Preparation method of puerarin sustained-release dropping pill
A technology for sustained-release and sustained-release dropping pills of puerarin, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. problems such as high irritation, to achieve broad development and application prospects, great clinical application value, and the effect of improving medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
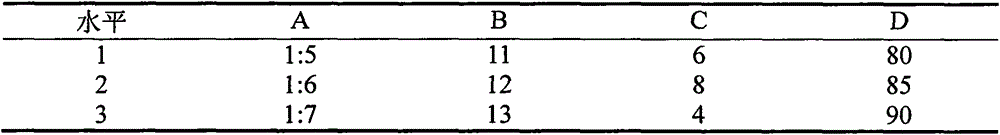
[0027] The preparation method of the puerarin sustained-release dropping pills in the present embodiment, its concrete steps are as follows:
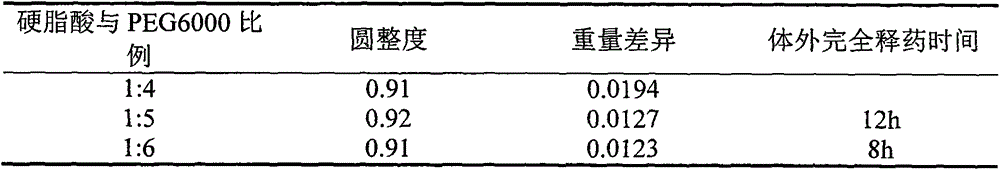
[0028] (1) Precisely weigh the hydrophobic matrix and the hydrophilic matrix according to the proportion, fully melt and mix them under the condition of heating in a water bath, then add puerarin powder according to the proportion, stir continuously for 30 minutes under the condition of heating, and fully mix evenly;
[0029] (2) Start the dripping pill machine, preheat for 30 minutes, set the temperature of the feed liquid in the instrument, the temperature of the chassis of the feed filling and the condensation temperature or the cooling temperature, and start the control buttons of each temperature to make it reach the displayed value; the temperature of the feed liquid and the temperature of the chassis should be consistent;
[0030] (3) Start the stirring system of the dripping pill machine, control the stirring speed, enter the fe...
Embodiment 2
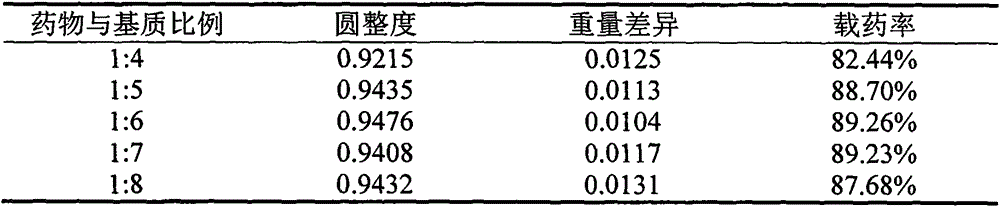
[0065] Under the optimal preparation process conditions in Example 1, three batches of puerarin sustained-release dripping pills were respectively prepared, the batch numbers were: 14042501, 14042502, 14042503, and the roundness, weight difference, and drug loading rate were used as investigation indicators. [17] , for investigation, the results are shown in Table 6.
[0066] Table 6 Determination results of three batches of puerarin sustained-release dropping pills
[0067]
[0068] The results show that the RSD values of the weight difference, roundness and drug loading rate of the sustained-release dropping pills prepared under the optimal preparation process conditions of the three batches are all less than 2%. It means that all of them meet the requirements of the Pharmacopoeia, and it can be verified that the optimal preparation process conditions are stable and feasible.
Embodiment 3
[0070] In this embodiment, 0.9% sodium lauryl sulfate solution is used as the release medium, and the dissolution volume is 900mL, which is injected into each dissolution cup, and the temperature of the dissolution tester is adjusted to 37±0.5°C. At (37±0.5)°C, the rotation speed is 100r / min, and the dissolution time is set to 12h. A blank sustained-release dropping pill was used as a control.
[0071] Get each 15 of 3 batches of puerarin sustained-release dripping pills prepared under the best preparation process conditions in Example 1, the total weight is respectively 0.510g, 0.489g, 0.497g, drop into 3 drying baskets, take blank Several slow-release dropping pills, with a total weight of 0.431g, were put into the rotating basket and placed in the dissolution cup as a blank control. Immediately start the rotation and start timing, take samples after 2h, 4h, 6h, 8h, 10h, and 12h, and absorb 5mL of the solution each time. After sampling, add 5mL of 0.9% sodium dodecyl sulfat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com