Stable vitamin C sustained release preparation and preparation method thereof
A technology of slow-release preparations and vitamins, applied to non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of reducing to 16-40%, and achieve easy operation and process design Reasonable, bioavailability-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
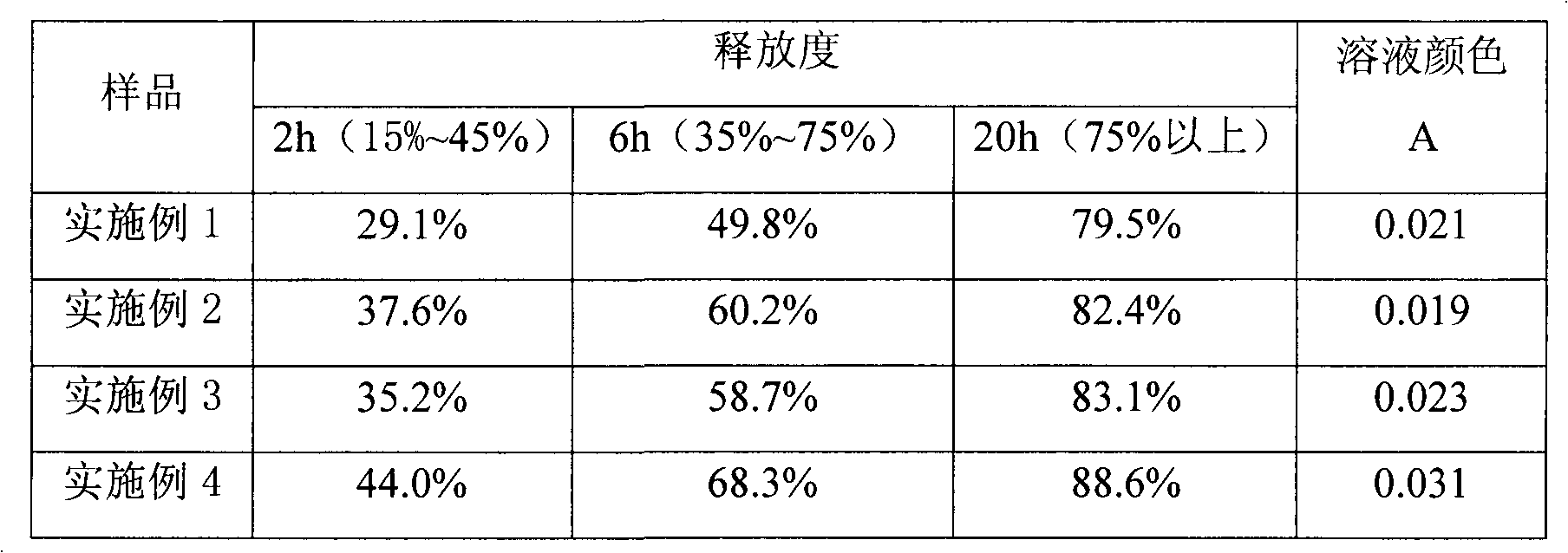
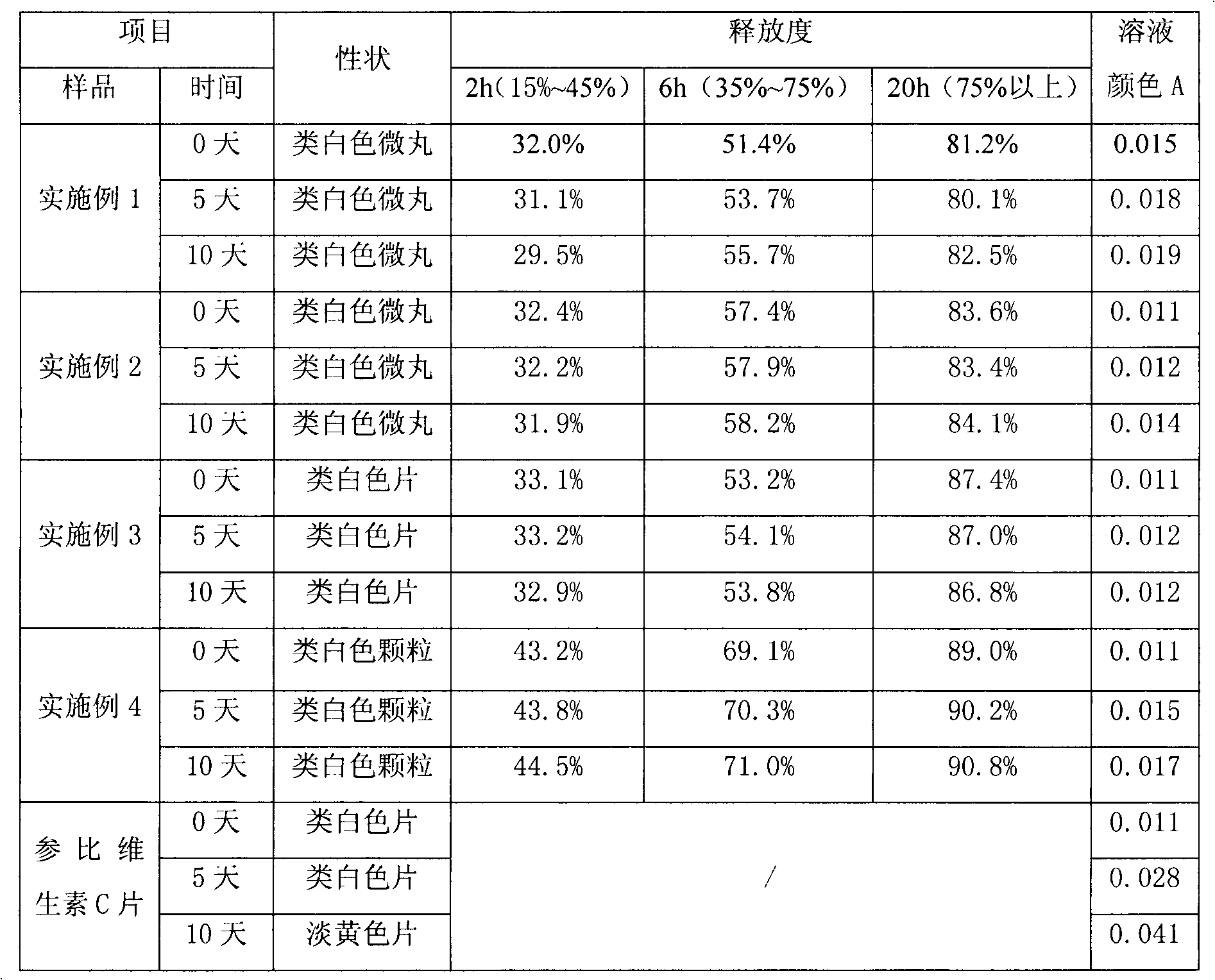
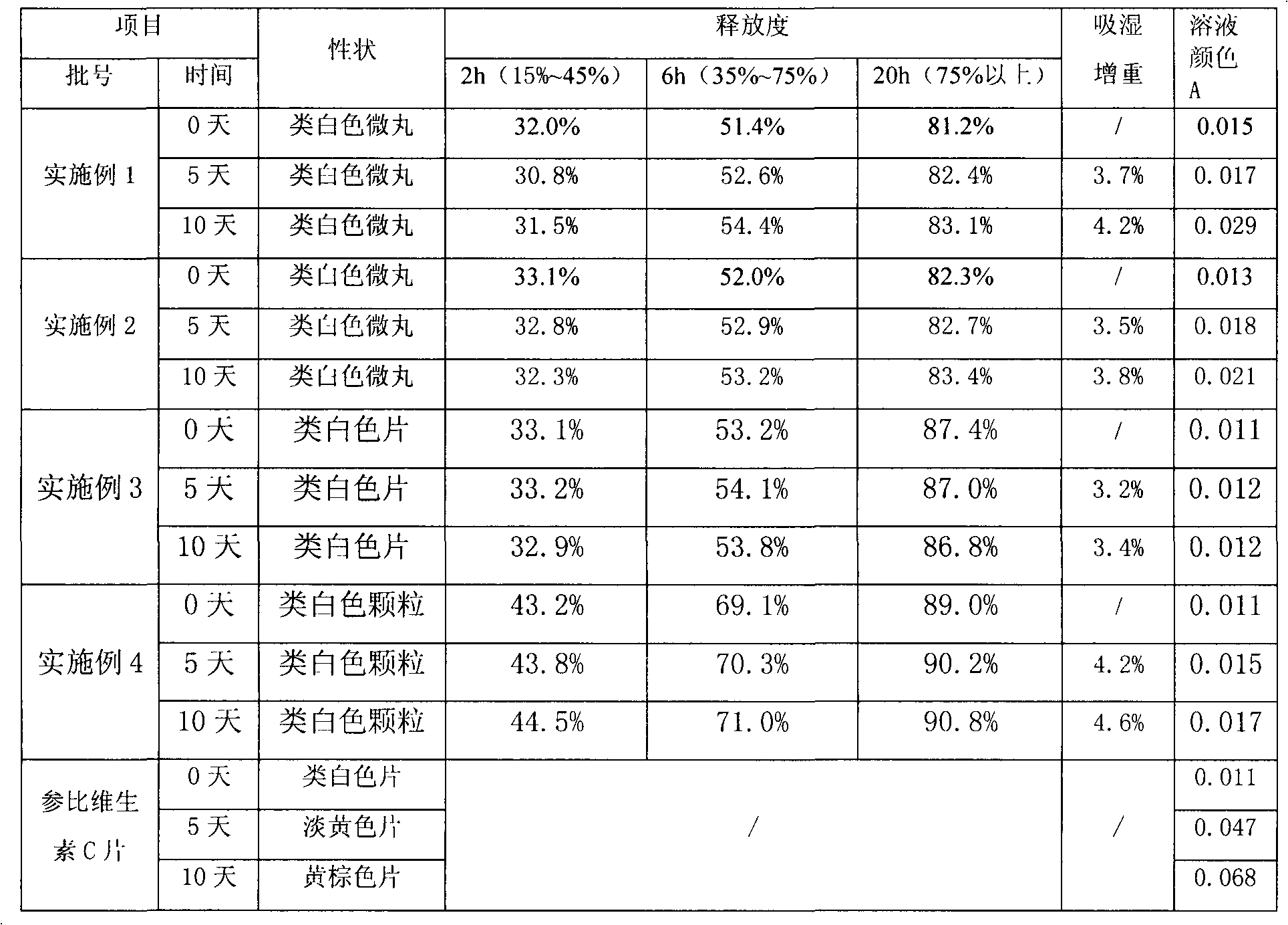
Examples
Embodiment 1
[0024] Example 1: Preparation of vitamin C sustained-release pellets.
[0025] Prescription: Vitamin C 1000g, sucrose core 150g, povidone 40g, tartaric acid 40g, edetate disodium 0.4g, L-cysteine 40g, ethyl cellulose 60g, diphthalate Ethyl ester 6g, talcum powder 6g.
[0026] Preparation Process:
[0027] 1) Load the main drug: put the sucrose pellets in the centrifugal granulator, adjust the parameters of the centrifugal granulator, the air intake temperature is 20-30°C, the rotating speed of the turntable is 150-200rpm, the spray liquid is 5-15rpm, and the spray contains Tartaric acid, disodium edetate in 4% povidone 