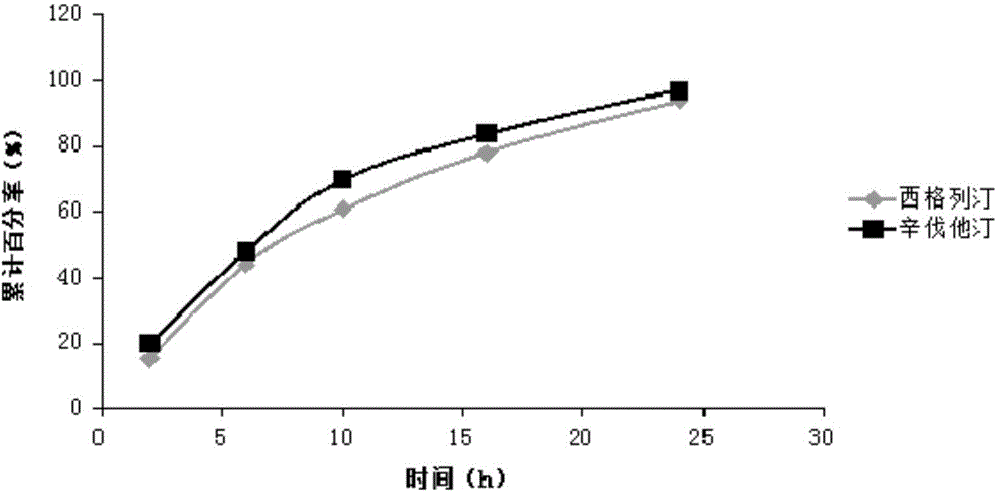
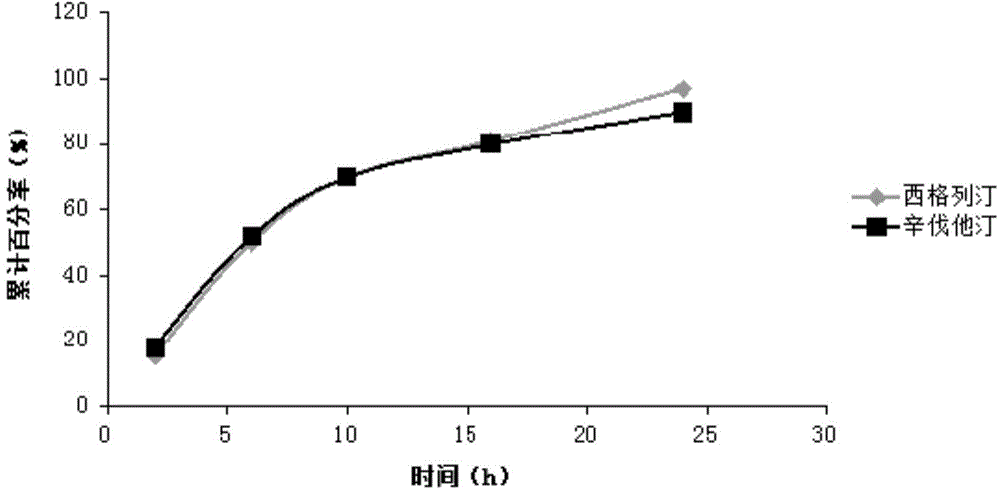
Sustained-release composition of sitagliptin and simvastatin
InactiveCN104473929AThe prescription process is simpleSmall toxicityOrganic active ingredientsMetabolism disorderSustained release pelletsSitagliptin
The invention belongs to the field of medicine preparation science, and particularly relates to a sustained-release composition of sitagliptin and simvastatin, a preparation method of the sustained-release composition, and a sustained-release preparation of sitagliptin and simvastatin. The sustained-release composition of the sitagliptin and the simvastatin disclosed by the invention is composed of a sitagliptin coated pellet and a simvastatin sustained-release pellet at the mass ratio of (10:1) to (1:1). The sustained-release composition of the sitagliptin and the simvastatin is simple in prescription and process; the sitagliptin and the simvastatin can be slowly released within 24 hours; the sitagliptin and the simvastatin always work together, so that the plasma drug concentration can be well stabilized; the peak valley phenomenon of the plasma drug concentration is avoided; the toxic and side effects on a human body caused by the medicine are reduced; the bioavailability of the medicine is improved; the medication frequency is reduced; and the compliance of a patient is improved. The preparation method of the sustained-release composition of the sitagliptin and the simvastatin disclosed by the invention is simple to operate, and is suitable for industrialized production.
