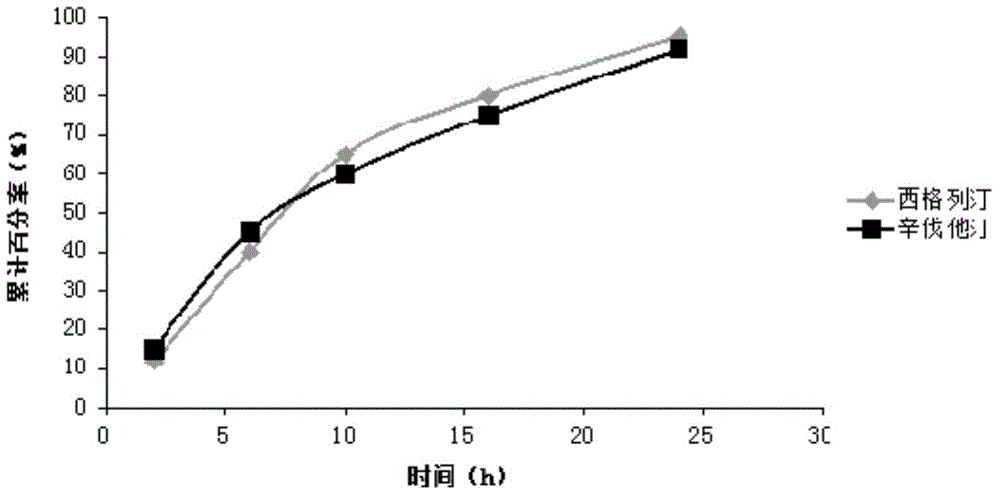
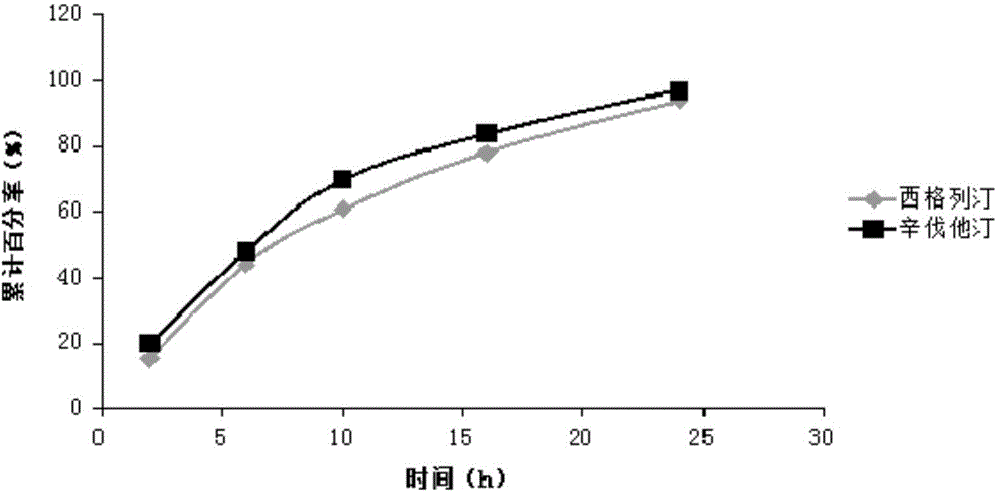
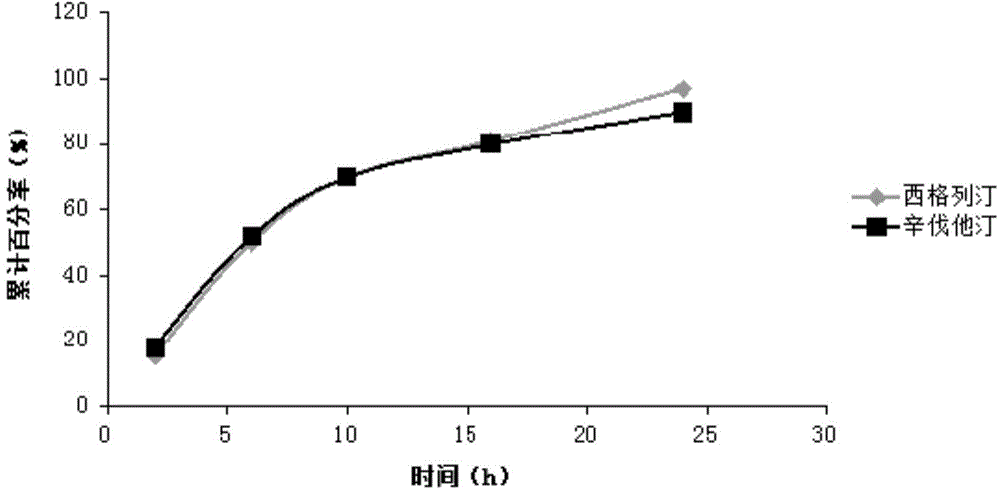
Sustained-release composition of sitagliptin and simvastatin
A slow-release composition, simvastatin technology, applied in drug combination, drug delivery, pill delivery, etc., can solve the problems of patients prone to adverse reactions to drugs, low bioavailability, frequent medication times, etc., to improve biological Utilization, stable blood drug concentration, and the effect of avoiding peak and valley phenomena
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] In some embodiments, the preparation method of the sitagliptin coated pellets specifically includes the following steps:
[0076] a. The sitagliptin and the filler are pulverized and sieved separately, mixed evenly and then mixed with the binder, and the sitagliptin-containing pellet core is prepared by the extrusion spheronization method.
[0077] B, the Surelease aqueous dispersion is mixed with water to prepare the Surelease aqueous dispersion coating solution;
[0078] c, get the ball core containing sitagliptin and place it in the fluidized bed, and coat the ball core containing sitagliptin with Surelease aqueous dispersion coating solution;
[0079] Wherein, step a and step b are in no particular order.
[0080] Wherein, in some preferred embodiments, in the preparation method of the sitagliptin-coated pellets, the sieving in step a is 100-mesh sieve.
[0081] In some preferred embodiments, in the preparation method of the sitagliptin coated pellets, the specifi...
Embodiment 1
[0097] Embodiment 1, sitagliptin simvastatin sustained-release capsules
[0098] (1) Pill core containing sitagliptin:
[0099] sitagliptin