65% ethanol solution. At the same time, the main drug vitamin C was gradually added in an appropriate amount until all the main drugs were added, then taken out, and the drug-loaded pellets were dried in a hot air circulation oven at 60°C for 6 hours.
[0028] 2) Antioxidant layer: Dissolve L-cysteine in 4% povidone 65% ethanol solution, place the drug-l...
Embodiment 2
[0031] Example 2: Preparation of vitamin C sustained-release pellets.
[0032] Prescription: 850 grams of vitamin C, 50 grams of sucrose ball core, 60 grams of shellac, 40 grams of tartaric acid, 0.4 grams of disodium edetate, 45 grams of L-cysteine, and 6 grams of talcum powder.
[0033] Preparation Process:
[0034] 1) Loading the main drug: put the sucrose pellet core in the centrifugal granulator, adjust the parameters of the centrifugal granulator, the air intake temperature is 15-25°C, the rotating speed of the turntable is 150-200rpm, the spray liquid is 5-20rpm, and the spray contains Tartaric acid, disodium edetate in 15% shellac 95% ethanol solution. At the same time, the main drug vitamin C was gradually added in an appropriate amount until all the main drugs were added, then taken out, and the drug-loaded pellets were dried in a hot air circulation oven at 60°C for 3 hours.
[0035] 2) Anti-oxidation layer: put the drug-loaded pellets in the centrifugal granulat...
Embodiment 3
[0038] Embodiment 3: Preparation of vitamin C sustained release tablet
[0039]Vitamin C 1000g Hypromellose K4M 360g
[0040] Microcrystalline Cellulose 260g Talc 12g
[0041] Silica 12g Tartaric acid 1.5g
[0042] Edetate disodium 1g ethanol appropriate amount
[0043] making process:
[0044] First, dissolve tartaric acid and edetate disodium in an appropriate amount of 75% ethanol; weigh vitamin C, hypromellose K4M, and microcrystalline cellulose in a wet mixing granulator and mix evenly, then add the above ethanol solution Granulation. Dry the wet granules in an oven at 50°C, and sieve the dry granules through a 20-mesh sieve for granulation; then weigh talcum powder and silicon dioxide, add them to the above granules, mix evenly, and press into tablets to obtain vitamin C sustained-release tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com