Owner:HYBIO PHARMA
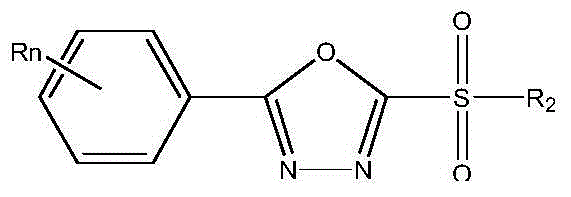
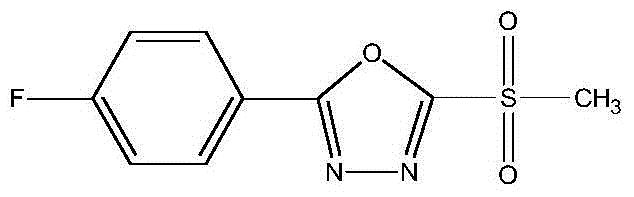
Compound composition containing methanesulfonyl bacteria oxazole and preparation
ActiveCN104542624AGood synergyDelay drug resistanceBiocideOrganic chemistryAvermectinActive component
The invention discloses a compound composition and a preparation. The compound composition contains two active components; the first active component is methanesulfonyl bacteria oxazole; the second active component is selected from any one of fosthiazate, abamectin and metaldehyde; the chemical name of the methanesulfonyl bacteria oxazole is 2-(p-fluorophenyl)-5-methylsulfonyl-1,3,4-oxadiazole. Compared with a single dosage, the compound composition and the preparation thereof disclosed by the invention have obvious synergistic effects; the prevention and treatment effects on crop diseases and pests are prevented and treated; the use of the pesticide is reduced; the cost is reduced; the lasting period is long; the medication frequency is reduced; and environmental pollution is facilitated.
Owner:GAUNGXI TIANYUAN BIOCHEM
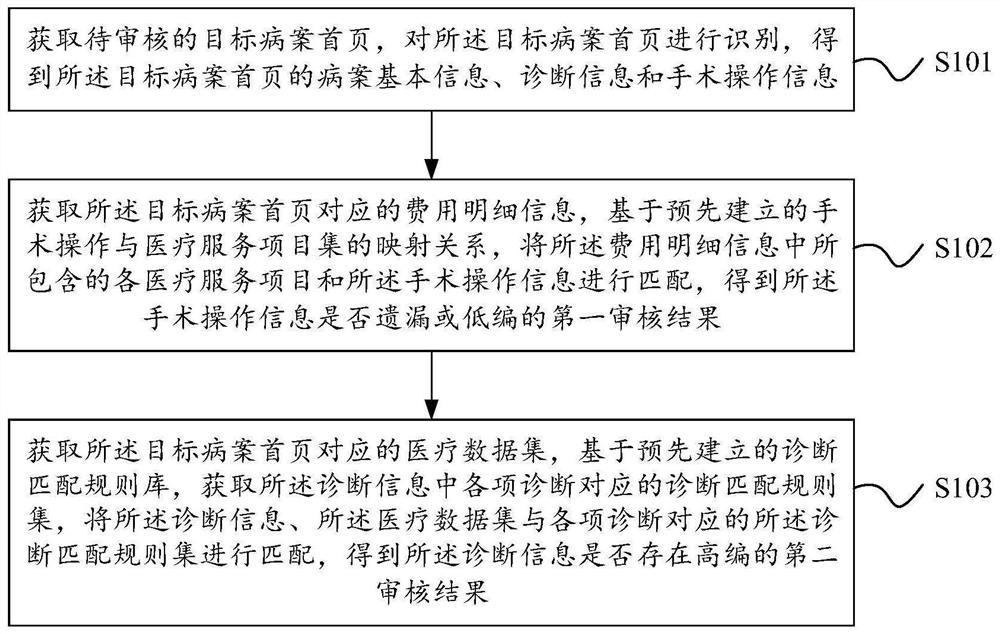
Method, device and equipment for reasonably controlling fees in medical process
PendingCN114141340AAvoid over-medicationLow costDrug and medicationsHealthcare resources and facilitiesMedical recordSurgical operation
The embodiment of the invention discloses a method, device and equipment for reasonably controlling fees in a medical treatment process, and the method comprises the steps: carrying out the statistics of the use conditions of medicines of all diseases based on a big data technology, and obtaining the use frequencies of all medicines of all diseases, adding the drugs with the use frequency exceeding the drug use frequency threshold value to a necessary drug catalog of each disease; performing prediction grouping according to the diagnosis information of the current medical record and the surgical operation information to obtain a predicted disease type; and acquiring a medication instruction of a doctor, judging whether each target drug in the medication instruction is included in the necessary drug catalog corresponding to the predicted disease category, and if not, acquiring the medication frequency of the target drug to generate first prompt information. By the adoption of the mode, the medication condition of a doctor can be restrained, excessive medical treatment on a patient is avoided, and meanwhile the cost of a hospital is reduced.
Owner:深圳市康比特信息技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com