50 copies
80 copies
200 copies
[0100] Coating solution:
[0101]
[0102] Grind sitagliptin, sucrose or lactose, and microcrystalline cellulose separately, pass through a 100-mesh sieve, mix evenly, use distilled water as a binder, and prepare by extrusion spheronization. The extrusion rate is 25rpm, and the spheronization speed is 2100rpm. The time is 5 minutes, the finished product is dried at 35°C, and the pellets between 700-900 μm are sieved to obtain the drug-containing pellets;
[0103] prescription amount Aqueous dispersion coating liquid, get above-mentioned drug-containing micropill and place in fluidized bed and carry out coating: fluidization temperature is 35 ℃, and fluidization pressure is 0.4MPa, and spray pressure is 0.15MPa, peristaltic p...
Embodiment 2
[0109] Embodiment 2, sitagliptin simvastatin sustained-release capsules
[0110] (1) Pill core containing sitagliptin:
[0111] sitagliptin
75 copies
100 copies
175 copies
[0112] Coating solution:
[0113]
[0114]Grind sitagliptin, sucrose or lactose, and microcrystalline cellulose separately, pass through a 100-mesh sieve, mix evenly, use distilled water as a binder, and prepare by extrusion spheronization. The extrusion rate is 25rpm, and the spheronization speed is 2300rpm. The time is 6 minutes, the finished product is dried at 40°C, and the pellets between 700-900 μm are screened to obtain the drug-containing pellets;
[0115] prescription amount Aqueous dispersion coating solution, get above-mentioned drug-containing micropill and place in fluidized bed and carry out coating: fluidization temperature is 37 ℃, and fluidization pressure is 0.35MPa, and spray pressure is 0.20MPa, peristal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com